Abstract
Background:
Higher testosterone contributes to imaging-confirmed nonalcoholic fatty liver disease (NAFLD) in women, but whether testosterone influences their disease severity is unknown.
Methods:
The association of free testosterone (free T) with nonalcoholic steatohepatitis (NASH) was determined in pre-menopausal women with biopsy-confirmed NAFLD (n=207). Interaction testing was performed for age and free T given decline in testosterone with age, and association of aging with NASH. Regression models adjusted for abdominal adiposity, diabetes, and dyslipidemia.
Results:
Median age was 35 yrs (IQR 29-41); 73% were white, 25% Hispanic; 32% had diabetes, 93% abdominal adiposity, and 95% dyslipidemia. 69% had NASH, 67% any fibrosis, and 15% advanced fibrosis. Higher free T levels were associated with NAFLD severity in younger women (interaction p values <0.02). In the youngest age quartile, free T was independently associated with NASH (OR 2.3, 95% CI 1.2-4.4), NASH fibrosis (2.1, 95% CI 1.1-3.8), and higher fibrosis stage (OR 1.9, 95% CI 1.1-3.4), p values ≤ 0.02. In these women, the proportion with NASH (from 27% to 88%) and NASH fibrosis (from 27% to 81%) steadily rose with higher free T quartiles (p<0.01). Free T was additionally associated with abdominal adiposity among all pre-menopausal women (OR 2.2, 95%CI 1.2-4.1, p=0.015).
Conclusions:
In young women with NAFLD, higher testosterone levels conferred a two-fold higher risk of NASH and NASH fibrosis, and increased risk of abdominal adiposity, supporting a potential mechanistic link of abdominal fat on testosterone-associated liver injury. Testosterone may represent an early risk factor for NASH progression in young women, prior to their onset of more dominant, age-related metabolic risk factors.
Keywords: androgens, hepatic inflammation, sex hormones, nonalcoholic fatty liver disease, abdominal adiposity
INTRODUCTION:
Nonalcoholic fatty liver disease (NAFLD) occurs in 25-30% of individuals globally1, and the epidemiologic landscape of NAFLD is shifting, with a growing number of women and young adults now affected.2,3 The most rapid rise in incident NAFLD is among those under 40 years of age4, and consequently cirrhosis from nonalcoholic steatohepatitis (NASH) is the most rapidly growing indication for transplant in young adults.2 Moreover, compared to men, female patients are disproportionately affected by nonalcoholic steatohepatitis (NASH) 5,6, with higher risk of progression to cirrhosis and end-stage liver disease.3 To date there remain no approved drugs for treatment of NASH and there is urgent need to identify modifiable risk factors for NASH, particularly in young women.
Testosterone, traditionally considered a “male” sex hormone, plays important physiologic roles in women, including effects on metabolic health. Importantly, testosterone has well established sexually dimorphic effects on metabolic disease, with testosterone deficiency in men promoting diabetes and visceral adiposity, whereas higher testosterone levels are associated with these risks in women.7-10 We and others have further shown that unlike in men11,12, higher testosterone levels in young women increase their risk for NAFLD13,14, independent of other metabolic risk factors. Whether testosterone also contributes to liver injury (i.e. NASH) in young women with NAFLD, is not known. If identified, testosterone may serve as a potential therapeutic target for NAFLD in women.
To address existing knowledge gaps, we evaluated the association of free testosterone measured in pre-menopausal women from the multicenter NASH Clinical Research Network (NASH CRN) on biopsy-confirmed findings of NAFLD, including presence of NASH and NASH fibrosis.
METHODS:
Study Design and Patient Population-
Pre-menopausal women (n=210) in the multicenter NASH Clinical Research Network (NASH CRN) were selected at random to include the full spectrum of NAFLD, from simple steatosis to NASH with varying severity of fibrosis. Women older than the average national age at menopause (> 51 years)15 who reported no menstrual periods within the past 5 years were excluded (n=3) to ensure no inadvertent inclusion of post-menopausal women.
