The greatest cost associated with terrestrial photosynthesis is maintaining hydration in the presence of phenomenal evaporative forces from the atmosphere (Wong et al., 1979). Without the capacity to maintain internal water reserves, vascular plants (tracheophytes) would never have escaped the soil boundary layer (Raven, 1977). Two key adaptations enable homoiohydry in vascular land plants: (i) a means to rapidly conduct water over long distances via xylem and (ii) the ability to regulate water use by stomata (Raven, 1977). Xylem alone has long been credited for the evolutionary success of tracheophytes. Trees are only found in this clade, with most ‘non-vascular’ land plants (bryophytes) confined to the soil boundary layer and relying on vegetative desiccation-tolerance to survive drought (Proctor et al., 2007). In contrast, stomata which predate xylem in the fossil record and are found in most extant land plant clades, are often relegated to a level of lesser importance for driving the evolution of homoiohydric land plants (Raven, 2002). We would argue that physiological data, particularly from bryophytes, challenge this conventional wisdom rooted in morphological observation, and suggest that the evolution of stomatal function was a critical innovation for the evolution of large plants.
WHY ARE BRYOPHYTES SMALL?
Across biomes, taller growth maximizes the capture of light substantially increasing individual productivity. In vascular plants there is abundant evidence that growing tall confers a selective benefit, from the adaptive advantage of a fast growth rate in forest tree seedlings (Walters & Reich, 2000), convergent evolution of woody growth in all major lineages (Stewart & Rothwell, 1983; Lens et al., 2013), as well as competition for light explaining the evolution of canopy structures in forests (Falster & Westoby, 2003). A perplexing question then is why bryophytes have remained apparently immune to this competition, with most species growing within the substrate boundary layer and no evidence of extinct bryophyte trees? Today a majority of bryophyte species are highly adapted to ecological niches devoid of or with minimal competition from vascular plants, or indeed any other photosynthetic organism (Shaw & Renzaglia, 2004), making the idea of them competing with vascular plants for light moot. This argument is somewhat problematic considering that the bryophyte ancestor likely emerged prior to the appearance of vascular plants (Wellman et al., 2003) (although there are no fossils of bryophytes in the Rhynie Chert, one of the oldest known macrofossil land plant assemblages), yet did not evolve to fill the ecological niche rapidly occupied later by early vascular plants. Reproductive limitations may play a role, with dominant bryophyte gametophytes relying on liquid water to transport motile spermatozoids to the female egg (Glibert, 2000). Yet similar reproductive limitations have been overcome in vascular plants (e.g. the evolution of pollen), and in bryophytes spermatozoids can travel vast distances in water (Pressel & Duckett, 2019) or by insect dispersal (Gibson & Miller-Brown, 1927). Furthermore flagellate sperm have not limited the ecological diversity of other vascular plant groups, including ferns (Watkins et al., 2007). While the ecological specialization of most extant bryophytes renders solving the absence of tall bryophytes intractable, the Polytrichales provide a notable exception as the tallest bryophytes (species of Dawsonia, the tallest in this group, reach 0.7 m) (van Zanten, 1973) (Figure 1).
Figure 1.

(A) Polytrichum commune Hedw. has an internal vascular system and is one of the tallest mosses (scale bar = 100 mm), yet this species is dwarfed by vascular plants (B and C). (B) Note the height of the surrounding forest in comparison to the Polytrichum bearing sporophytes in the foreground. (C) Hummocks of Polytrichum (most visible in the bottom right of the image) are often invaded and overtopped by tracheophytes, in this case moncots (seen in the top left of the image).
