In 19241 Ludwig Aschoff, the direct linear intellectual descendant of Rudolph Virchow, expounding on whether wall damage alone could lead to thrombosis wrote “Covering this point of endothelial damage, the importance of which is always accepted without question, and which is always given great prominence in the literature, we know in reality practically nothing” (p. 266). Are we farther along 100 years later?
Two carefully performed studies in this issue of Circulation, each professing to leverage endothelial biology to enhance repair after endovascular stenting, disappointed when compared to standard of care. These papers seized on long held beliefs harnessing endothelial biology for optimization of endovascular devices including recapitulating the endothelium de novo with circulating endothelial progenitor cells, enhancing endothelial repair by stimulating local recovery from remnant islets of cells, and preserving endothelium to start. The idea of the first two are that one can overcome the most brutal of vessel injury by resurrecting the damaged endothelial monolayer. The last idea holds that it is best to limit injury to start and allow physiological healing, for nothing we can do is better than nature2.
Jakobsen et al3 tested the first two approaches. The stainless-steel COMBO sirolimus-eluting stent coated luminally with CD34+ antibody was designed to capture endothelial progenitor cells and yet had higher, not lower or equivalent, target lesion revascularization than a current generation cobalt-chrome stent that only eluted sirolimus. In a separate study Lanksy et al4 examined an approach which was touted as enhancing endothelial recovery, and compared two cobalt-chromium stents, the PIONEER with a rapidly eroding PLGA delivering sirolimus and the XIENCE with durable fluoridehexafluoropropylene eluting everolimus over a longer period of time. The early erosion of material and release of drug was thought to allow earlier endothelial recovery enhancing vascular response. Non-inferiority of the rapid release was demonstrated but rather than hints of superiority there were signs of inferiority – here too target lesion revascularization was problematic, now 2.5-fold higher. This could be an incidental finding, but there was not a metric that favored the newer over the established device, and all of this in straightforward lesions. Most lesions had one stent of near 3 mm in diameter and 20 mm in length – what would have been seen in more demanding lesions that required smaller, longer or overlapping devices?
So, is the idea of endothelium as central to vascular repair misguided in device biology. Let’s first look at what we learned from these studies and whether the studies actually achieved what they set out to prove. The COMBO and ORSIRO stents differ in many regards (strut dimensions, stent backbone materials, coating chemistry and duration of degradation and rate of drug release) and not unexpectedly had different vascular reactivity. What was undesired was that this difference was not overcome by attempted recruitment of endothelial cells. Careful though - denouncing the reparative aspects of the endothelium is predicated on the presumption that the recruitment of these endothelial cells accelerates endothelial restoration and this central assumption was never validated. In a similar fashion one cannot indict the role of the endothelium in the failure of rapid coating dissolution of the PIONEER stent to keep pace with target lesion revascularization unless we are sure that this device restores endothelium more effectively than predicate devices. Here too the primary presumption was never proved. The major lesson from these studies then is indeed primum reducere nocere, above all minimize injury, especially to the magic lining of the blood vessel but do not forget Karnovsky’s dictum. Morris Karnovsky was one of the leading lights in vascular biology over the last half of the 20th century5 and held that the dumbest endothelial cell was smarter than the smartest vascular biologist. Both papers we are considering purport to leverage endothelial biology and yet the endothelium is far more complicated than either device presumes, and rather than being disappointing or unexpected both results followed what one would have anticipated.