Study predictors:
Our primary predictor was free testosterone (free T) measured from banked serum within 6 months of liver biopsy. Free testosterone was analyzed in quartiles to manage outliers.
Histologic outcomes:
All biopsies were centrally evaluated by a panel of NASH CRN pathologists. Liver biopsy was assessed for steatosis, NAFLD Activity Score (NAS) and fibrosis. The NAS is a composite score ranging from 0 to 8 points composed of steatosis (0–3), hepatocyte ballooning (0–2) and lobular inflammation scores (0–3).16 Fibrosis stage ranged from 0-4, and advanced fibrosis was defined as stage 3 or 4 disease. The presence of NASH was categorized as definite, possible/borderline, or absent based on central NASH CRN pathology review. Our primary study outcomes were 1) presence of definite or borderline NASH (as compared to nonalcoholic fatty liver (NAFL)) and 2) presence of any fibrosis (stage 1 or greater versus stage 0).
Cohort characteristics and covariates:
Demographics included age, race, and ethnicity. Metabolic risk factors were captured from most recent timepoint within 6 months of biopsy, including body mass index (BMI), waist circumference, fasting lipids (low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides), and homeostatic model assessment for insulin resistance (HOMA-IR). Hypertension and type 2 diabetes mellitus (DM2) reflected self-report of ever having this diagnosis. Abdominal adiposity was defined as > 88cm and dyslipidemia defined as triglycerides ≥150mg/dL, HDL < 50mg/dL, and/or LDL ≥100mg/dL. Polycystic ovary syndrome (PCOS) was determined by self-report, and separately considered as “suspected PCOS” if women had elevated free testosterone levels (>10pg/mL17) and report of irregular or rare menstrual periods within the past 5 years.18
Sex hormone assays:
Total testosterone was measured using the gold standard methodology of liquid chromatography tandem mass spectrometry. The sensitivity of the testosterone assay (limit of quantification) was 0.0024 ng/ml. Sex hormone binding globulin (SHBG) concentrations were measured by Quantikine enzyme immunoassay (R&D Systems) with intra-assay coefficient of variation (CV) of 9.71%, assay sensitivity of 0.006 nmol/L and the inter-assay CV of 7.45%. These measures were performed by the Assay Services at the Wisconsin National Primate Research Center. Free testosterone was calculated by validated equation using measured total testosterone and SHBG levels.19,20
Statistical Analysis:
The primary multivariate models included covariates selected a priori based on known associations with the study outcome (age, diabetes, dyslipidemia, and abdominal adiposity). Logistic regression was used to assess the association between testosterone and presence of NASH (versus NAFL) and presence of any fibrosis (versus none), while ordinal logistic regression was used to assess the association with severity of fibrosis, ranging from stages 0-4. P-values < 0.05 were considered statistically significant.
Interaction testing between age and free testosterone was performed given the physiologic decline of testosterone levels with age in pre-menopausal women21, and the established association of aging with NAFLD severity. P-values < 0.05 were also considered statistically significant for interaction terms. Study outcomes were then analyzed by quartiles of age due to identified age by testosterone interactions. Test of trend was performed to evaluate the proportion of women with NASH and NASH fibrosis by free testosterone quartiles. Formal mediation analysis (Stata mediation package)22 was performed to evaluate the association of metabolic co-morbidities on the association of free testosterone with study outcomes.