Recent work in these giants of the bryophyte world has found that the internal water-conducting hydroids in the moss Polytrichum, while of completely independent origin, are functionally analogous to xylem, being capable of transporting considerable volumes of water under negative tension in the vegetative gametophyte (Brodribb et al., 2020; Duckett & Pressel, 2020). This observation raises an intriguing conundrum, why are these tall mosses -that are capable of transporting water though a vascular system- still so small compared to vascular plants? In tracheophytes, stomata are found on the surface of leaves; in mosses, stomata, when present, are confined to the solitary spore capsule in the unbranched sporophyte (Paton & Pearce, 1957). Polytrichum leaves with lamellae only one cell thick means that, while hydraulic conductivity is the same as many tracheophytes, evaporation is poorly regulated. This poses no problem under humid conditions, however when vapor pressure deficit (VPD) increases, the excessive water loss, despite a thick cuticular and wax investiture, results in a negative water potential sufficient to cause embolism, ending water transport (Brodribb et al., 2020). These observations suggest that stomata on leaves were indeed essential for the evolution of homoiohydric land plants, with stomatal closure at high VPD in vascular plants able to reduce significant declines in water potential and thereby prevent embolism (Brodribb et al., 2017).
UNIQUE BRYOPHYTE STOMATA?
If the greatest limitation to Polytrichum competing with vascular plants is simply a lack of stomata on vegetative organs, then why do the leaves of Polytrichales not have stomata? No extant gametophytes have stomata, yet stomata are found on stems below reproductive structures in both sporophyte and gametophyte generations of the extinct pre-vascular plant Aglaophyton (Edwards et al., 1998), suggesting that the dominant life history stage of bryophytes is not in itself a limitation. We argue that while stomata are structurally superficially similar across all land plants, typically taking the form of two guard cells surrounding a pore; considerable evolution in stomatal function across land plant lineages is the reason why, although some bryophytes have highly elaborate vascular tissue, they do not utilize stomata to regulate leaf water loss. In contrast to tracheophytes that bear stomata on anatomically complex leaves and stems, bryophyte stomata are exclusively located on sporangia and contribute to a coordinated process that results in spore production and dispersal rather than to general assimilation (Renzaglia et al., 2017; Duckett & Pressel, 2018). Among bryophytes, stomata are absent in all extant liverworts (Renzaglia et al., 2007; Duckett & Pressel, 2018; Renzaglia et al., 2020), an observation consistent with the maturation of the sporophyte within gametophyte protective tissue. Stomata on sporangia of mosses and hornworts, in contrast to tracheophytes, play an important role in promoting water loss for spore maturation and release (Lucas & Renzaglia, 2002; Duckett et al., 2009; Pressel et al., 2014; Field et al., 2015; Chater et al., 2016; Renzaglia et al., 2017). Once open, thickened cell walls of mature bryophyte stomata render them physically incapable of closing, rendering them useless for mitigating excessive water loss. The capsules of bryophytes are relatively short-lived compared to the subtending gametophytes, consequently the selective pressures to maintain water relations during the growing season that drove the evolution of complex stomatal opening and closing capacity and signals in tracheophytes did not play a role in bryophyte diversification.
Despite these compelling data there still remains a pervasive alternative view that when stomata first appeared, they were already in possession of the full suite of signaling and molecular operating machinery found in modern angiosperms and thus stomatal function was the same as in modern angiosperms (Chater et al., 2011). In a recent example, Zhao et al. (2019) claim that the colonization of land was enabled by an omnipresent chloroplast retrograde signal that closes all stomata during water stress. This paper is similar in conclusion to a body of literature dating back more than a decade professing that all stomata respond to the hormone abscisic acid (ABA) (Chater et al., 2011; Ruszala et al., 2011; Cai et al., 2017). Levels of this hormone increase in angiosperms when water status declines, triggering a signaling cascade that actively closes stomata (Geiger et al., 2009; Lee et al., 2009; Ma et al., 2009; Park et al., 2009; McAdam & Brodribb, 2015). Arguments in support of universal stomatal functional across all land plants deserve close scrutiny, as they imply stomata were irrelevant for plant adaptation, diversification or massive ecological transitions over the past 400 million years, and cannot explain why mosses with efficient hydroids such as Polytrichum have not capitalized on stomata to regulate leaf water loss.
