Abstract
Sex differences in METH use exist among human METH users and in animal models of METH addiction. Herein, we tried to identify potential differences in gene expression between female and male rats after Methamphetamine self-administration (METH SA). Rats were trained to self-administer METH using two 3-hours daily sessions for 20 days. Cue-induced drug seeking was measured on withdrawal days 3 (WD3) and 30 (WD30). Rats were euthanized twenty-four hours after WD30. Prefrontal cortex (PFC) and hippocampus (HIP) were dissected to measure mRNA expression. Both female and male rats increased their METH intake and showed increased METH seeking during withdrawal. Female had higher basal level expression of hypocretin receptor 1 (Hcrtr1) and prodynorphin (Pdyn) mRNAs in the PFC and HIP. Basal corticotropin releasing hormone receptor 1 (Crhr1), Crh receptor 2 (Crhr2), hypocretin receptor 2 (Hcrtr2) and opioid receptor kappa 1 (Oprk1) mRNA levels were higher in the PFC of females. Male rats had higher basal levels of Crh and Crhr1 in HIP. METH SA was associated with increased Crh and Crhr1 in the HIP of both sexes and Crhr2 only in female HIP. Importantly, increased Crh and Crhr1 mRNA levels correlated positively with incubation of METH craving in both sexes, supporting their potential involvement, in part, in the regulation of this behavioral phenomenon. When taken together, our results identified sexual dimorphic baseline differences in rats. We also detected dimorphic responses in animals that had self-administered METH. These observations highlight the importance of understanding the molecular neurobiology of sex differences when therapeutic interventions are planned against METH addiction.
Keywords: Addiction, Sexual dimorphism, Methamphetamine Self-administration, Hippocampus, Prefrontal cortex
INTRODUCTION
METH use disorder (MUD) is a biopsychosocial disorder that impacts both men and women negatively (DSM–5, 2013; Zhang et al., 2013; Radfar and Rawson, 2014; Kogachi et al., 2017). METH abusers experience cognitive dysfunctions (Radfar and Rawson, 2014; Cadet and Bisagno, 2015) that are due to METH-induced neuropathological changes in various brain regions including the prefrontal cortex (PFC) and hippocampus (HIP) (Cadet et al., 2014a; London et al., 2015; Proebstl et al., 2018). Women appear to become more dependent on METH and may experience more frequent relapses during periods of abstinence (Gonzales et al., 2010; Reichel et al., 2012). There also appear to be some sex differences in the severity in METH-induced cognitive deficits and structural alterations in METH using individuals (Becker and Hu, 2008; Dluzen and Liu, 2008; He et al., 2013; Zhang et al., 2013; Radfar and Rawson, 2014; Kogachi et al., 2017).
Sex differences in animal models of METH use disorder also exist (Roth and Carroll, 2004; Reichel et al., 2012; Cox et al., 2013; Ruda-Kucerova et al., 2015; Venniro et al., 2017; Lynch, 2018; Daiwile et al., 2019). Male rats self-administer more METH at higher doses (Ruda-Kucerova et al., 2015; Daiwile et al., 2019) whereas female rats take more METH at lower doses (Roth and Carroll, 2004; Reichel et al., 2012). Nevertheless, there have been only few reports on the potential biochemical and molecular bases for these behavioral differences (Zuloaga et al., 2014; Johansen and McFadden, 2017; McFadden et al., 2018; Pena-Bravo et al., 2019). Johansen and McFadden, (2017) reported increased BDNF expression in the HIP of only male rats after METH SA. In addition, Pena-Bravo et al., (2019) found that only females upregulated GluN2B-lacking NMDA receptors (NMDAR) in the PFC after METH self-administration (SA). Moreover, Daiwile et al. (2019) reported that METH SA induced sex-related differential changes in prodynorphin (Pdyn), hypocretin receptor 1 (Hcrtr1), hypocretin receptor 2 (Hcrtr2), corticotropin releasing hormone (Crh) and arginine vasopressin (Avp) mRNA levels in the nucleus accumbens (NAc). These observations support the importance of elucidating the molecular substrates of sex differences in animal models of MUD because they could better inform the development of pharmacological treatments against this public health problem (Becker and Chartoff, 2019; Cadet et al., 2019).
Distinct but interconnected brain regions including the PFC and HIP play pivotal roles in neuronal processes such as learning, decision making and memory that are important to the behavioral manifestations of drug addiction (Goldstein and Volkow, 2011; Volkow et al., 2012; Everitt, 2014; Cadet et al., 2015; Kutlu and Gould, 2016; Hu et al., 2019). In the present study, we focused on identifying potential sex differences in the expression of several PFC and HIP neuropeptides that have been implicated in psychostimulant addiction (Cadet et al., 2019).
EXPERIMENTAL PROCEDURES
Animals and SA Procedures
We performed SA training procedures (METH SA and withdrawal day) and used METH dose according to our previous publications (Cadet et al., 2016; Torres et al., 2017; Daiwile et al., 2019, Hu et al., 2019; Job et al., 2020). Briefly, twenty-four female and male Long Evans rats weighing 350–500 g and 450–600 g, respectively, were used in these experiments. Rats were procured from NIDA breeding facility, Baltimore, MD, USA (RRID: RGD_1566430). Ketamine and xylazine (50 and 5 mg/kg, i.p., respectively) were used to anesthetize rats. As previously described a silastic catheter (SAI Infusion Technologies, Lake Villa, IL, USA) was then implanted into the right jugular vein (Daiwile et al., 2019). Drug-naive rats were used in the study and they were also not food-trained, before the start of the METH SA experiment. Rats were arbitrarily assigned to either METH (n = 18) and saline (n = 6) groups. After recovery, rats were trained to self-administer METH (0.1 mg/kg/injection, i.v.) in Med Associates SA chambers (Fairfax, VT, USA) for the period of two 3-hours sessions/day (separated by a 30-minute off interval) for 20 days using a FR-1 schedule with 20-second timeouts. We did not limit the number of infusions per 3h session but each 3h session was separated by a 30 min break during which the house light was turned off and the active lever was retracted. Rats were trained to self-administer METH, 5 days a week, with weekends off. Rats remained in SA chambers during weekends off and were disconnected from i.v. SA lines. Control (Ct) group rats self-administered saline under similar condition. After completion of training phase (20 days), rats were individually housed in vivarium with no access to METH. On withdrawal days 3 (WD3) and 30 (WD30) rats were assessed for cue-induced drug seeking. Rats were brought back to their respective SA chambers on each test day, to assess cue-induced drug seeking during withdrawal period. Drug seeking test was consisted of a 3-hour session, during which press on METH-associated lever resulted in the tone and light cues previously paired with METH infusions, but no METH infusions. Rats put to the test on WD3 were also tested on WD30. Animal procedures were approved and conducted according to the Guide for the Care and Use of Laboratory Animals (ISBN 0–309-05377–3) by the National Institute of Drug Abuse Animal Care and Use Committee (NIDA-ACUC). As previously reported, after completion of METH SA training, we further divided both female and male rats into two separate groups based on their METH intake (Daiwile et al., 2019). METH High Takers (MHT) significantly increased their METH intake from the second week onwards whereas the METH Low Takers (MLT) did not exhibit escalation.
Tissue Collection and RNA Extraction
To assess potential sex and brain region-specific differences in gene expression after drug abstinence, rats were killed 24 hours after the WD30 (30 days after the last METH SA session) following rapid decapitation with guillotine. PFC and HIP tissues were dissected from the brains using specific neuroanatomical coordinates based on rat Atlas, immediately snap-frozen on dry ice and stored at −80°C. Total RNA was isolated using Qiagen RNeasy Mini kits and treated against genomic DNA contamination by using Qiagen RNase-free DNase kits (Qiagen, Germantown, MD, USA) following the manufacturer’s protocol. RNA quantity and purity were then assessed using a NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
Quantitative RT-PCR
Five hundred nanograms (500 ng) of total RNA was reverse transcribed to cDNA using oligo dT primer from the Advantage RT for PCR kit (Clontech, Takara Bio, Mountain View, CA, USA). Gene-specific qPCR primers were purchased from the Synthesis and Sequencing Facility of Johns Hopkins University, Baltimore, MD, USA. 20 μL qPCR reactions comprising of cDNA, gene-specific primers, nucleic-acid free water, and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) were conducted in LightCycler 480 II instrument (Roche, South San Francisco, CA, USA) using standard curve method. Primer sequences for Pdyn, Prodynorphin; Penk, Proenkephalin; Oprk1, Opioid receptor kappa 1; Oprm1, Opioid receptor mu 1; Oprd1, Opioid receptor delta 1; Hcrtr1, Hypocretin receptor 1; Hcrtr2, Hypocretin receptor 2; Crh, Corticotropin releasing hormone; Crhr1, Crh receptor 1; Crhr2, Crh receptor 2 used in the study are listed in supplementary table 1. Melting curve analysis was used to check the purity of each amplicon. We used standard curve method for qPCR. Expression for each gene was normalized to the reference genes (housekeeping genes) 18S and Clathrin and the analyzed gene data was represented as relative fold change.
Statistical Analyses
METH intake per week, withdrawal day (Active lever presses), and gene expression analysis were analyzed using 2-way ANOVA followed by Fisher’s protected least significance difference post-hoc test using GraphPad Prism 8. We used baseline gene expression [raw read values of gene obtained after qPCR without normalizing to the two housekeeping genes, which also were referred as mRNA expression raw read (arbitrary unit) in graph] analysis to account for any possible sexual dimorphism in basal expression which might be missed if fold changes were used in these preliminary analyses. For the regression analysis, levels of expression of each genes were reported as relative fold changes calculated as the ratios of normalized gene expression data of METH SA groups compared with data of the saline group. For all analyses, the null hypothesis was rejected at P ≤ 0.05.
RESULTS
Sex Differences in Meth intake during self-administration training but not in METH seeking behavior after prolonged withdrawal
Patterns of METH intake per week for female and male rats are illustrated in Fig. 1A. As previously reported (Daiwile et al., 2019), two-way ANOVA between female METH SA vs male METH SA rats showed significant effects of sex [F (1, 30) = 6.531, P = 0.0159], METH intake [F (3, 90) = 37.44, P < 0.0001], and METH intake x sex interactions [F (3, 90) = 2.758, P = 0.0469], thus indicating that male rats took more METH than females. Post-hoc test revealed that male rats self-administered more METH during the second (P = 0.0227) and third (P = 0.0092) weeks (Fig. 1A). The comparison between female saline vs male saline showed no significant effect of sex or saline intake x sex interactions, details are provided in supplementary figure 1.
Fig. 1. Female and male rats escalate their METH intake during METH SA.