In a post-hoc analysis, we additionally performed binary logistic regression models to explore the association of free testosterone with metabolic co-morbidities (DM2, abdominal adiposity, and/or dyslipidemia) as potential pathways by which testosterone may affect NAFLD disease severity. To determine whether observed findings were driven by presence of PCOS, we additionally performed sensitivity analyses following exclusion of women with self-reported or suspected PCOS as defined above. All analyses were conducted using Stata 15.0
RESULTS:
Cohort characteristics
This study included 207 pre-menopausal women with biopsy-confirmed NAFLD with a median age of 35 years (IQR 29-41); 73% were White and 25% Hispanic (Table 1). Most women were obese (81%), had abdominal adiposity (93%), and dyslipidemia (96%), while 32% had DM2, and 31% had hypertension. Twenty-one percent of women had self-reported PCOS. Median free T was 3.6 pg/mL (IQR 1.9-5.4) and median total testosterone level was 210 pg/mL (IQR 120-290), with steady decline with increasing age (test of trend p values <0.04). Two thirds of women had borderline or definite NASH (69%) and 67% had evidence of fibrosis (91% among those with NASH). Advanced fibrosis (stages 3-4) was present in 15% of all women and in 20% of women with NASH. Characteristics by age included lower LDL levels, less hypertension, and a higher proportion of women with PCOS in the youngest compared to the oldest age quartiles (Supplemental Table 1).
Table 1.
Cohort Characteristics Among Pre-Menopausal Women With NAFLD (n=207)
| Age, median years (IQR) | 35 (29-41) |
| Race, n (%) | |
| White | 150 (72.5) |
| Asian | 13 (6.3) |
| Black | 9 (4.4) |
| Hispanic, n (%) | 52 (25.1) |
| Body mass index (BMI), median kg/cm2 (IQR) | 36.4 (31.8-41.2) |
| Obesity (BMI ≥ 30 kg/cm2), n (%) | 167 (80.7) |
| Waist circumference, median cm (IQR) | 112 (99-120) |
| Abdominal adiposity (waist > 88cm), n (%) | 192 (92.8) |
| Hypertension, n (%) | 65 (31.4) |
| Total cholesterol, median mg/dL (IQR) | 190 (167-215) |
| HDL, median mg/dL (IQR) | 42 (35-50) |
| LDL, median mg/dL (IQR) | 117 (94-138) |
| Triglycerides, median mg/dL (IQR) | 146 (115-196) |
| Dyslipidemia | 198 (95.7) |
| Type 2 diabetes, n (%) | 67 (32.4) |
| HOMA-IR, median mg/dL (IQR) | 4.6 (2.8-8.0) |
| Polycystic ovary syndrome (PCOS), self-report, n (%) | 43 (20.8) |
| Total testosterone, median pg/mL (IQR) | 210 (120-290) |
| Free testosterone (Free T), median pg/mL (IQR)* | 2.8 (1.6-4.8) |
| Sex hormone binding globulin, median nmol/L (IQR) | 37.8 (24.9-62.9) |
| NASH (borderline or definite), n (%) | 144 (69.6) |
| NAFLD Activity Score, median (IQR) | 4 (3-6) |
| NAFLD Activity Score among those with NASH | 5 (4-7) |
| Any fibrosis, n (%) | 140 (67.6) |
| Any fibrosis among those with NASH | 131 (91.0) |
| Advanced fibrosis (stages 3-4), n (%) | 31 (15.0) |
| Advanced fibrosis among those with NASH | 30 (20.8) |
Normal range for pre-menopausal populations 0.6-9.8pg/mL17
We identified a significant interaction between quartiles of age and free T on presence of NASH (OR 0.69, 95% CI 0.53-0.91, p=0.007) and NASH fibrosis (OR 0.71, 95% CI 0.55-0.93, p=0.012), indicating a more positive association of free T on histologic outcomes with decreasing age. Stratified by age, the association of free T with histologic outcomes was driven by the testosterone effect among women in the youngest age quartile (median age 25 years, IQR 22-27) (Table 2), while this effect was not seen in older age groups. Likewise, among women in the youngest age quartile, the proportion with NASH and with NASH fibrosis steadily increased from 27% to 88%, and 21% to 81%, respectively (p values <0.02) with increasing quartiles of free T (Figure 1), though differences by free T were not seen in older age groups (p values ≥0.5).