QUESTIONING A UNIVERSAL STOMATAL RESPONSE TO CHLOROPLAST RETROGRADE SIGNALS
Observations of stomatal aperture responses in the moss Sphagnum fallax are central to the theory of Zhao et al. (2019) that a proposed chloroplast retrograde signal, 3’-phosphoadenosine 5’-phosphate (PAP) has closed stomata in response to water deficit for the past 500 million years. These observations are perplexing given that the stomata of Sphagnum species are highly distinct from those of other land plants, and have been described as pseudostomata (Duckett et al., 2009; Merced, 2015). Sphagnum pseudostomata lack pores and subtending intercellular air spaces, and are covered by a calyptra throughout capsule development (Figure 2). The guard cells of Sphagnum never separate to form a discrete pore; they simply collapse when cell volume and turgor decline (Figure 2). Consequently, pseudostomata do not function in the dynamic regulation of gas exchange, as guard cell collapse is irreversible (Duckett et al., 2009; Merced, 2015). Even if PAP drives guard cell re-joining in Sphagnum then the mechanism must have facilitated guard cell inflation, a converse function to the Zhao et al. (2019) model. It should also be noted that water-conducting cells are absent in Sphagnum.
Figure 2.
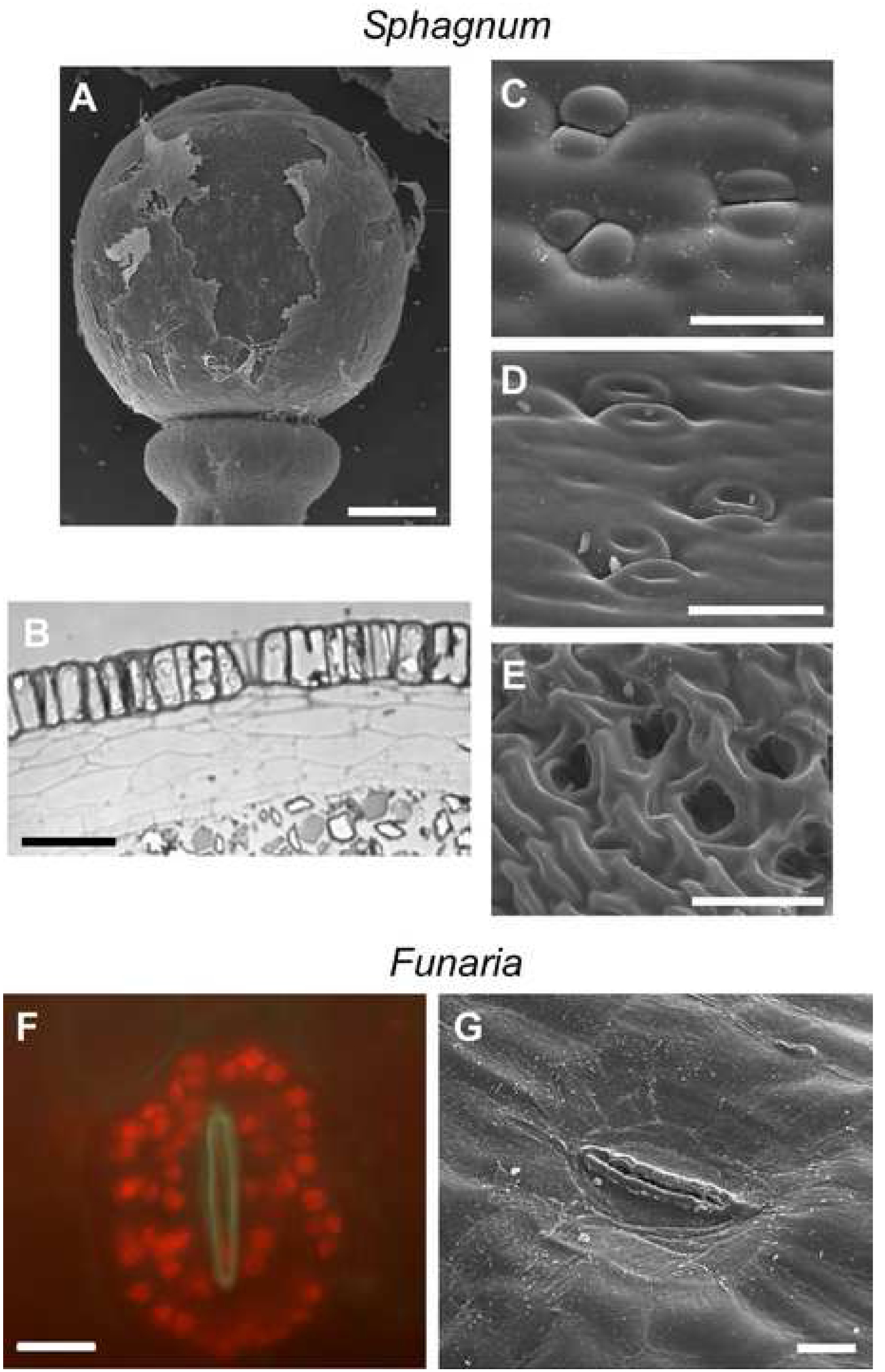
The pseudostomata of Sphagnum are anatomically and functionally unique amongst land plants. (A) Pseudostomata are found on the sporophyte capsule and are covered in a calyptra that ruptures once the sporophyte has reached maturity (scale bar = 300 μm), (B) pseudostomata are not subtended by intercellular air spaces (scale bar = 75 μm). (C) Turgid pseudostomata can be found on a mature sporophyte under the calyptra. (D) As the sporophyte begins to dehisce the guard cells begin to lose turgor. (E) By the time the calyptra has ruptured and the capsule has dehisced the guard cells have shrunken