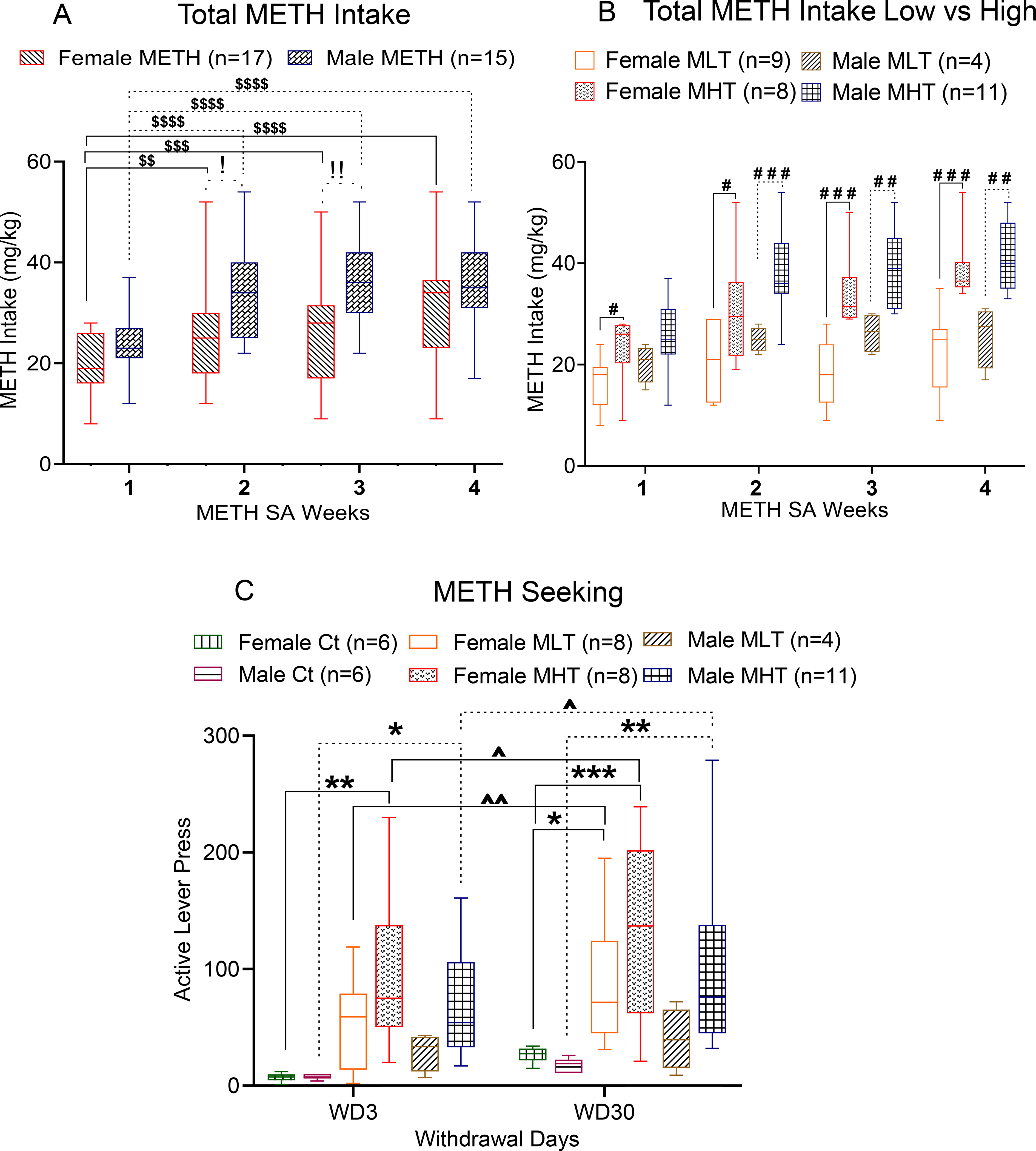
(A) Patterns of weekly METH taking behaviors by female and male rats. (B) Drug intake by low [(female MLT, n = 9) and (male MLT, n = 4)] and high [(female MHT, n = 8) and (male MHT, n = 11)] METH taker rats. (C) METH-seeking behaviors by female and male rats at WD3 and WD30. Key to statistics: *P < 0.05, **P < 0.01, ***P < 0.001, female and male METH groups compared with respective saline groups; #P < 0.05, ##P <0.01, ###P < 0.001, comparison of male METH group with female METH group; $P <0.05, $ $P < 0.01, $ $ $P < 0.001, comparison of weekly METH intake of female and male rats with respect to METH intake on the first week of training; !P < 0.05, !!P < 0.01, !!!P < 0.001, comparison between female vs male METH takers. ^P < 0.05 within sex comparison of lever pressing at WD3 and WD30. All values represent means + SEM of number of animals indicated in the figure. Ct, Control Saline Rats; MLT, METH Low Takers; MHT, METH High Takers.
We were also able to divide both female and male rats into two separate groups based on their METH intake. Rats that significantly increased their METH intake from the second week onwards: METH High Takers (MHT) and the other rats: METH Low Takers (MLT), hereafter they were referred as MLT or MHT (Fig. 1B). Two-way ANOVA comparing female and male MLTs revelated significant effects of METH intake (F (3, 33) = 4.674, P = 0.0079), no significant effect of sex (F (1, 11) = 2.262, P = 0.1607) and no significant interaction (F (3, 33) = 0.8708, P = 0.4660)]. Similar analysis of MHTs showed significant effects of METH intake (F (3, 51) = 42.52, P < 0.0001), no significant sex effect (F (1, 17) = 1.817, P = 0.1953) nor significant interaction (F (3, 51) = 1.823, P = 0.1547)]. There were significant differences in METH intake by female MHTs compared to female MLTs from the beginning to the end of the SA experiments, with significant effects of METH intake (F (3, 45) = 16.95, P < 0.0001), group (F (1, 15) = 16.23, P = 0.0011) and group x METH interaction (F (3, 45) = 4.843, P = 0.0053)] (Fig. 1B). In the case of male rats, differences were observed after the second week of SA, with significant effects of METH intake (F (2.883, 37.48) = 18.26, P < 0.0001), group (F (1, 13) = 11.97, P = 0.0042) and their interaction (F (3, 39) = 3.768, P = 0.0181)] (Fig. 1B). Interestingly, 47% (8/17) of females while 73% (11/15) of males were high METH takers, in a manner consistent with observations in some patient populations (He et al., 2013; Zhang et al., 2103).
Earlier, we had reported the drug seeking behavior of both male and female rats to correlate positively between total METH intake and active lever presses on WD3 and 30 (Daiwile et al., 2019). Herein, we tested if there any sex differences in METH seeking behavior in MHT and MLT rats after prolonged withdrawal, we compared cue-induced active lever responses on WD3 and 30. Two-way ANOVA for female vs male MLT rats showed no effect of sex (F (1, 10) = 2.431, P = 0.1500), withdrawal day (F (1, 10) = 4.847. P = 0.0523) and their interaction (F (1, 10) = 1.388, P = 0.2660). However, for female vs male MHT rats, 2-way ANOVA showed significant effects of withdrawal day (F (1, 17) = 7.270, P = 0.0153) but no effect of sex (F (1, 17) = 1.429, P = 0.2484) or their interaction (F (1, 17) = 0.01041, P = 0.9199). We also compared METH seeking data for Ct, MLT, MHT female rats and found significant effects of withdrawal day (F (1, 19) = 16.48, P = 0.0007), group (F (2, 19) = 6.694, P = 0.0063), but not their interaction (F (2, 19) = 0.4569, P = 0.6400). Similar comparisons for male Ct, MLT, and MHT rats revealed significant effects of group (F (2, 18) = 6.745, P = 0.0065), but no effect of withdrawal day (F (1, 18) = 2.627, P = 0.1224) or their interaction (F (2, 18) = 0.5705, P = 0.5751). Post hoc analysis showed greater cue-induced lever pressing by female MLT and MHT rats, but only male MHT rats showed incubation on WD30 compared to WD3 (Fig. 1C).
METH-induced changes in dynorphin and enkephalin mRNAs in PFC and HIP.
Dynorphin (Shippenberg et al., 2007; Butelman et al., 2012) and enkephalin (Cadet et al., 2016; Mongi-Bragato et al., 2018) neuropeptides are thought to play important roles in addiction to psychostimulants. To identify any potential sex and regional differences in their expression, we compared the levels of prodynorphin (Pdyn) and proenkephalin (Penk) mRNAs in the PFC and HIP of drug naive and METH-exposed female and male rats after 30 days of withdrawal from METH (Fig. 2). Two-way ANOVA for Pdyn mRNA revealed significant effects of sex [F (1, 14) = 51.17, P < 0.0001], METH intake [F (2, 17) = 16.61, P = 0 .0001] and their interactions [F (2, 17) = 8.739, P = 0.0025] in the PFC. In the HIP, we observed significant effects of sex [F (1, 13) = 12.25, P < 0.0039] and METH intake [F (2, 17) = 10.21, P = 0.0012], but not their interactions [F (2, 17) = 1.048, P = 0.3724]. Post-hoc tests showed that female rats had significantly higher basal levels of Pdyn mRNA than males in both brain regions (Fig. 2A, B). In addition, both males and females had low levels of Pdyn mRNA in the HIP.
Fig. 2. Expression of Prodynorphin (Pdyn) and Proenkephalin (Penk) after METH SA and withdrawal.