Table 2.
Free Testosterone is Associated With NASH and NASH Fibrosis in Younger Pre-Menopausal Women with NAFLD (n=207)
| NAFLD Comparisons |
1st Age Quartile n=52 Median 25 years (IQR 22-27) |
2nd Age Quartile n=52 Median 32 years (IQR 31-34) |
3rd Age Quartile n=52 Median 39 years (IQR 37-40) |
4th Age Quartile n=51 Median 47 years (IQR 44-50) |
|---|---|---|---|---|
| Adjusted OR (95% CI), p-value | ||||
| NASH (vs NAFL) |
2.28 (1.18-4.39) p=0.013 |
0.95 (0.55-1.61) p=0.836 |
0.88 (0.45-1.71) p=0.700 |
0.82 (0.44-1.52) p=0.520 |
| Any fibrosis (vs none) |
2.08 (1.13-3.82) p=0.018 |
0.78 (0.47-1.30) p=0.350 |
0.81 (0.43-1.51) p=0.502 |
0.78 (0.40-1.51) p=0.448 |
|
Higher fibrosis stage (from stages 0-4) |
1.94 (1.12-3.38) p=0.019 |
0.72 (0.47-1.11) p=0.137 |
0.67 (0.41-1.08) p=0.101 |
0.84 (0.50-1.41) p=0.523 |
Adjusted for DM2, abdominal adiposity, and dyslipidemia. From lowest to highest age quartiles, NASH was present in n=34, n=37, n=39, and n=34 participants, and any fibrosis was present in n=32, n=34, n=37, and n=37 participants, respectively.
Figure 1.
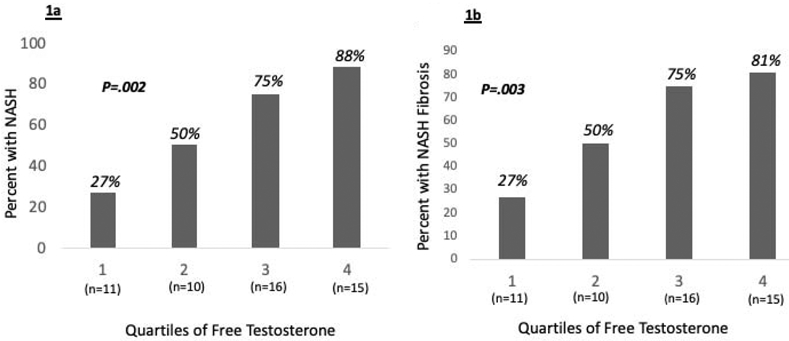
Proportion of pre-menopausal women in the youngest age quartile (n=52) with NASH (Figure 1a) and NASH fibrosis (Figure 1b) by increasing quartiles of free testosterone. Test of trend p-values shown.
Given the higher proportion of women with PCOS in the youngest versus oldest age quartiles, we also explored whether exclusion of self-reported and suspected PCOS may alter findings. Though sample size was limited (n=39), the association of free T with any NASH (adjusted OR 2.28, 95% CI 1.06-4.93, p=0.035) and NASH fibrosis (adjusted OR 1.97, 95% CI 1.03-3.79, p=0.041) persisted (Table 3).
Table 3.
Association of Free Testosterone with NASH and NASH Fibrosis in Youngest Age Quartile After Excluding Polycystic Ovary Syndrome (n=39)
| NAFLD Comparisons | Unadjusted OR (95% CI), p value |
Adjusted OR* (95% CI), p value |
|---|---|---|
| NASH (vs NAFL) | 2.80 (1.35-5.79), p=0.006 |
2.29 (1.06-4.93), p=0.035 |
| Any fibrosis | 2.27 (1.16-4.43), p=0.016 |
1.97 (1.03-3.79), p=0.041 |
| Higher fibrosis stage | 2.28 (1.23-4.22), p=0.009 |
1.93 (0.95-3.90), p=0.069 |
PCOS defined as self-report or having elevated free testosterone and irregular menses.
