TO THE EDITOR: Macrophage checkpoint inhibition, an approach in which the phagocytic clearance of cancer cells is reactivated, represents a new paradigm in immuno-oncology. In parallel, a defect in “efferocytosis” (i.e., the removal of inflamed and dying cells by phagocytosis) is now recognized as a hallmark of atherosclerotic disease, which is caused in part by pathologic up-regulation of the antiphagocytic signal molecule CD47.1,2 A recent phase 1b–2 trial of a humanized anti-CD47 antibody (magrolimab) showed promising results in tumor reduction, as measured by 18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography and computed tomography in patients with relapsed or refractory lymphoma.3 Given that anti-CD47 therapies reduced atherosclerotic burden and plaque rupture in preclinical studies,1 we hypothesized that magrolimab might reduce vascular inflammation, as quantified by 18F-FDG uptake, in the carotid arteries of these participants.4,5
Details of the methods used in this retrospective study are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org. The baseline characteristics of the nine study patients are shown in Table S1 in the Supplementary Appendix. The patients’ ages ranged from 59 to 81 years, and two of the patients were women. Cardiovascular risk factors were common: four patients had diabetes mellitus, and eight had hypertension. Six patients had known atherosclerotic disease at baseline, including two with previous myocardial infarction. Seven patients had coronary-artery calcification, including four with moderate to severe scores.
After 9 weeks of treatment with magrolimab, we observed a significant reduction in 18F-FDG uptake, measured as maximum standardized uptake values (mean [±SD], 2.68±0.59 vs. 2.06±0.52; P = 0.02) and target-to-background ratios (mean, 1.56±0.22 vs. 1.28±0.11; P = 0.01) in the most diseased segment of the index carotid artery (Fig. 1). Data for individual patients are shown in Table S2. Of note, we did not observe any effect of magrolimab on physiological 18F-FDG uptake elsewhere in the body (Fig. S1A) or on two available traditional cardiovascular risk factors — fasting serum glucose level and blood pressure (Fig. S1B and Table S3).
Figure 1. Vascular 18F-FDG Uptake before and after Magrolimab Treatment.
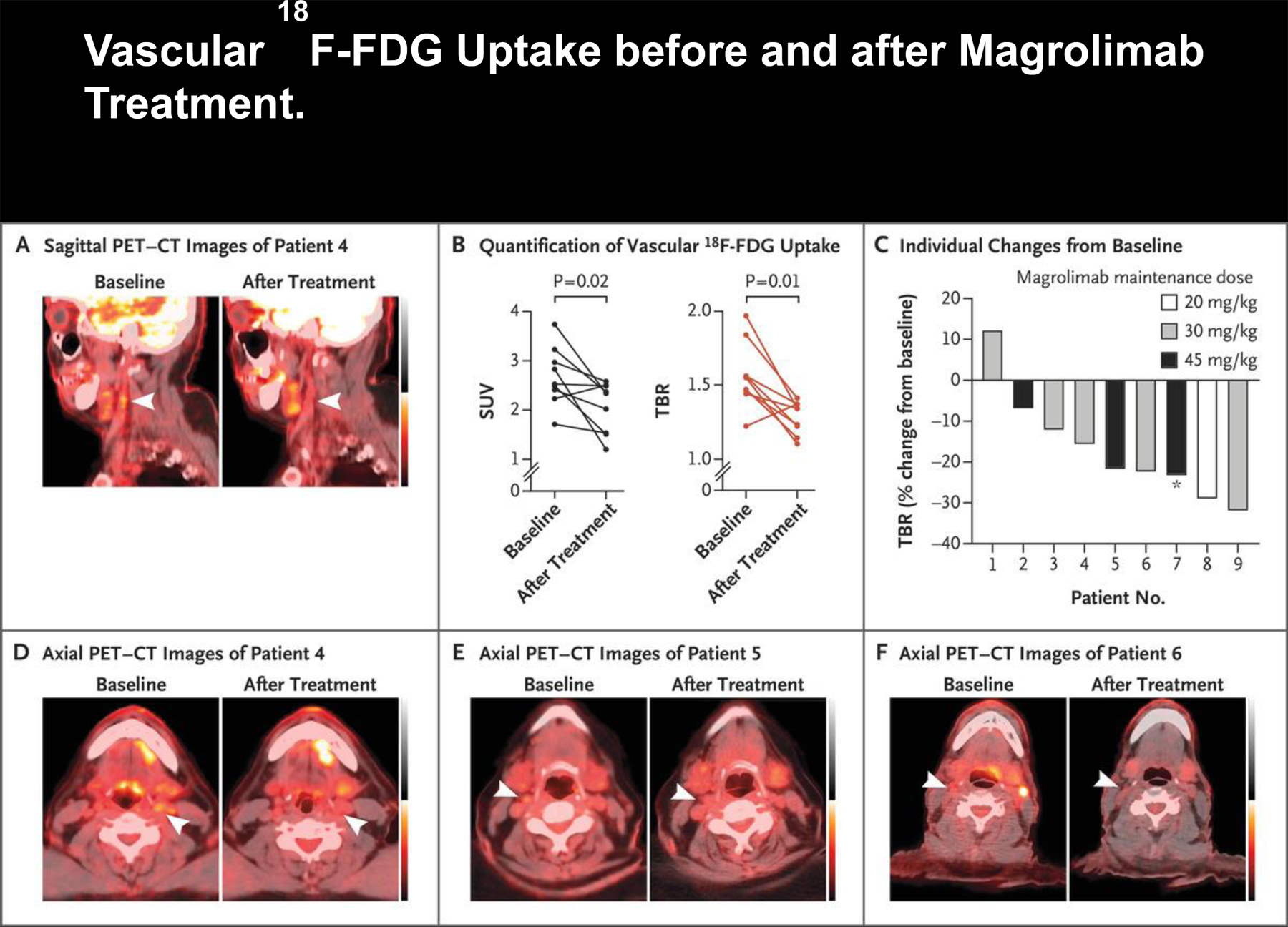
Panel A shows 18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography and computed tomography (PET–CT) scans (sagittal view) of Patient 4, who had a reduction in vascular 18F-FDG uptake in the index carotid artery (arrowheads) after magrolimab treatment. Panel B shows the maximum standardized uptake values (SUVs) and target-to-background ratios (TBRs) in the most diseased segment of the index carotid artery in all nine patients at baseline and after