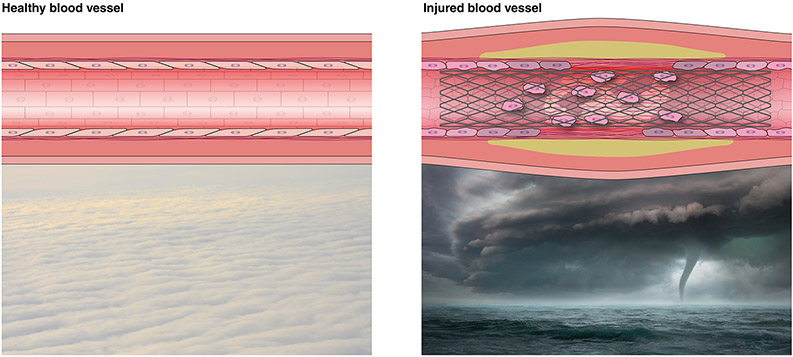
Neither study is surprising for as Karnovsky would have explained the endothelial cells and endothelium acted just as they have evolved to do in controlling vascular repair. While endothelial cells are the building blocks of the organ that is the endothelium, the cells and the organ should not be confused with each other. Endothelial cells are stress-sensing phenotype-switching cells which by definition are sided with an up and a down, a front and back all relative to flow. No other definition aside from flow responsiveness captures all endothelial cells – there is no one universal specific cell differentiation marker or specific form, rather endothelial cells are often substratum-adherent and always flow sensitive. These cells do not create an endothelium simply by being together. There is not the organ until all the cells are intimately linked – the cells and the organ are distinct entities. The organ is the intact monolayer that covers the inner portion of blood vessels and it is only intact when physically confluent and chemically connected - if these ties are broken or disrupted there is no longer an endothelium, the organ reverts to a collection of cells. The intact endothelium ensures quiescence through barrier function and by the balanced expression of factors that stabilize thrombosis and hemostasis, interaction with circulating cells of the immune system, vasomotor tone, proliferation and metabolism. With injury or stress all of these aspects of vascular biology drive to restoring homeostasis even at the expense of initially promoting what might be viewed as seemingly adverse cellular events and even sacrificing pristine architecture (Figure). Endothelial cells, even adherent and even in subjacent islands are not endothelium and can promote proliferation, activate metabolism, vasoconstriction and local thrombosis in a chaotic drive to heal6. The endothelium is like an orderly arrayed carpet of cumulus clouds which in face of a tempest is displaced by chaotic cumulonimbus towering harbingers of thunderstorms. Recruiting endothelial cells does not reconstitute endothelium just as recruiting more thunderclouds cannot evade storms, and retarding and then releasing vascular injury is not synonymous with promotion of endothelial recovery or resurrection of homeostasis.
FIGURE. The endothelium as an organ is more than a collection of endothelial cells.
Like the orderly arrayed carpet of cumulus clouds in calm the cells of the quiescent endothelium appear in an ordered arrangement, and in this configuration support expression of critical factors that keep thrombosis, vasomotor tone, inflammation, proliferation and metabolism in check. Endothelial cells in the face of vascular injury resemble chaotic cumulonimbus clouds that appear as dispersed towering harbingers of thunderstorms. The stresses and damage to endothelium with vascular injury (e.g. stenting) not only disrupts the monolayer barrier but leaves behind ill-appearing remnant endothelial cells and recruits progenitor cells of a clearly different phenotype, both capable of expressing factors that promote what might be viewed as adverse cellular events in a drive to restore homeostasis.
Indeed, the down side of attracting endothelial cells to sites of injury without achieving endothelial cell confluence has been seen in the past with devices coated with antibodies that can bind endothelial precursor cells. As Jakobsen notes, previous versions of endovascular stents and vascular grafts with immobilized CD34 antibody did attract endothelial cells, but neither recapitulated the endothelium and then neither reduced vascular reactivity7. In contrast to uncoated grafts which were devoid of endothelial cells 3 days after implantation, over 95% of the surface of CD34 antibody coated grafts had identifiable endothelial cells, and yet by one month while the uncoated graft coverage had risen to 32%, the antibody covered grafts fell to 85%. The resultant intimal hyperplasia was greater in coated grafts with more of these subconfluent endothelial cells – earlier but not complete coverage led to excess not reduced hyperplasia and reactivity. Yet-to-be confluent endothelial cells promote and do not inhibit local cell and tissue growth. Attracting cells that do not reach confluence should stimulate thrombosis and indeed promote platelet deposition, enhance adhesion of inflammatory cells and evince proliferation of local smooth muscle cells. Rather we need the realization and then stabilization of an intact endothelium as an organ that imposes health and quiescence which cannot be accomplished with the COMBO stent.