Adjusted for DM2 and abdominal adiposity (waist >88cm). Dyslipidemia fell out of model as all women with the limited number of fibrosis (n=22) and NASH (n=23) events also had dyslipidemia.
We conducted several post-hoc analyses to better understand how testosterone may promote NAFLD severity. First, we analyzed the association of free T with key metabolic risk factors for NAFLD severity including abdominal obesity, DM2, and dyslipidemia. Adjusted for age, there was no association of free T with DM2 or dyslipidemia (Table 4). However, there was a strong association of free T with abdominal adiposity, including after adjustment for age, dyslipidemia, and DM2 (OR 2.16, 95% CI 1.22-3.81, p=0.007). Moreover, there was no interaction between age and free T on presence of abdominal adiposity (p=0.572) indicting that the association of free T with abdominal obesity persisted across age groups of pre-menopausal women.
Table 4.
Association of Free Testosterone with Metabolic Risk Factors for NASH in Pre-Menopausal Women with NAFLD (n=207)
| Outcome | Age-adjusted OR (95% CI) p value |
Fully adjusted* OR (95% CI) p value |
|---|---|---|
| Type 2 Diabetes (DM2) | 1.02 (0.78-1.33) p=0.892 |
---- |
| Dyslipidemia | 0.99 (0.53-1.84) p=0.973 |
---- |
| Abdominal adiposity (waist circumference > 88cm) |
2.16 (1.23-3.82) p=0.008 |
2.15 (1.22-3.79) p=0008 |
Adjusted for Age, DM2, and dyslipidemia (defined as elevated LDL, triglycerides, and/or low HDL)
We then conducted formal mediation analyses to determine whether the effect of free T on NASH or NASH fibrosis in the youngest age quartile of women was mediated, or explained by, the presence of metabolic comorbidities. The estimated mediating effect of abdominal adiposity on the relationship between free T and NASH was approximately 9.5% (95% CI 0.5.6–36.9%) with wide confidence intervals in light of small sample size. A similar mediating effect of 9.5% (95% CI 0.5.5-35.7%) was noted for DM2, with a minimal mediating effect for dyslipidemia of 1.7% (95% CI 0.9-6.8). Slightly lower mediating effects were seen for abdominal adiposity and DM2 on the relationship between free T and NASH fibrosis, with estimated effects for abdominal adiposity of 7.9% (95% 4.9-28.0), and for DM2 of 7.1% (95% CI 4.3-25.4). There was again a minimal effect of dyslipidemia at 1.2% (95% CI 0.7-4.6).
We additionally re-ran our final models among women in the youngest age quartile using HOMA-IR instead of DM2, and continuous measure of waist circumference instead of abdominal obesity. These point estimates remained consistent for study outcomes, though with attenuation of the p value for NASH fibrosis from 0.02 to 0.07. (Supplemental Table 2).
DISCUSSION:
Prior studies have demonstrated an association between testosterone and imaging-confirmed steatosis in women, and to our knowledge this is the first study to evaluate the association of testosterone levels with histologic measures of NAFLD severity in women. Leveraging the multi-center NASH CRN cohort of pre-menopausal women with biopsy-confirmed NAFLD, we found a more than two-fold higher risk of NASH, as well as NASH fibrosis, with increasing quartiles of free T, independent of metabolic risk factors. Importantly, there was a striking interaction between age and free testosterone on histologic outcomes, with findings driven by the association of free testosterone with NAFLD histology in the youngest age quartile. Given the rising risk of NASH in young adults and women, the current findings may offer a key modifiable risk factor for disease progression in young women.