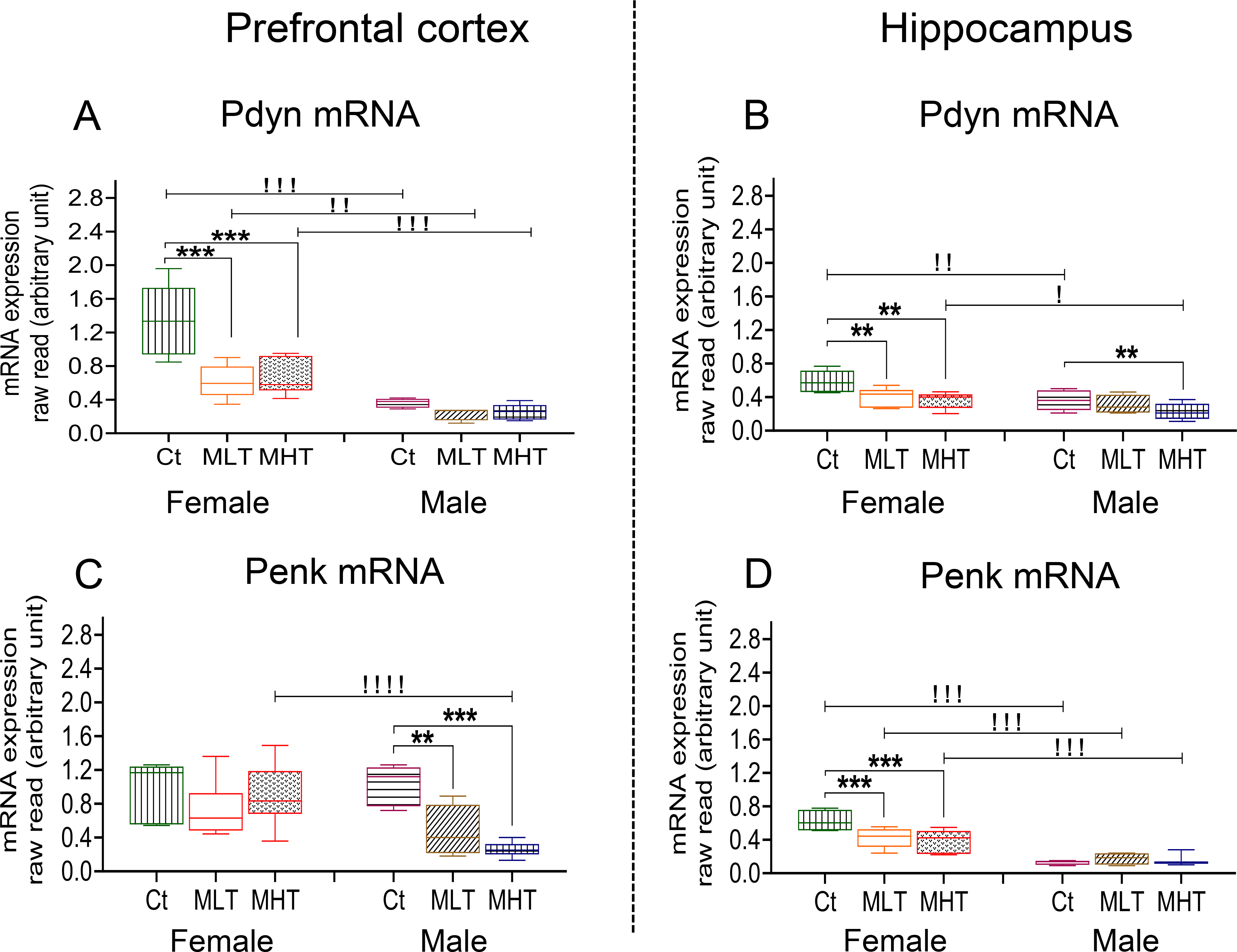
Relative Pdyn mRNA levels in female and male METH rats compared to controls in the (A) prefrontal cortex (PFC) and (B) hippocampus (HIP) after 30 days of withdrawal from METH SA. Relative Penk mRNA levels in female and male METH rats compared with controls are shown in (C) PFC and (D) HIP. Key to statistics: Key to statistics: *P < 0.05, **P < 0.01, ***P < 0.001, comparison control vs low and high METH taker groups; #P < 0.05, ##P < 0.01, ###P < 0.001, comparison between low and high METH taker groups; !P < 0.05, !!P < 0.01, !!!P < 0.001, comparison between female and male. Ct, Control Saline Rats, MLT, METH Low Takers; MHT, METH High Takers.
In the PFC, two-way ANOVA for Penk mRNA levels revealed significant effects of sex [F (1, 15) = 6.154, P = 0.0255], METH intake [F (2, 17) = 8.318, P = 0.0030], and their interactions [F (2, 17) = 6.049, P = 0.0104]. In the HIP, there were also significant effects of sex [F (1, 28) = 93.37, P < 0.0001], METH intake [F (2, 28) = 3.702, P = 0.0375] and their interactions [F (2, 28) = 6.174, P = 0.0060]. Post-hoc tests showed that female rats had significantly higher basal levels of Penk mRNA than males in the HIP but not the PFC, with male rats showing very low level of expression of Penk mRNA levels in the HIP (Fig. 2C, D).
The effects of METH intake are also observed on the expression profile of these genes. Female METH takers experienced significant decreases in PFC Pdyn mRNA levels in comparison to control (Fig. 2A). In the HIP, both female and male METH takers showed significant decreases compared to control (Fig. 2B). Only male METH rats showed dose-dependent decreases in the expression of PFC Penk mRNA levels in reference to control (Fig. 2C) whereas only female rats showed decreases in the expression of Penk mRNA expression in the HIP (Fig. 2D).
To test if there were any relationships between METH seeking behaviors and changes in Pdyn or Penk mRNA levels, we ran regression analysis between fold-changes in mRNA levels and active lever presses after a month of forced withdrawal from METH SA. There were significant negative correlations between PFC Pdyn and lever presses in both female (r = −0.6202, P = 0.0027) and male (r = −0.5306, P = 0.0235) rats (Fig. 3A). There were also significant a negative correlation (r = −0. 5861, P = 0.0170) between HIP Pdyn and lever presses in only male rats (Fig. 3B). Interestingly, changes in PFC Penk was negatively correlated (r = −0. 5580, P = 0.0161) with the number of lever presses only in male rats (Fig. 3C), with there being no significant correlations between changes in HIP Penk and METH seeking (Fig. 3D).
Fig. 3. Correlation of Prodynorphin (Pdyn) and Proenkephalin (Penk) mRNA with METH seeking behavior.
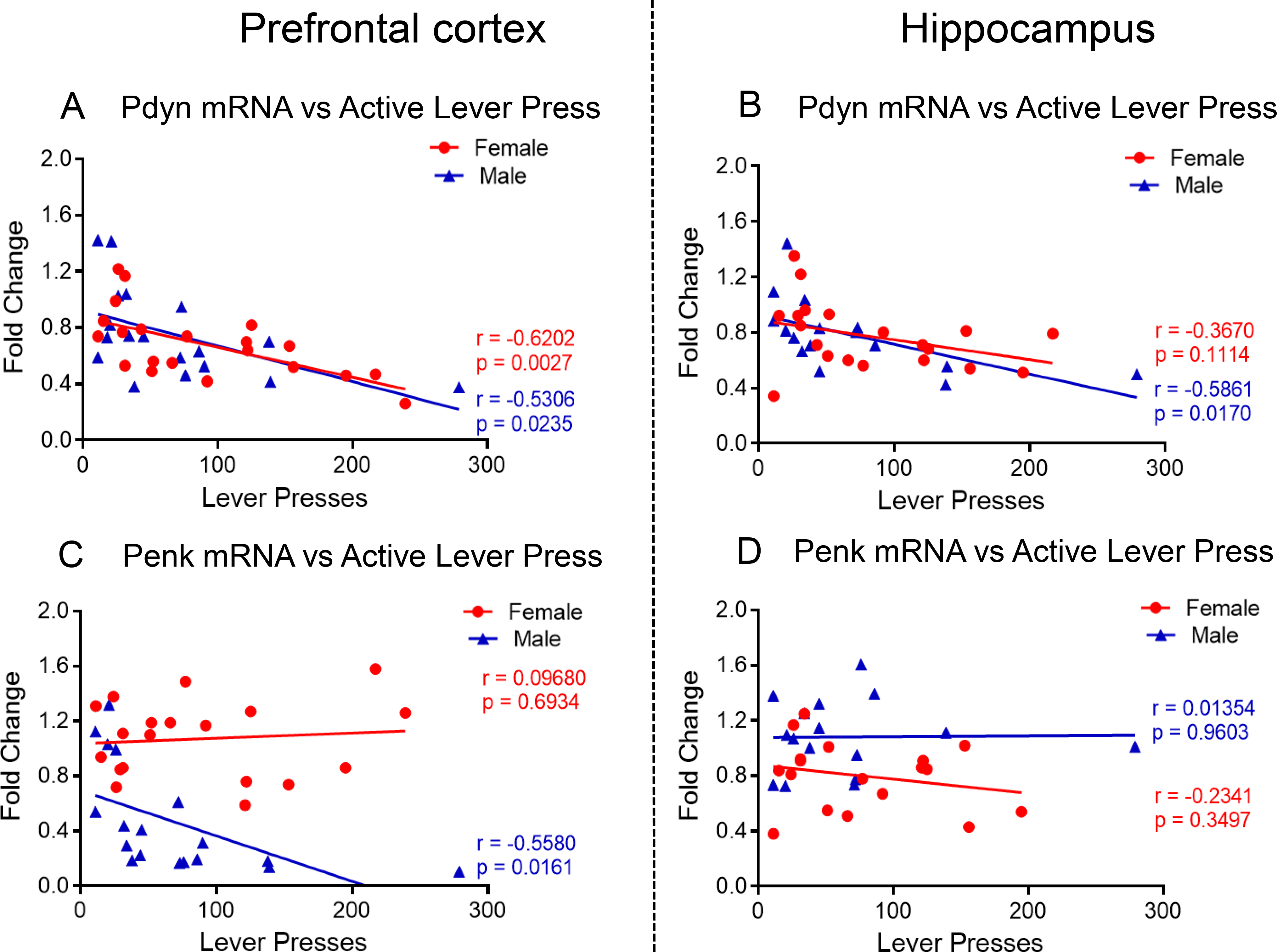
(A) Pdyn mRNA levels are negatively correlated with active levels presses in both female and male METH SA rats at WD30 in PFC but only (B) in male rats in the HIP. (C) Penk mRNA levels are negatively correlated with active levels presses in male METH SA rats at WD30 in PFC. (D) No correlation was found between Penk mRNA levels and active levels presses in male and female METH SA rats in HIP.
Changes in the expression of opioid receptor mRNAs after METH SA
Because we found significant sexual differences in the expression of Pdyn and Penk and because endogenous opioid neuropeptides interact with the 3 classical delta, mu, and kappa opioid receptors (Stein, 2016; Darcq and Kieffer, 2018), we therefore measured the expression of Oprd1, Oprk1, and Oprm1 mRNA levels in the PFC and HIP of these rats (Fig. 4, 5).
Fig. 4. Expression of Opioid receptors (Oprk1, Oprm1 and Oprd1) after METH SA and withdrawal.