Similarly the failure of the rapid eluting PIONEER stent to perform as well as a device that elutes drug longer cannot be ascribed to the inability of a more rapidly restored endothelium to achieve earlier quiescence for this was never proved. The preclinical work reported on this device does not support the idea of providing greater time for endothelial recovery, only that as a drug-eluting device it induced less intimal hyperplasia than its bare metal counterpart8. As with most drug-eluting devices inflammation was a bit heightened and though the endothelium did ultimately recover no faster than any other device used and in fact slower than the bare metal counterpart – endothelial recovery was uncoupled in the animal model from intimal hyperplasia and was not accelerated with short term drug release. Thus, lesser injury and earlier recovery might well have led to better results but neither was evidenced here – there is in fact no link of the performance of this device to endothelial health or enhanced endothelial growth, only a response dictated by stent design and drug kinetics. As to whether drug was eluted long enough or well enough to benefit the spectrum of clinical use cases remains to be proved.
We are farther along than Aschoff claimed in 1924 and know a lot more about the endothelium and vascular biology 100 years later. We have learned that we should continue to celebrate the endothelium as a critical element in vascular biology, striving above all to minimize endothelial injury (primum reducere nocere) upon intervention, enhance its repair and until such time of recapitulation seek to provide protection by way of medication in lieu of endothelial products. This we knew 25 years ago when we proposed in Circulation that more flexible and stronger devices of inert materials produce more favorable clinical results because they induce less injury9, and that more inspired means of locally delivering drugs that seek uniform drug concentration can reduce overexuberant repair. What is new though is that as we test emerging devices we must recognize that it is not sufficient to define initial injury and subsequent repair using pharmacology and stent-based metrics. We must speak the language of the endothelium and directly measure endothelium function and vascular quiescence in declaring innovation in this space. The papers by Jakobsen and Lansky emphasize that the endothelium does a wonderful job of ensuring health, but we must be precise in defining the difference between islands of cells and an intact organ and we cannot forget Karnovsky’s dictum – the endothelium is smart not just good-looking.
Footnotes
DISCLOSURE STATEMENT
none
REFERENCES
- 1.Aschoff Thrombosis L, p. 266, in Lectures on Pathology: (delivered in the United States, 1924) P.B. Hoeber Inc. New York [Google Scholar]
- 2.Edelman ER, Rogers C. Hoop Dreams: Stents Without Restenosis. Circulation 1996;94(6):1199–1202. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen
- 4.Lansky
- 5.Edelman ER, Castellot J, D’Amore P, Raviola Libby P, Raviola E. Obituary. Harvard Medical School home page. 2020. https://fa.hms.harvard.edu/files/hmsofa/files/memorialminute_karnovsky_morris_j.pdf [Google Scholar]
- 6.Nugent MA, Karnovsky MJ, Edelman ER. “Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells.” Circulation Research. 1993. December;73(6):1051–60. doi: 10.1161/01.res.73.6.1051. PMID: 8222077 [DOI] [PubMed] [Google Scholar]
- 7.Rotman JO, Heyligers JMM, Verhagen HJM, Velema E, Nagtegaal MM, de Kleijn DPV, de Groot FG, Stroes ESG, Pasterkamp G In Vivo Cell Seeding With Anti-CD34 Antibodies Successfully Accelerates Endothelialization but Stimulates Intimal Hyperplasia in Porcine Arteriovenous Expanded Polytetrafluoroethylene Grafts 10.1161/CIRCULATIONAHA.104.504407 Circulation. 2005;112:12–18 [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Wang XG, Zheng B, Peng HY, Zhang XY, Zhang B and Huo Y. Investigation of long-term implantation of BuMA stent in a porcine coronary model. Chin Med J (Engl). 2012;125:4083–7. [PubMed] [Google Scholar]
- 9.Rogers C, Parikh S, Seifert P, Edelman ER. “Endogenous cell seeding. Remnant endothelium after stenting enhances vascular repair.” Circulation. 1996. December 1; 94(11):2909–14. doi: 10.1161/01.cir.94.11.2909. PMID: 8941120 [DOI] [PubMed] [Google Scholar]