apart at the top, appearing open (scale bars = 60 μm). The stomata of mosses outside the Sphagnopsida, like Funaria hygrometrica Hedw. (F), also open and become locked in that state due to a completely inflexible, thickened wall surrounding the pore (G) which renders them immobile (scale bars = 10 μm).
While questions might arise surrounding the taxonomic validity of the moss used in the study by Zhao et al. (2019), even if another moss species, such as the most likely candidate Funaria (based on the single stomatal image provided), was used in their study, major differences in stomatal function between bryophytes and angiosperms further preclude any conclusion of universal mechanistic homology. Consistent with a role in sporophyte maturation and desiccation, a function that is antithetic to that of tracheophyte stomata, hornwort and moss stomata, including those of Funaria (Figure 2F), open and become locked in that state due to guard cell wall chemistry and architecture preventing subsequent closure (Merced, 2015; Merced & Renzaglia, 2014; Merced & Renzaglia, 2017; Merced & Renzaglia, 2019; Duckett & Pressel, 2018; Pressel et al., 2018). Whereas mature stomata in angiosperms are responsive to a variety of environmental and endogenous cues including light intensity, water status, ABA, plasmolysis and physical damage, those of bryophytes remain unchanged (Duckett & Pressel, 2018; Pressel et al., 2018). Also running contrary to functional congruence across land plants are considerable differences in stomatal numbers and sizes in bryophytes that are unrelated to taxonomy, ecology and genome sizes, and atmospheric CO2 levels (Field et al., 2015; Duckett & Pressel, 2018). Indeed, the loss of stomata in two hornwort clades and at least 60 times in mosses indicates that they are essentially disposable in bryophytes unlike their near universality in vascular plants (Renzaglia et al., 2020).