magrolimab treatment. Panel C shows a waterfall plot of maximum TBR in all nine patients according to the maintenance dose received. Of note, for Patient 7 (asterisk), treatment was initiated at a maintenance dose of 45 mg per kilogram of body weight, but the dose was later changed to 30 mg per kilogram. Panels D, E, and F show 18F-FDG PET–CT scans (axial view) of Patients 4, 5, and 6, respectively. Arrowheads indicate the index vessel (carotid artery) at baseline and after magrolimab treatment. Data from each patient before and after treatment were compared and analyzed with a Wilcoxon matched-pairs signed-rank test (two-tailed).
This retrospective analysis was limited by the inclusion of only a small number of patients at a single institution, and the study was neither randomized nor placebo-controlled. Although seven of the nine patients happened to have coronary-artery calcification, it is important to emphasize that the patients in this analysis had lymphoma and that magrolimab was used in combination with rituximab. Lastly, the present study did not allow an estimation of whether plaque composition or intraplaque efferocytosis rates were modified by magrolimab treatment.
This study showed that the CD47-targeting macrophage checkpoint inhibitor magrolimab may reduce arterial 18F-FDG uptake and suppress vascular inflammation. These preliminary observations require confirmation in a prospective trial.
Supplementary Material
THIS WEEK’S LETTERS.
| 382 | Effect of CD47 Blockade on Vascular Inflammation |
| 384 | Dasatinib-Blinatumomab for Ph-Positive ALL |
| 384 | Empagliflozin in Heart Failure |
| 388 | Dapagliflozin in Patients with Chronic Kidney Disease |
Acknowledgments
Supported by grants from the National Institutes of Health (R35 HL144475, to Dr. Leeper), the Deutsche Forschungsgemeinschaft (JA 2869/1-1:1, to Dr. Jarr), the Deutsche Herzstiftung (S/09/19, to Dr. Jarr), the American Heart Association (EIA34770065, to Dr. Leeper), the Greathouse Family Foundation (to Dr. Leeper), and Ludwig Cancer Research (to Dr. Weissman).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Kojima Y, Volkmer J-P, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 2016; 536: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojima Y, Weissman IL, Leeper NJ. The role of efferocytosis in atherosclerosis. Circulation 2017; 135: 476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Advani R, Flinn I, Popplewell L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N Engl J Med 2018; 379: 1711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tawakol A, Fayad ZA, Mogg R, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol 2013; 62: 909–17. [DOI] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002; 105: 1135–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