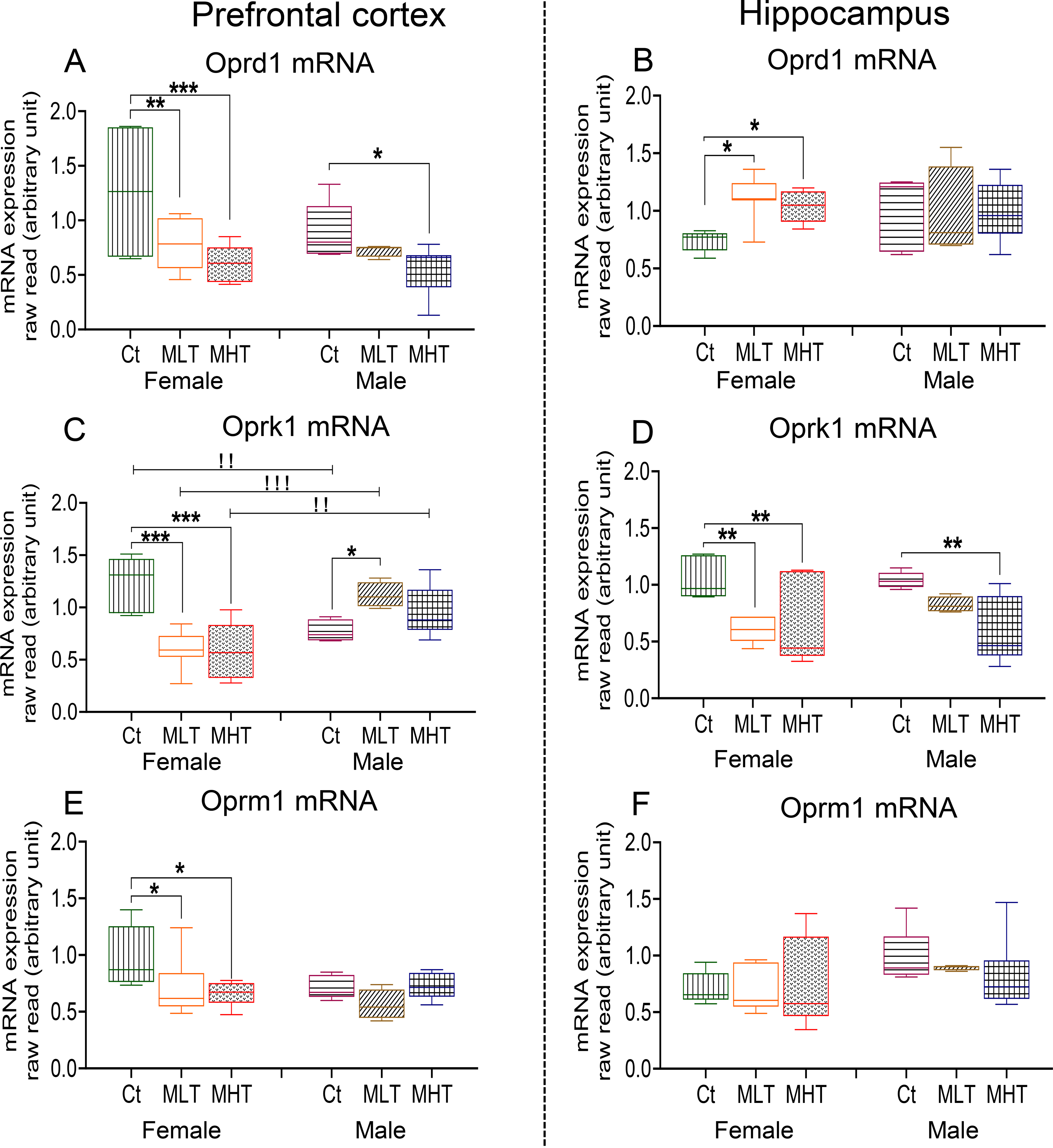
Oprd1 mRNA levels in the (A) PFC and (B) HIP of female and male rats. Oprk1 mRNA levels in the (C) PFC and (D) of female and male rats. Oprm1 mRNA levels in the (E) PFC and (F) of female and male rats. Keys to statistics are as in Fig. 2.
Fig. 5. Correlation between active lever presses on WD30 and expression of opioid receptors.
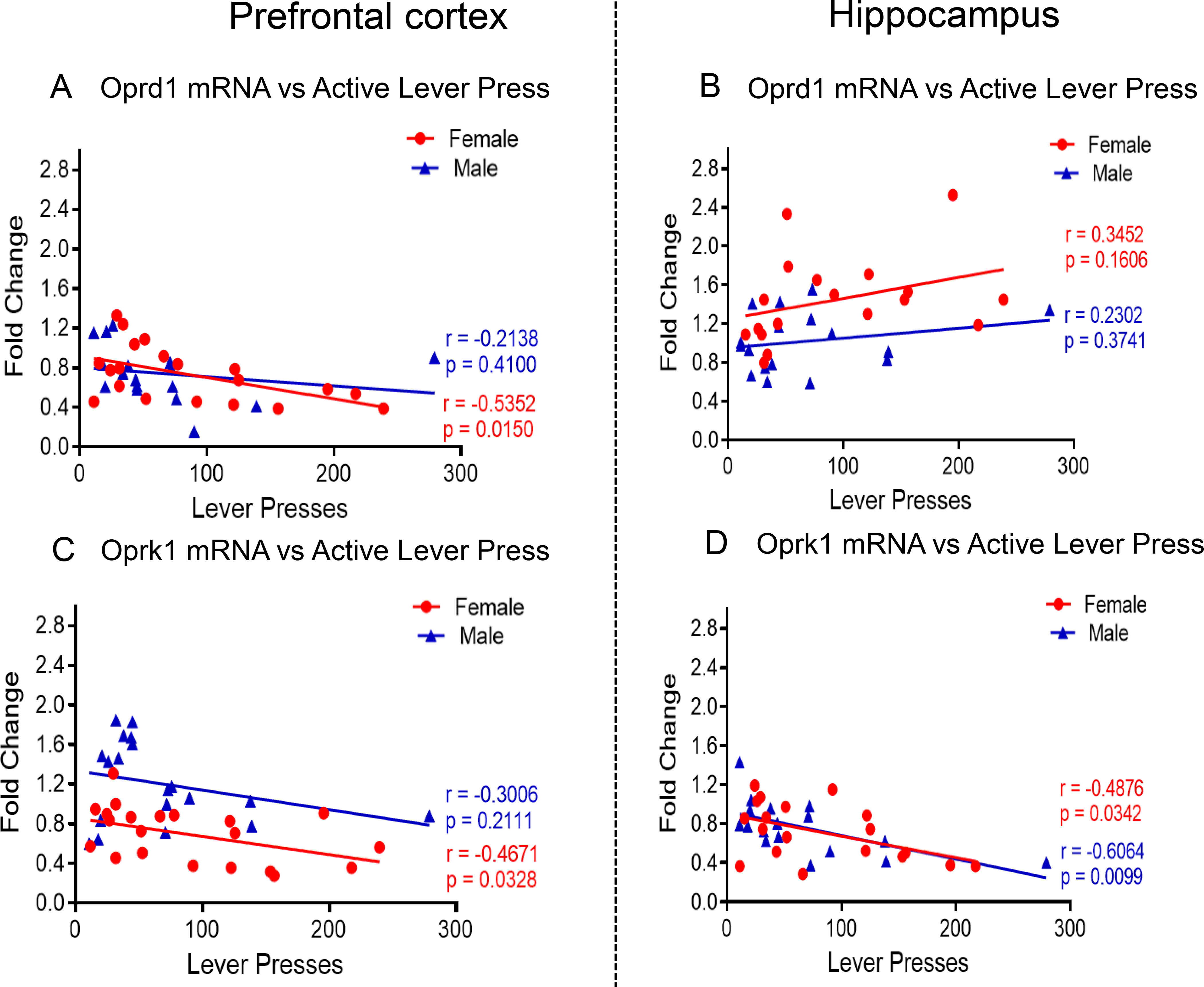
(A) Oprd1 mRNA levels are negatively correlated with active levels presses in female METH SA rats at WD30 in PFC. (B) No correlation was found between Oprd1 mRNA levels and active levels presses in male and female METH SA rats in HIP. Oprk1 mRNA levels are negatively correlated with active levels presses in (C) female METH SA rats at WD30 in the PFC and (D) both female and male METH SA rats in HIP
PFC Oprd1 mRNA levels display significant effects of METH intake [F (2, 33) = 9.609, P = 0.0005], but non-significant effects of sex [F (1, 33) = 2.848, P = 0.1009] and their interactions [F (2, 33) = 1.158, P = 0.3266]. There were no baseline sex differences in expression of PFC Oprd1 mRNA levels (Fig. 4A). In the HIP, there were no significant effects of METH intake [F (2, 29) = 1.437, P = 0.2541], sex [F (1, 29) = 0.06705, P = 0.7975], nor their interactions [F (2, 29) = 1.931, P = 0.1631] (Fig. 4B). There were also no sex differences in baseline expression of HIP Oprd1 expression. Both female and male METH rats showed significant decreases in PFC Oprd1 expression in comparison to their respective controls (Fig. 4A). Whereas, in the HIP, only female METH rats showed increases in the level of Oprd1 mRNA compared to respective controls (Fig. 4B). Regression analysis revealed significant negative correlation between PFC Oprd1 expression and METH seeking in females (r = −0.5352, P = 0.0150) but not in males (r = −0. 2138, P = 0.4100) (Fig. 5A). In the HIP, no significant correlations were observed in Oprd1 mRNA levels to METH seeking in either females (r = −0.3452, P = 0.1606) or males (r = −0.2302, P = 0.3741) (Fig. 5B).
For PFC Oprk1 mRNA levels, there were no significant effects of sex [F (1, 31) = 3.809, P = 0.0601], but significant effects of METH intake [F (2, 31) = 3.482, P = 0.0432] and their interactions [F (2, 31) = 15.29, P = 0.0001], with female METH rats showing decreased expression but male METH rats exhibiting increased expression compared to male controls (Fig. 4C). We also detected significant negative correlation between PFC mRNA expression and drug seeking in females (r = −0.4671, P = 0.0328) but not males (r = 0.3006, P = 0.2111) (Fig. 5C). In the HIP, there were significant effects of METH intake [F (2, 30) = 12.23, P = 0.0001] but not effect of sex [F (1, 30) = 0.4585, P = 0.5035] or their interactions [F (2, 30) = 1.195, P = 0.3166] on Oprk1 expression. Both female and male METH rats showed decreased mRNA levels compared to their respective controls (Fig. 4D). There were significant negative correlations between mRNA levels and drug seeking in females (r = −0.4876, P = 0.0342) and males (r = −0.6064, P = 0.0099) (Fig. 5D).
For PFC Oprm1 expression, there were no significant effects of METH intake [F (2, 31) = 2.558, P = 0.0937] or sex [F (1, 31) = 1.984, P = 0.1689], but their interactions were significant [F (2, 31) = 3.377, P = 0.0471]. Only female METH rats showed decreased expression of Oprm1 (Fig. 4E). In addition, there were no significant correlations in either in females (r = −0.3455, P = 0.1474) or in males (r = −0.2391, P = 0.3394). For HIP Oprm1, we observed no significant effects of METH intake [F (2, 29) = 0.1746, P = 0.840], sex [F (1, 29) = 4.340, P = 0.062], nor their interaction [F (2, 29) = 0.3792, P = 0.6878] (Fig. 4F).