In mosses and hornworts, ion changes in the guard cells have been found to occur concurrently with similar ion changes in epidermal cells (Duckett et al., 2009; Duckett & Pressel, 2018). Consequently, we cannot conclude that the ion flux data presented by Zhao et al. (2019) were guard cell-specific without epidermal cell controls. Furthermore, the Zhao et al. (2019) model for universal stomatal closure by PAP does not consider evolution in ion channels or their guard cell-specificity (Sussmilch et al., 2019a). These evolutionary transitions have occurred in ion channels that play a critical role in angiosperm guard cell movements: such as the absence of outward- and inward-rectifying Shaker potassium channel genes in bryophyte and lycophyte genomes (Gomez-Porras et al., 2012; Sussmilch et al., 2019a), respectively, and major differences in the activation of S-type anion channels across tracheophytes (McAdam et al., 2016). Importantly, it is yet to be shown if chloroplast signals specifically change guard cell gene expression outside of angiosperms.
EVOLUTION OF STOMATAL FUNCTION IN TRACHEOPHYTES
While the behavior of bryophyte stomata is undoubtedly divergent from the behavior of angiosperm stomata, it has recently been suggested that the ancestor of land plants possessed stomata that functioned like those of the model, annual, angiosperm herb Arabidopsis and that bryophyte stomatal function is highly derived (Rich & Delaux, 2020; Harris et al. 2020). We would argue that evolution of stomatal responses across tracheophyte lineages challenges this view as well as the concept of a universal stomatal closure model by PAP. The in situ stomata of lycophytes and ferns respond to changes in leaf water status as highly predictable passive-hydraulic valves (Brodribb & McAdam, 2011). The stomata of angiosperms do not respond in this way (Buckley, 2019). Contrary to some reports that extremely high levels of exogenous ABA slightly reduces aperture in some fern and lycophyte species (Ruszala et al., 2011; Cai et al., 2017; Hõrak et al., 2017), there is no evidence that endogenous ABA produced by a plant during drought, or any other endogenous metabolic signal like PAP, drives functional stomatal closure under drought stress in species from these lineages (Brodribb & McAdam, 2011; McAdam & Brodribb, 2012; Cardoso et al., 2019; Cardoso et al., 2020). These results suggest that the stomata of the ancestor of vascular land plants responded to leaf water status as passive hydraulic valves and the evolution of a functional stomatal response to ABA (driven by evolution in the interaction of key signaling proteins (Sussmilch et al., 2019b)) arose in the common ancestor of the seeds plants, and was instrumental in the evolutionary success of this lineage of plants.
CONCLUSION
Retrograde signaling may be ancient, but like most plant hormone signaling pathways, neofunctionalization, diversity and cell specificity (e.g. action in guard cells) are likely to have evolved gradually through time (Sussmilch et al., 2019b; Blázquez et al., 2020; Cannell et al., 2020; McAdam & Sussmilch, 2020), not in a single event 500 million years ago. The importance of PAP signals in regulating Arabidopsis stomatal response to water stress was established using mutants (Pornsiriwong et al., 2017); based on current data, it is far from parsimonious to conclude that this signal closes the stomata of all land plants. Nevertheless, this work highlights the critical need to study how diversity in stomatal function has influenced the macroevolution of land plant lineages. This is indeed a critical future endeavor as there is evidence that evolution in these simple structures was instrumental not only in the evolution of homoiohydry and tall stature (Brodribb et al., 2020), or anatomical adaptations that enabled survival during drought (Cardoso et al., 2020), but also the ability of trees to survive in seasonally dry environments (Brodribb et al., 2014), and leaves to attain high rates of photosynthesis (Rockwell & Holbrook, 2017). Furthermore, differences in stomatal function underlie differences in ecological strategies across tracheophytes, particularly with regards to light environment (Doi et al., 2015) or soil water availability (Martínez-Vilalta & Garcia-Forner, 2017). While it is an impactful claim to state a single signal has ruled stomata for all of time (Zhao et al., 2019) or that Arabidopsis physiological function reflects a land plant ancestral state (Rich & Delaux, 2020), such approaches to physiological evolution will never reveal why, for instance, with very similar xylem physiology (Brodribb et al., 2020) and a selective pressure to grow tall (McNickle et al., 2016), Polytrichum does not overtop Sequoia.
ACKNOWLEDGEMENTS
We thank the two reviewers and Jarmila Pittermann for helpful comments. FS would like to acknowledge funding from the Australian Research Council (DE200101133).
LITERATURE CITED
- Blázquez MA, Nelson DC, Weijers D. 2020. Evolution of plant hormone response pathways. Annual Review of Plant Biology 71: 327–353. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Carriquí M, Delzon S, McAdam SAM, Holbrook NM. 2020. Advanced vascular function discovered in a widespread moss. Nature Plants 6(3): 273–279. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SA, Carins Murphy MR. 2017. Xylem and stomata, coordinated through time and space. Plant, Cell and Environment 40(6): 872–880. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. 2011. Passive origins of stomatal control in vascular plants. Science 331(6017): 582–585. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Martins SCV. 2014. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proceedings of the National Academy of Sciences of the United States of America 111(40): 14489–14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN. 2019. How do stomata respond to water status? New Phytologist 224(1): 21–36. [DOI] [PubMed] [Google Scholar]
- Cai S, Chen G, Wang Y, Huang Y, Marchant B, Wang Y, Yang Q, Dai F, Hills A, Franks PJ, et al. 2017. Evolutionary conservation of ABA signaling for stomatal closure in ferns. Plant Physiology 174: 732–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell N, Emms DM, Hetherington AJ, MacKay J, Kelly S, Dolan L, Sweetlove LJ. 2020. Multiple metabolic innovations and losses are associated with major transitions in land plant evolution. Current Biology 30: 1783–1800. [DOI] [PubMed] [Google Scholar]
- Cardoso AA, Randall JM, McAdam SAM. 2019. Hydraulics regulate stomatal responses to changes in leaf water status in the fern Athyrium filix-femina. Plant Physiology 179: 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso AA, Visel D, Kane CN, Batz TA, García Sánchez C, Kaack L, Lamarque LJ, Wagner Y, King A, Torres-Ruiz JM, et al. 2020. Drought-induced lacuna formation in the stem causes hydraulic conductance to decline before xylem embolism in Selaginella. New Phytologist doi: 10.1111/nph.16649. [DOI] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, Fleming A, Cuming AC, Gray JE, Beerling DJ. 2011. Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology 21(12): 1025–1029. [DOI] [PubMed] [Google Scholar]
- Chater CC, Caine RS, Tomek M, Wallace S, Kamisugi Y, Cuming AC, Lang D, MacAlister CA, Casson S, Bergmann DC, et al. 2016. Origin and function of stomata in the moss Physcomitrella patens. Nature Plants 2: 16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Kitagawa Y, Shimazaki K-i. 2015. Stomatal blue light response is present in early vascular plants. Plant Physiology 169(2): 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett JG, Pressel S. 2018. The evolution of the stomatal apparatus: intercellular spaces and sporophyte water relations in bryophytes—two ignored dimensions. Philosophical Transactions of the Royal Society B: Biological Sciences 373(1739). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett JG, Pressel S. 2020. Of mosses and vascular plants. Nature Plants 6(3): 184–185. [DOI] [PubMed] [Google Scholar]
- Duckett JG, Pressel S, P’Ng KMY, Renzaglia KS. 2009. Exploding a myth: the capsule dehiscence mechanism and the function of pseudostomata in Sphagnum. New Phytologist 183(4): 1053–1063. [DOI] [PubMed] [Google Scholar]
- Edwards D, Kerp H, Hass H. 1998. Stomata in early land plants: an anatomical and ecophysiological approach. Journal of Experimental Botany 49: 255–278. [Google Scholar]
- Falster DS, Westoby M. 2003. Plant height and evolutionary games. Trends in Ecology and Evolution 18(7): 337–343. [Google Scholar]
- Field KJ, Duckett JG, Cameron DD, Pressel S. 2015. Stomatal density and aperture in non-vascular land plants are non-responsive to above-ambient atmospheric CO2 concentrations. Annals of Botany 115: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. 2009. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proceedings of the National Academy of Sciences of the United States of America 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RJH, Miller-Brown D. 1927. Fertlization of Bryophyta. Polytrichum commune. (Preliminary Note.). Annals of Botany 41: 190–191. [Google Scholar]