Expression of Crh and its receptors
Sexual dimorphism in the expression of Crh and its receptors has been reported in various brain regions (Weathington et al., 2014; Lukkes et al., 2016; Bangasser and Wiersielis, 2018). In addition, we have previously reported significant changes in the expression of genes that participate in the Crh system of male rats treated exposed to METH given contingently or non-contingently (Cadet et al., 2014b; Jayanthi et al., 2018; Daiwile et al., 2019). In the present study, low and comparable PFC Crh mRNA levels were measured in female and male rats (Fig. 6A). However, basal HIP Crh mRNA expression was significantly higher in males compared to females (Fig. 6B). In contrast, basal PFC Crhr1 and Crhr2 mRNA levels were higher in female controls compared to male controls (Fig. 6C, E). However, male rats showed higher basal mRNA level of HIP Crhr1 than female rats (Fig. 6D) while no sex differences existed in basal HIP Crhr2 mRNA levels (Fig. 6F).
Fig. 6. Expression of Crh system in response to METH SA and withdrawal.
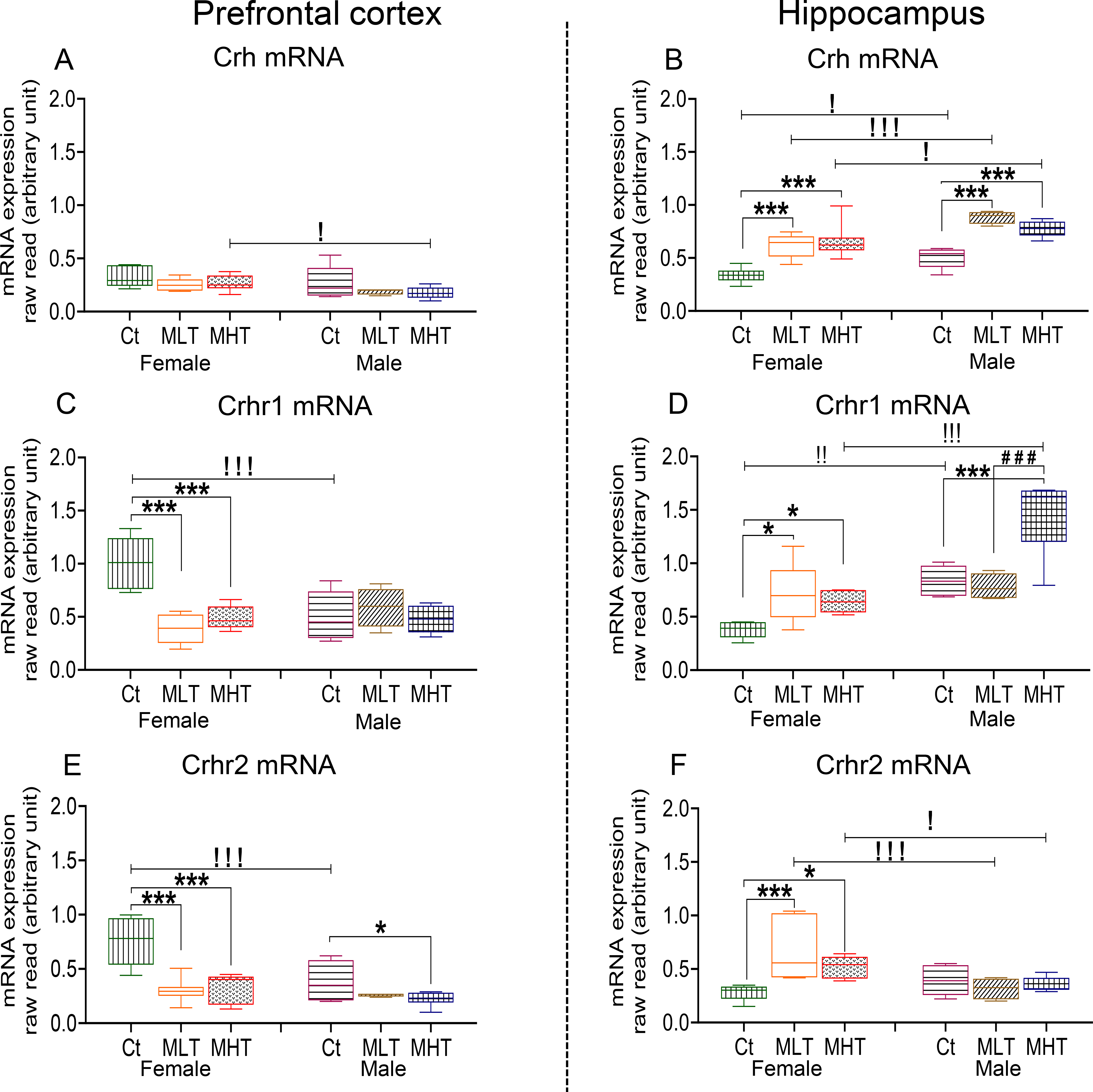
Crh mRNA levels in the (A) PFC and (B) HIP of female and male rats. Crhr1 mRNA levels in the (C) PFC and (D) HIP of female and male rats. Crhr2 mRNA levels in the (E) PFC and (F) HIP of female and male rats. Key to statistics is as in Fig. 2.
Two-way ANOVA for PFC Crh expression showed significant effects of sex [F (1, 30) = 6.578, P = 0.0156], but non-significant effects of METH intake [F (2, 30) = 2.988, P = 0.0656] and interaction [F (2, 30) = 0.1232, P = 0.8845]. Post-hoc tests showed no changes in the levels of PFC Crh in female and male METH rats compared to controls (Fig. 6A). For HIP Crh mRNA, ANOVA showed significant effects of sex [F (1, 31) = 26.38, P < 0.0001] and METH intake [F (2, 31) = 33.61, P < 0.0001] but their interaction was not significant [F (2, 31) = 1.635, P = 0.2114]. Post-hoc analyses revealed that both females and males showed significant increases in HIP Crh mRNA levels in METH groups (Fig. 6B). Regression analysis showed negative correlation between active lever presses at WD30 and PFC Crh expression only in male (r = −0. 5853, P = 0.0107) (Fig. 7A). There were, importantly, significant positive correlations between HIP Crh expression and active lever presses in both females (r = 0. 4965, P = 0.0306) and males (r = 0. 7961, P = 0.0001) (Fig. 7D).
Fig. 7. Correlation between Crh and its receptor with active lever presses on WD30.
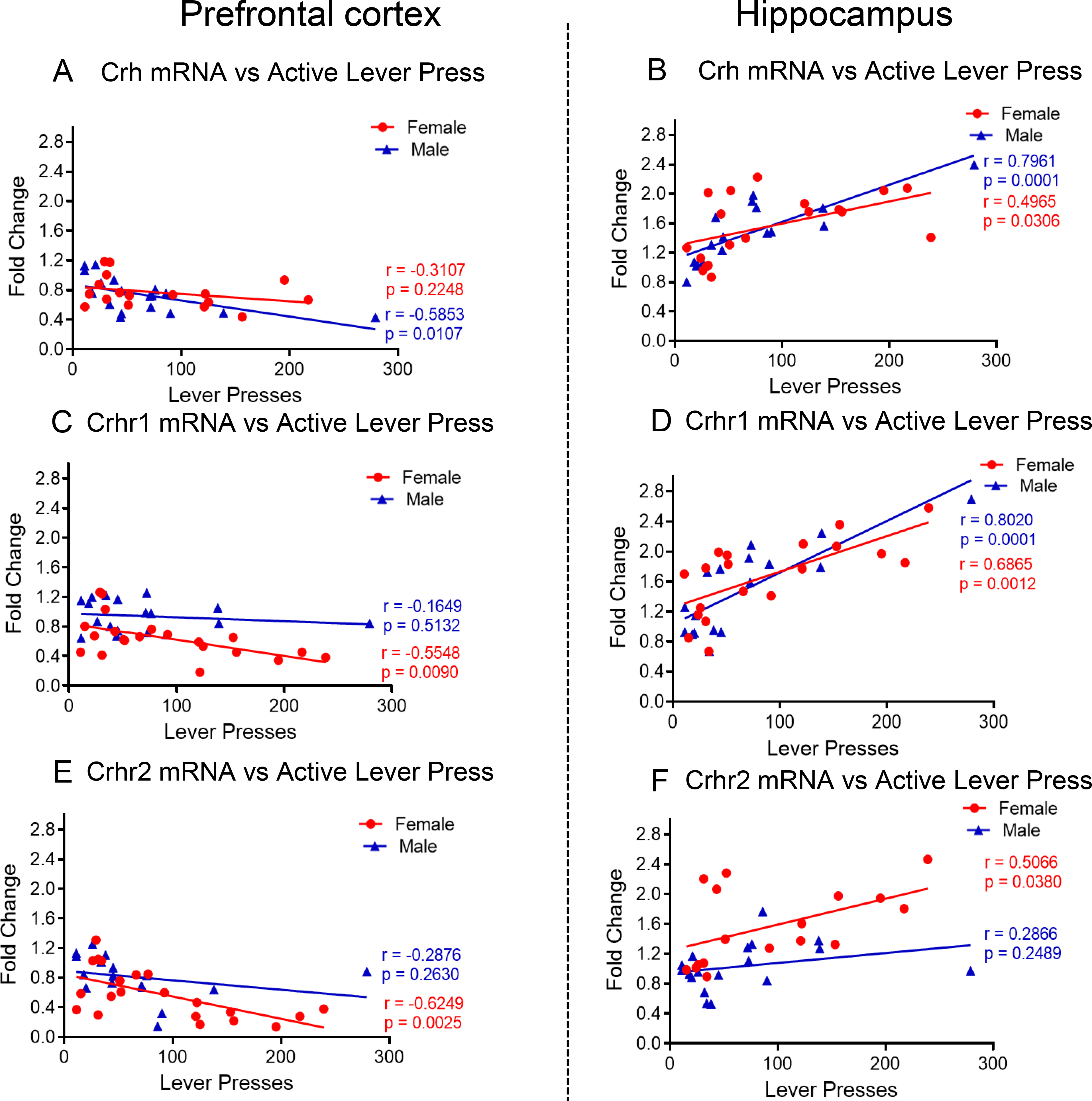
(A) Changes in PFC Crh mRNA levels are negatively correlated with active levels presses only in male METH SA rats at WD30. (B) Changes in HIP Crh mRNA levels showed positive correlation with active levels presses in female and male METH SA rats at WD30. (C) PFC Crhr1 mRNA levels are negatively correlated with active levels presses in female METH SA rats at WD30. (D) Changes in HIP Crhr1 mRNA levels are positively correlated with active levels presses in female and male METH SA rats at WD30. (E) Crhr2 mRNA levels are negatively correlated with active levels presses in female METH SA rats at WD30 in PFC. (F) Changes in HIP Crhr2 mRNA levels are positively correlated with active levels presses in female METH SA rats at WD30.
PFC Crhr1 showed significant effects of METH intake [F (2, 18) = 10.79, P = 0.0008] and interaction [F (2, 18) = 13.24, P = 0.0003], but nonsignificant effects of sex [F (1, 15) = 2.913, P = 0.1085]. Post-hoc tests revealed significant decreases in PFC Crhr1 in female but not in male, rats METH rats compared to their respective controls (Fig. 6C). In case of HIP Crhr1, there were significant effects of sex [F (1, 15) = 35.21, P < 0.0001], METH intake [F (2, 17) = 13.90, P = 0.0003] and their interactions [F (2, 17) = 9.781, P = 0.0015]. Both female and male METH rats showed significant increases in HIP Crhr1 compared to controls (Fig. 6D). A significant correlation between active lever presses and PFC Crhr1 expression was observed only in female rats (r = −0. 5548, P = 0.0090) (Fig. 7C). Of interest, positive correlations between HIP Crhr1 expression and active lever presses were measured in both female (r = 0. 6865, P = 0.0012) and male (r = 0.8020, P = 0.0001) (Fig. 7D).
For PFC Crhr2, there were significant effects of sex [F (1, 12) = 8.217, P = 0.0142], METH intake [F (2, 17) = 26.65, P <0.0001] and their interactions [F (2, 17) = 6.105, P = 0.0100]. Significant decreases in PFC Crhr2 expression were observed in female and male METH rats compared to control rats (Fig. 6E). Changes in HIP Crhr2 also showed significant effects of sex [F (1, 30) = 8.107, P = 0.0079] and METH*Sex interaction [F (2, 30) = 6.839, P = 0.0036] but a trend for METH intake [F (2, 30) = 3.210, P = 0.0546]. Female but not male METH rats showed significant increases in HIP Crhr2 mRNA levels compared to control rats (Fig. 6F). In the PFC, only females (r = −0.6249, P = 0.0025) showed a negative correlation between active lever presses and Crhr2 expression (Fig. 7E). In the HIP, lever presses correlated with increased Crhr2 expression in only female rats (r = 0.5066, P = 0.0380) (Fig. 7F).
Expression of orexin/hypocretin (Hcrt) receptors
The orexin/hypocretin system appears to play important roles in addiction (Baimel et al., 2015; James et al., 2017). Sex-related differences in the expression of orexin and its receptors have also been reported in rats (Johren et al., 2001, 2002). To assess the effects of METH on the expression of orexin receptors, we measured the mRNA expression of orexin/hypocretin receptors in the PFC and HIP (Fig. 8). Compared to males, female rats expressed higher basal mRNA levels of Hcrtr1 in both the PFC and HIP (Fig. 8A, B). Females also have higher basal levels expression of Hcrtr2 in the PFC but not in the HIP (Fig. 8C, D).
Fig. 8. Expression of Hypocretin receptors after METH SA and withdrawal.
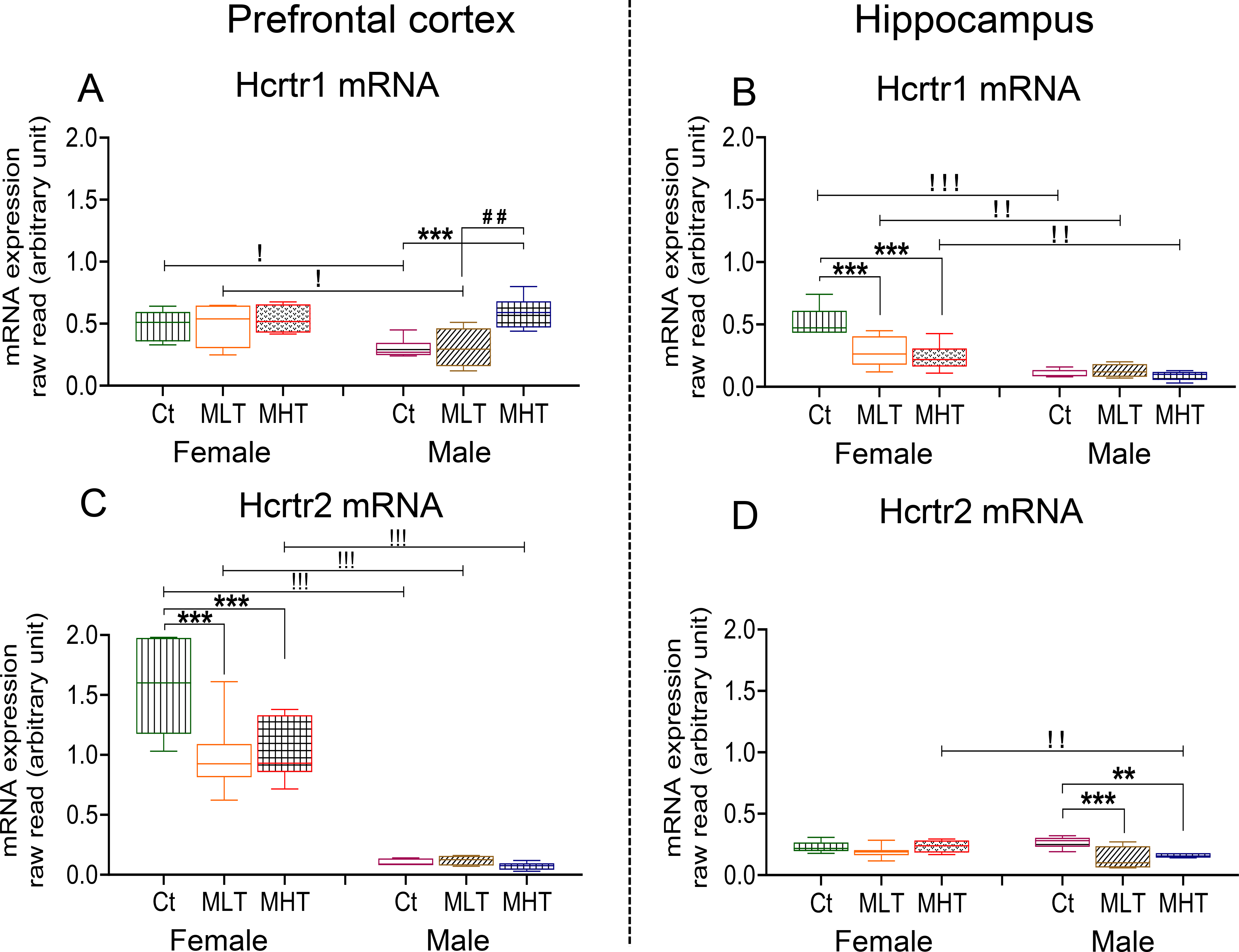
Relative Hcrtr1 mRNA levels in the (A) PFC and (B) HIP of female and male rats. Hcrtr2 mRNA levels the (C) PFC and (D) HIP of female and male rats. Key to statistics is as in Fig. 2.
In the PFC, we observed significant effects of sex [F (1, 14) = 5.973, P = 0.0284], METH intake [F (2, 18) = 7.802, P = 0.0036], and their interaction [F (2, 18) = 3.927, P = 0.0384] for Hcrtr1 expression. Post-hoc tests showed significant increases in the expression of PFC Hcrtr1 only in male high METH takers compared to other groups (Fig. 8A). In the HIP, there were significant effects of sex [F (1, 30) = 60.97, P < 0.0001], METH intake [F (2, 30) = 8.231, P = 0.0014], and their interaction [F (2, 30) = 7.500, P = 0.0023]. Post-hoc tests revealed significant decreased expression of HIP Hcrtr1 in female METH groups (Fig. 8B). Regression analysis revealed a significant positive correlation (r = 0.7128, P = 0.0006) between PFC Hcrtr1 expression and active lever presses in males (Fig. 9A) but not in females (r = 0.05952, P = 0.8032). Moreover, HIP Hcrtr1 expression correlated negative with active lever presses in both females (r = −0.4918, P = 0.0382) and males (r = −0.4594, P = 0.0551) (Fig. 9B).
Fig. 9. Correlation between active lever presses on WD30 and expression level of hypocretin receptors.
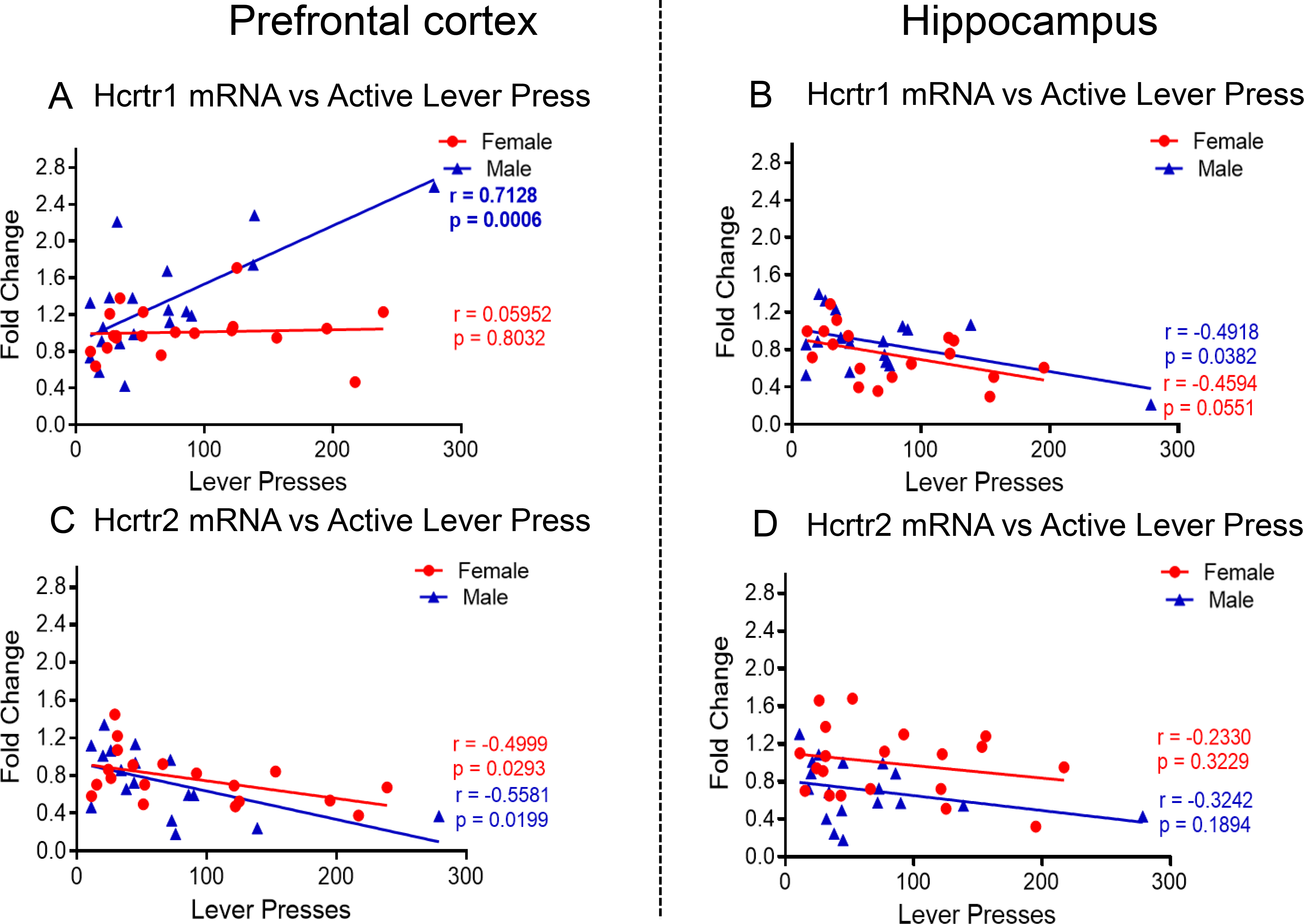
(A) PFC Hcrtr1 mRNA levels are positively correlated with active levels presses in male METH SA rats at WD30. (B) HIP Hcrtr1 mRNA levels are negatively correlated active levels presses in female and male METH SA rats. (C) PFC Hcrtr2 mRNA levels are negatively correlated with active levels presses in female and male METH SA rats at WD30. (D) No correlation was found between Hcrtr2 mRNA levels and active levels presses in male and female METH SA rats in the HIP.