- Glibert SF. 2000. Developmental Biology. Sunderland, MA, USA: Sinauer Associates. [Google Scholar]
- Gomez-Porras JL, Riaño-Pachón DM, Benito B, Haro R, Sklodowski K, Rodríguez-Navarro A, Dreyer I. 2012. Phylogenetic analysis of K+ transporters in bryophytes, lycophytes, and flowering plants indicates a specialization of vascular plants. Frontiers in Plant Science 3: 167–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BJ, Harrison CJ, Hetherington AM, Williams TA. 2020. Phylogenomic evidence for the monophyly of bryophytes and the reductive evolution of stomata. Current Biology 30: 2001–2012. [DOI] [PubMed] [Google Scholar]
- Hõrak H, Kollist H, Merilo E. 2017. Fern stomatal responses to ABA and CO2 depend on species and growth conditions. Plant Physiology 174: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. 2009. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proceedings of the National Academy of Sciences of the United States of America 106: 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Davin N, Smets E, Arco Md. 2013. Insular Woodiness on the Canary Islands: A Remarkable Case of Convergent Evolution. International Journal of Plant Sciences 174: 992–1013. [Google Scholar]
- Lucas JR, Renzaglia KS. 2002. Structure and function of hornwort stomata. Microscopy and Microanalysis 8: 1090CD. [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. 2009. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 324: 1064–1068. [DOI] [PubMed] [Google Scholar]
- Martínez-Vilalta J, Garcia-Forner N. 2017. Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant, Cell and Environment 40: 962–976. [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ. 2012. Stomatal innovation and the rise of seed plants. Ecology Letters 15: 1–8. [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ. 2015. The evolution of mechanisms driving the stomatal response to vapour pressure deficit. Plant Physiology 167: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ, Banks JA, Hedrich R, Atallah NM, Cai C, Geringer MA, Lind C, Nichols DS, Stachowski K, et al. 2016. Abscisic acid controlled sex before transpiration in vascular plants. Proceedings of the National Academy of Sciences of the United States of America 113: 12862–12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC. 2020. The evolving role of abscisic acid in cell function and plant development over geological time. Seminars in Cell & Developmental Biology doi: 10.1016/j.semcdb.2020.06.006. [DOI] [PubMed] [Google Scholar]
- McNickle GG, Wallace C, Baltzer JL. 2016. Why do mosses have height? Moss production as a tragedy of the commons game. Evolutionary Ecology Research 17: 75–93. [Google Scholar]
- Merced A 2015. Novel insights on the structure and composition of pseudostomata of Sphagnum. American Journal of Botany 102: 329–335. [DOI] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2015. Developmental changes in guard cell wall structure and pectin composition in the moss Funaria: implications for function and evolution of stomata. Annals of Botany 114: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merced A, Renzaglia KS. 2017. Structure, function and evolution of stomata from a bryological perspective. Bryophyte Diversity and Evolution 39: 7–20. [Google Scholar]
- Merced A, Renzaglia KS. 2019. Contrasting pectin polymers in guard cell walls of Arabidopsis and the hornwort Phaeoceros reflect physiological differences. Annals of Botany 123: 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow T-F, et al. 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JA, Pearce JV. 1957. The occurrence, structure and functions of the stomata in British bryophytes. Transactions of the British Bryological Society 3: 228–259. [Google Scholar]