For PFC Hcrtr2, there were significant effects of sex [F (1, 30) = 187.7, P <0.0001], METH intake [F (2, 30) = 5.557, P = 0.0089] and their interactions [F (2, 30) = 5.146, P = 0.0120]. These were due to significant METH-induced decreases in PFC Hcrtr2 mRNA levels in females but not in males (Fig. 8C). In the HIP, there were no significant effects of sex [F (1, 13) = 2.714, P = 0.1234] but significant effects of METH intake [F (2, 17) = 8.047, P = 0.0035] and interaction [F (2, 17) = 4.312, P = 0.0306], with male rats experiencing significant METH-associated decreased HIP Hcrtr2 mRNA expression (Fig. 8D). Regression analysis also revealed a significant negative correlation between PFC Hcrtr2 expression and active lever presses in females (r = 0.4999, P = 0.0293) and male (r = 0. 5581, P = 0.0199) (Fig. 9C). There were no significant correlations in the HIP (Fig. 9D).
Figures 8B and 8C show basal expression (raw read) of Hcrtr1 and Hcrtr2 and no significant changes were observed in the male rats probably because of very-low mRNA copy numbers. However, in the correlation analysis, there were significant correlations between gene expression changes (fold change) and active lever presses (Figures. 9B and 9C).
DISCUSSION
Sex differences in METH use disorders have been reported in humans and in METH intake in animal models of addiction (Gonzales et al., 2010; Reichel et al., 2012; Cox et al., 2013; Radfar and Rawson, 2014; Ruda-Kucerova et al., 2015; Daiwile et al., 2019). The more salient results in the present study are: (1) Both male and female rats escalated their METH intake and exhibited similar incubation of METH seeking; (2) female rats had higher basal level expression of Pdyn and Hcrtr1 in both the PFC and HIP; (3). Basal Crhr1, Crhr2, Hcrtr2, Oprk1 mRNA levels were higher in the PFC whereas higher basal of Penk were observed in the HIP of females; (4) Crh and Crhr1 mRNA levels were higher in the HIP of males; (5) females exhibited significant METH-induced decreases in Pdyn mRNA levels in the PFC and HIP while males showed METH-induced decreases in Pdyn mRNA expression only in the HIP of males; (6) METH SA increased Crh and Crhr1 in the HIP of both sexes and Crhr2 only in the female HIP; and (7) Incubation of METH seeking showed strong positive correlation with increased Crh and Crhr1 mRNA levels in the HIP of both sexes.
Sex differences in METH Intake
We observed that male rats took more METH intake than females during the second and third weeks of METH SA training. Those results are consistent with the report of Ruda-Kucerova et al., 2015 who found that males self-administered more METH during the last 5 days of SA during a 15-day SA experiment. In addition, Job et al., 2020, using mathematical modeling, showed that male rats acquire METH SA faster than females. Together, these observations may be of clinical relevance because He et al., 2013 had also reported that 81% of hospitalized individuals with a MUD diagnosis were men, thus suggesting that human males may suffer from more severe forms of MUD because of greater consumption of the drug.
It is noteworthy that our results are different from another study in which it was shown that female rats take more METH than males (Reichel et al., 2012). The differences between the two studies might be attributable to the METH doses used by the different investigators. For example, we used approximately about 5 times higher dose of METH (0.1 mg/kg) than Reichel et al., 2012 used for males and females (0.02 and 0.0175 mg/kg, respectively). Differences in the behavioral paradigms used may be another contributing factor. Reichel et al., 2012 trained rats to SA METH 1-hour daily for the first 7 days followed by 6-hours extended access to METH for another 7 days whereas we trained rats to self-administer METH 6-hours from the beginning of our study. The above issues are important and will need to be addressed in future experiments. We used METH (0.1 mg/kg) in the present study based on previous studies in which we showed that this dose led to male rats exhibiting reliable METH SA and incubation of METH seeking (Cadet et al., 2016 and 2017; Krasnova et al., 2017; Daiwile et al., 2019; Hu et al., 2019; Job et al., 2020;).
Sex and brain region-specific differences in opioid systems
In our study, we found that female showed higher basal level expression of Pdyn and Penk in the PFC and HIP. These finding is consistent with our recent report of higher basal levels of Pdyn mRNA in the nucleus accumbens (NAc) of female rats (Daiwile et al., 2019), these observations indicate that female rodents have higher brain dynorphinergic tone than males. Our findings of decreased Pdyn mRNA in the HIP of male and female rats during withdrawal from METH SA are consistent with the results of a previous paper that reported decreased Pdyn mRNA in the striatum of male Sprague-Dawley rats following 10 days withdrawal from cocaine (Svensson and Hurd, 1998). Our observations are consistent with a paper that reported that human METH users had decreased DYN protein levels in the medial pulvinar thalamus and in Brodmann’s cortical areas 22 and 39 but of M-ENK in the caudate and putamen obtained from postmortem brain specimens (Frankel et al., 2007).
Several studies have implicated dynorphin and enkephalin peptides in the manifestations of substance use disorder through their interactions with opioid receptors (Shippenberg et al., 2007; Gorelick et al., 2008; Butelman et al., 2012; Chung and Kieffer, 2013; Blackwood et al., 2019). Because of the possibility that sex differences in opioid receptors might account for some of the differences in gender-specific clinical courses of SUD, we also measured their expression in the PFC and HIP. We found higher basal levels of Oprk1 in the PFC of female rats compared to males, but no other differences in basal expression of these receptors. Female rats that self-administered METH exhibited decreased PFC Oprk1 mRNA whereas male rats exhibit increased Oprk1 mRNA expression. These data implicate the DYN-Kappa system in METH use disorder as discussed for other psychostimulants and opioid drugs (Shippenberg et al., 2007; Butelman et al., 2012).
Sex differences in the CRH system in response to withdrawal from METH self-administration.
Stress systems, including CRH signaling, are known to play important roles in the course of substance use disorders in a sex-dependent fashion (Iredale et al., 2000; Nawata et al., 2012; Cadet et al., 2014b; Koob and Schulkin, 2019). The present study further documents the existence of sexual dimorphism in the CRH system in that male rats showed higher basal expression of PFC Crh and Crhr1 mRNAs compared to females whereas female rats had higher basal HIP Crhr2 mRNA level compared to males. Lukkes et al., 2016 had also reported higher level of Crhr1 and Crhr2 in the dorsal raphe nuclei of female rodents.
Of significant interest are our observations that withdrawal from METH SA is accompanied by increased HIP Crh and Crhr1 mRNA levels in both female and male rats. These changes correlated positively with incubation of METH seeking in both sexes, thus implicating the HIP CRH system in that process. In contrast, we had not found any significant positive correlations between Crh and Crhr1 in the nucleus accumbens (NAc) and incubation of METH seeking in either sex (Daiwile et al., 2019). There was only a positive correlation between incubation and NAc Crhr2 only in female rats (Daiwile et al., 2019), suggesting that hippocampal Crh and Crhr1 might serve as regulators of incubation of METH seeking in both sexes. This reasoning is, in part, compatible with ideas pushed forward by other investigators who have implicated increased Crh expression in the amygdala of male rats in footshock-induced METH seeking (Nawata et al., 2012). Our findings are consistent, in part, with those of previous papers that had reported higher CRH peptide levels in the amygdala (Georgiou et al., 2016), PVN (paraventricular nucleus of the hypothalamus), and ovBNST (oval region of the bed nucleus of stria terminalis) after 7 days of withdrawal from METH (García-Carmona et al., 2018). These suggestions are also consistent with the demonstration that systemic administration of CP-154–526, a Crh receptor 1 antagonist, can attenuate cue-and methamphetamine-induced reinstatement of extinguished METH seeking in rats (Moffett and Goeders, 2006). Together, these data implicate a hippocampal-amygdala circuit in withdrawal-induced METH craving. Firm conclusions about the role of this circuit in METH seeking will await future studies using viral vectors to manipulate expression of Crh and Crhr1 expression in the rat amygdala and/or hippocampus. Injections of specific CRH receptor antagonists will also be helpful.
METH SA and regional expression of orexin/hypocretin receptors
The potential role of hypocretin/orexin systems in animal models of psychostimulant use disorders has been reported (James et al., 2017, Schmeichel et al., 2017, 2018). Human studies have also implicated the orexin system in addiction since METH users have been reported to have higher plasma level of hypocretin (Chen et al., 2016). In the present study, we found that female rats had higher basal expression of PFC Hcrtr1 and Hcrtr2 compared to male rats that showed very low basal expression of Hcrtr2 mRNA. Basal Hcrtr1, but not of Hcrtr2, mRNA expression was also higher in the HIP of female rats, with male rats expressing very low levels of HIP Hcrtr1. These results are consistent with our previous observations in the NAc (Daiwile et al., 2019). Similarly, decreased levels of plasma HCRT (Orexin-A) were observed in individual after subacute withdrawal from METH (Lee et al., 2020). Sexual dimorphism in orexin/hypocretin expression also exists in the rat hypothalamus (Johren et al., 2001, 2002; Grafe et al., 2017). The sexual dimorphic responses of PFC and HIP Hcrtr1 and Hcrtr2 to withdrawal from METH SA are consistent with the report that orexin is a mediator of sex differences in responses to stress (Grafe et al., 2017). Withdrawal from drug self-administration has been conceptualized as a stressful event (see Koob and Schulkin, 2019).