- Pornsiriwong W, Estavillo GM, Chan KX, Tee EE, Ganguly D, Crisp PA, Phua SY, Zhao C, Qiu J, Park J, et al. 2017. A chloroplast retrograde signal, 3’-phosphoadenosine 5’-phosphate, acts as a secondary messenger in abscisic acid signaling in stomatal closure and germination. eLife 6: e23361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressel S, Duckett JG. 2019. Do motile spermatozoids limit the effectiveness of sexual reproduction in bryophytes? Not in the liverwort Marchantia polymorpha. Journal of Systematics and Evolution 57: 371–381. [Google Scholar]
- Pressel S, Goral T, Duckett JG. 2014. Stomatal differentiation and abnormal stomata in hornworts. Journal of Bryology 36: 87–103. [Google Scholar]
- Pressel S, Renzaglia KS, Clymo RS, Duckett JG. 2018. Hornwort stomata do not respond actively to exogenous and environmental cues. Annals of Botany 122: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, Alpert P, Stark LR, Cleavitt NL, Mishler BD. 2007. Desiccation-tolerance in bryophytes: a review. The Bryologist 110: 595–621. [Google Scholar]
- Raven JA. 1977. The evolution of vascular land plants in relation to supercellular transport processes. Advances in Botanical Research 5: 153–219. [Google Scholar]
- Raven JA. 2002. Selection pressures on stomatal evolution. New Phytologist 153: 371–386. [DOI] [PubMed] [Google Scholar]
- Renzaglia KS, Browning WB, Merced A. 2020. With over 60 independent losses, stomata are expendable in mosses. Frontiers in Plant Science 11: 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Schuette S, Duff RJ, Ligrone R, Shaw AJ, Mishler BD, Duckett JG. 2007. Bryophyte phylogeny: advancing the molecular and morphological frontiers. The Bryologist 110: 179–213, 135. [Google Scholar]
- Renzaglia KS, Villarreal JC, Piatkowski BT, Lucas JR, Merced A. 2017. Hornwort stomata: architecture and fate shared with 400-million-year-old fossil plants without leaves. Plant Physiology 174: 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich MK, Delaux P-M. 2020. Plant evolution: when Arabidopsis is more ancestral than Marchantia. Current Biology 30: R642–R644. [DOI] [PubMed] [Google Scholar]
- Rockwell FE, Holbrook NM. 2017. Leaf hydraulic architecture and stomatal conductance: a functional perspective. Plant Physiology 174: 1996–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM. 2011. Land plants acquired active stomatal control early in their evolutionary history. Curr Biology 21: 1030–1035. [DOI] [PubMed] [Google Scholar]
- Shaw J, Renzaglia K. 2004. Phylogeny and diversification of bryophytes. American Journal of Botany 91: 1557–1581. [DOI] [PubMed] [Google Scholar]
- Stewart WN, Rothwell GW. 1983. Paleobotany and the Evolution of Plants. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Sussmilch FC, Roelfsema MRG, Hedrich R. 2019a. On the origins of osmotically driven stomatal movements. New Phytologist 222: 84–90. [DOI] [PubMed] [Google Scholar]
- Sussmilch FC, Schultz J, Hedrich R, Roelfsema MRG. 2019b. Acquiring control: the evolution of stomatal signalling pathways. Trends in Plant Science 24: 342–351. [DOI] [PubMed] [Google Scholar]
- van Zanten BO. 1973. A taxonomic revision of the genus Dawsonia R. Brown. Lindbergia 2: 1–48. [Google Scholar]
- Walters MB, Reich PB. 2000. Seed size, nitrogen supply, and growth rate affect tree seedling survival in deep shade. Ecology 81: 1887–1901. [Google Scholar]
- Watkins JE, Mack MC, Sinclair TR, Mulkey SS. 2007. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytologist 176: 708–717. [DOI] [PubMed] [Google Scholar]
- Wellman CH, Osterloff PL, Mohiuddin U. 2003. Fragments of the earliest land plants. Nature 425: 282–285. [DOI] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. 1979. Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426. [Google Scholar]
- Zhao C, Wang Y, Chan KX, Marchant DB, Franks PJ, Randall D, Tee EE, Chen G, Ramesh S, Phua SY, et al. 2019. Evolution of chloroplast retrograde signaling facilitates green plant adaptation to land. Proceedings of the National Academy of Sciences of the United States of America 116: 5015–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]