CONCLUSIONS
In summary, we found significant sex difference in basal expression of Pdyn, Crh and its receptors, and hypocretin receptors in the PFC and HIP. Responses to withdrawal from METH SA were also sexually dimorphic. However, similar increases in Crh and Crhr1 mRNA levels occurred in the HIP of both male and female rats. In addition, these increases strongly correlated positively with METH seeking behaviors. These results implicate the hippocampal CRH system in the mediation of drug craving after prolonged withdrawal or abstinence. Further genetic and pharmacological manipulations are necessary to identify the specific and differential involvement of these molecular changes in observed sex differences in drug taking and relapse.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by the Department of Health and Human Services/ National Institutes of Health/ National Institute on Drug Abuse/ Intramural Research Program, Baltimore, MD, USA. The authors declare no conflicts of interest.
ABBREVIATIONS:
- METH
Methamphetamine
- SA
Self-administration
- PFC
Prefrontal cortex
- HIP
Hippocampus
- WD
Withdrawal day
- Pdyn
Prodynorphin
- Penk
Proenkephalin
- Oprk1
Opioid receptor kappa 1
- Oprm1
Opioid receptor mu 1
- Oprd1
Opioid receptor delta 1
- Hcrtr1
Hypocretin receptor 1
- Hcrtr2
Hypocretin receptor 2
- Crh
Corticotropin releasing hormone
- Crhr1
Crh receptor 1
- Crhr2
Crh receptor 2
- Ct
Control Saline Rats
- MLT
METH Low Takers
- MHT
METH High Takers
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL (2015) Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol 172:334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR (2018) Sex differences in stress responses: a critical role for corticotropin-releasing factor. Hormones (Athens) 17:5–13. [DOI] [PubMed] [Google Scholar]
- Becker JB, Chartoff E (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44:166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood CA, Hoerle R, Leary M, Schroeder J, Job MO, McCoy MT, Ladenheim B, Jayanthi S, Cadet JL (2019) Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence-induced incubation of drug seeking after escalated oxycodone self-administration. Molecular Neurobiology 56:3603–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Yuferov V, Kreek MJ (2012). Kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci 35:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM (2014a) Neuropathology of substance use disorders. Acta Neuropathol 127:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V (2015) Neuropsychological consequences of chronic drug use: relevance to treatment approaches. Front Psychiatry 6:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Jayanthi S, Krasnova IN (2015) Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat. Mol Neurobiol 51:696–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Krasnova IN, Jayanthi S, Ladenheim B, McCoy MT, Walther D, Godino A, Pirooznia M, Lee RS (2017) Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol Psychiatry 22:1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Ladenheim B, McCoy MT, Krasnova IN, Lehrmann E, Becker KG, Jayanthi S (2014b). Enhanced upregulation of CRH mRNA expression in the nucleus accumbens of male rats after a second injection of methamphetamine given thirty days later. Plos One 9:e84665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Walther D, Brannock C, Ladenheim B, McCoy MT, Collector D, Torres OV, Terry N, Jayanthi S (2016) Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats. Sci Rep 6:37002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Patel R, Jayanthi S (2019) Compulsive methamphetamine taking and abstinence in the presence of adverse consequences: Epigenetic and transcriptional consequences in the rat brain. Pharmacology, Biochemistry and Behavior 179:98–108. [DOI] [PubMed] [Google Scholar]
- Chen WY, Kao CF, Chen PY, Lin SK, Huang MC (2016) Orexin-A level elevation in recently abstinent male methamphetamine abusers. Psychiatry Res 239:9–11. [DOI] [PubMed] [Google Scholar]
- Chung PCS, Kieffer BL (2013) Delta opioid receptors in brain function and diseases. Pharmacology & Therapeutics 140:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM (2013) Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiwile AP, Subramaniam J, Ladenheim B, McCoy MT, Brannock C, Schroeder J, Cadet JL (2019) Sex differences in escalated methamphetamine self-administration and altered gene expression associated with incubation of methamphetamine seeking. International Journal of Neuropsychopharmacology 22:710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Kieffer BL (2018) Opioid receptors: drivers to addiction?. Nat Rev Neurosci 19:499–514. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Liu B (2008) Gender differences in methamphetamine use and responses: a review. Gend Med 5:24–35. [DOI] [PubMed] [Google Scholar]
- DSM–5 (2013) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition ed. American Psychiatric Association. [Google Scholar]
- Everitt BJ (2014) Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories – indications for novel treatments of addiction. Eur J Neurosci 40:2163–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ (2007) Brain levels of neuropeptides in human chronic methamphetamine users. Neuropharmacology 53:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Carmona JA, Georgiou P, Zanos P, Bailey A, Laorden LM (2018) Methamphetamine withdrawal induces activation of CRF neurons in the brain stress system in parallel with an increased activity of cardiac sympathetic pathways. Naunyn Schmiedebergs Arch Pharmacol 391:423–434. [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Garcia-Carmona J, Hourani S, Kitchen I, Laorden M, Bailey A (2016) Methamphetamine abstinence induces changes in μ-opioid receptor, oxytocin and CRF systems: Association with an anxiogenic phenotype. Neuropharmacology 105:520–532. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA (2010) The methamphetamine problem in the United States. Annu Rev Public Health 31:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, Dannals RF, Frost JJ (2008) Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 200:475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Cornfeld A, Luz S, Valentino R, Bhatnagar S (2017) Orexins mediate sex differences in the stress response and in cognitive flexibility. Biol Psychiatry 81:683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Xie Y, Tao J, Su H, Wu W, Zou S, Zhang J, Zhang J, Zhang H, Yang X, Guo J, Tang W, Zhang F, Liu J, Liu L, Chen Y, Wen N, Kosten TR, Zhang XY (2013) Gender differences in sociodemographic and clinical characteristics of methamphetamine inpatients in a Chinese population. Drug Alcohol Depend 130:94–100. [DOI] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Krasnova IN, Gu H, Lu H, Bonci A, Cadet JL, Stein EA, Yanga Y (2019) Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Proc Natl Acad Sci 116:9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale PA, Alvaro JD, Lee Y, Terwilliger R, Chen YL, Duman RS (2000) Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J Neurochem 74:199–208. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G (2017) A decade of orexin/hypocretin and addiction: where are we now? Curr Top Behav Neurosci 33:247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Gonzalez B, McCoy MT, Ladenheim B, Bisagno V, Cadet JL (2018) Methamphetamine induces TET1- and TET3- dependent DNA hydroxymethylation of Crh and Avp genes in the rat nucleus accumbens. Mol Neurobiol 2018 55:5154–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Chojnacki MR, Daiwile AP, Cadet JL (2020) Chemogenetic inhibition of dopamine d1-expressing neurons in the dorsal striatum does not alter methamphetamine intake in either male or female Long Evans rats. Neuroscience Letters 729:134987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen A, McFadden LM (2017) The neurochemical consequences of methamphetamine self-administration in male and female rats. Drug Alcohol Depend 178:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P (2001) Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology 142:3324–3331. [DOI] [PubMed] [Google Scholar]
- Johren O, Neidert SJ, Kummer M, Dominiak P (2002) Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides 23:1177–1180. [DOI] [PubMed] [Google Scholar]
- Kogachi S, Chang L, Alicata D, Cunningham E, Ernst T (2017) Sex differences in impulsivity and brain morphometry in methamphetamine users. Brain Struct Funct 222:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Schulkin J (2019) Addiction and stress: An allostatic view. Neurosci Biobehav Rev 106:245–262. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Gerra MC, Walther D, Jayanthi S, Ladenheim B, McCoy MT, Brannock C, Cadet JL (2017) Compulsive methamphetamine taking in the presence of punishment is associated with increased oxytocin expression in the nucleus accumbens of rats. Sci Rep 7:8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ (2016) Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem 23:515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Chen P, Kao C, Huang M (2020) Differences in serum orexin-A levels between the acute and subacute withdrawal phases in individuals who use methamphetamine. Exp Clin Psychopharmacol, doi: 10.1037/pha0000395. [DOI] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME (2015) Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res 1628:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Norman KJ, Meda S, Andersen SL (2016) Sex differences in the ontogeny of CRF receptors during adolescent development in the dorsal raphe nucleus and ventral tegmental area. Synapse 70:125–132. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2018) Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav 164:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Cordie R, Livermont T, Johansen A (2018) Behavioral and Serotonergic Changes in the Frontal Cortex Following Methamphetamine Self-Administration. Int J Neuropsychopharmacol 21:758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Goeders NE (2006) CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology 190:171–180. [DOI] [PubMed] [Google Scholar]
- Mongi-Bragato B, Avalos MP, Guzmán AS, Bollati FA, Cancela LM (2018) Enkephalin as a Pivotal Player in Neuroadaptations Related to Psychostimulant Addiction. Front Psychiatry 9:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawata Y, Kitaichi K, Yamamoto T (2012) Increases of CRF in the amygdala are responsible for reinstatement of methamphetamine-seeking behavior induced by footshock. Pharmacol Biochem Behav 101:297–302. [DOI] [PubMed] [Google Scholar]
- Pena-Bravo JI, Penrod R, Reichel CM, Lavin A (2019) Methamphetamine self-administration elicits sex-related changes in postsynaptic glutamate transmission in the prefrontal cortex. eNeuro 6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proebstl L, Kamp F, Koller G, Soyka M (2018) Cognitive deficits in methamphetamine users: how strong is the evidence? Pharmacopsychiatry 51:243–250. [DOI] [PubMed] [Google Scholar]
- Radfar SR, Rawson RA (2014) Current research on methamphetamine: epidemiology, medical and psychiatric effects, treatment, and harm reduction efforts. Addiction & Health 6:146–154. [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE (2012) Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 223:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME (2004) Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 172:443–449. [DOI] [PubMed] [Google Scholar]
- Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcova A (2015) Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front Psychiatry 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BE, Herman MA, Roberto M, Koob GF (2017) Hypocretin neurotransmission within the central amygdale mediates escalated cocaine self-administration and stress induced reinstatement in rats. Biol Psychiatry 81:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BE, Matzeu A, Koebel P, Vendruscolo LF, Sidhu H, Shahryari R, Kieffer BL, Koob GF, Martin-Fardon R, Contet C (2018) Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats. Neuropsychopharmacology 43:2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI (2007) Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther 116:306–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C (2016) Opioid Receptors. Annu Rev Med 67:433–451. [DOI] [PubMed] [Google Scholar]
- Svensson P, Hurd YL (1998) Specific reductions of striatal prodynorphin and D dopamine receptor 1 messenger RNAs during cocaine abstinence. Molecular Brain Research 56:162–168. [DOI] [PubMed] [Google Scholar]
- Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, Jean Lud Cadet JL (2017) Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav Brain Res 326:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, Caprioli D (2017) Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology 42:1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D (2012) Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol 52:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Hamki A, Cooke BM (2014) Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. J Comp Neurol 522:1284–1298. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu C, Zhang J, Hu L, Song H, Li J, Kang L (2013) Gender differences in abusers of amphetamine-type stimulants and ketamine in southwestern China. Addict Behav 38:1424–1430. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Johnson LA, Agam M, Raber J (2014) Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine. J Neurochem 129:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