Sexually dimorphic effects of testosterone on metabolic disease are well recognized, as testosterone deficient men have greater risk for diabetes and visceral adiposity, as well as NAFLD and NASH fibrosis.7,8,10-12 In contrast, higher testosterone levels in women promote diabetes7,10, visceral adiposity23,24, and hepatic steatosis13,14,25, with our current findings now demonstrating the association of testosterone with significant liver injury in young women. While our study focused on pre-menopausal women, a recent large Korean study evaluated the association of higher levels of testosterone with ultrasoundassessed NAFLD by menopausal status.13 The authors found consistent findings in pre-menopausal women, with no apparent association of testosterone with NAFLD in postmenopausal women. Expanding upon prior data, our study shows an increased risk of histologically-confirmed NAFLD severity with higher testosterone levels, while also highlighting differences by age, within a pre-menopausal population. While the reasons for this age effect are not clear, testosterone levels do decline with aging in pre-menopausal women21, and higher levels seen in the youngest group of women may help to set the stage for liver disease progression, prior to their onset of more dominant metabolic comorbidities that come with aging.
Most data on the association of testosterone with metabolic disease in women derive from populations with PCOS, a common, and classically hyperandrogenic condition.26 Androgens are an established risk factor for more severe metabolic features in women with PCOS26, including more severe hepatic steatosis in women with hyperandrogenic PCOS as compared to the less common PCOS phenotype marked by normal androgens.25 In the current study 21% (n=43) of pre-menopausal women had self-reported PCOS, which was more common in the youngest as compared to the oldest age quartile. While it is possible that a higher prevalence of PCOS in younger women contributed to observed differences by age, several lines of evidence suggest that our findings are not limited to women with PCOS. Prior data from the Coronary Artery Risk Development in Young Adults cohort14, as well as the recent large Korean cohort13, demonstrate a clear and independent association of testosterone with imaging-confirmed NAFLD in pre-menopausal women, including among women with normal range testosterone levels. In our current study, we did explore whether exclusion of women with self-reported or suspected PCOS by laboratory criteria and menstrual history would alter our findings. Although our sample size was limited, the association of free T with NASH and NASH fibrosis remained statistically significant following exclusion of PCOS. While PCOS was present in only 8% of women in the oldest age quartile, the proportion with PCOS in the first three age quartiles remained over 20%. These data therefore support a role of testosterone on histologic NAFLD severity that also extends beyond young women with androgen excess.
Current findings do raise the possibility of studying anti-androgen therapy as a novel therapy for NASH in young women. In mouse models of NAFLD, the anti-androgenic drug spironolactone, has been shown to improve hepatic triglycerides, serum lipid levels, as well as NASH histology.27 In a randomized controlled trial of NAFLD, 12 months of vitamin E plus spironolactone compared to vitamin E alone improved markers of hepatic steatosis and insulin resistance.28 In women with PCOS, spironolactone also improves insulin resistance, lipid profiles, and free fatty acid levels.29,30 These data underscore the need for dedicated evaluation of testosterone as a potential therapeutic target for NASH in young women.
The mechanisms by which testosterone may promote liver injury in women are likely multifactorial, though may relate to known androgenic effects on visceral adiposity. In a prior study we found that the relationship between testosterone and imaging-confirmed NAFLD in women was in large part mediated, or explained by, imaging-quantified visceral adiposity.14 In the current study, where waist circumference was used as a measure of abdominal adiposity, the mediating effect was modest. However, our post-hoc analyses demonstrated a strong association of testosterone with abdominal adiposity, which persisted among all pre-menopausal women. These findings are consistent with clinical observations of women taking exogenous testosterone24 including exogenous testosterone use in healthy young female to male transgender patients who preferentially redistribute fat from subcutaneous to visceral stores.23 In a randomized controlled trial of flutamide, a selective androgen receptor blocker, obese women with PCOS also experienced a reduction in visceral fat volume31. Thus, androgen-associated visceral adiposity might be an important contributing factor to NAFLD/NASH in women.
Visceral adiposity is an established risk factor for NASH progression, and acts through several established mechanisms including increasing insulin resistance, aberrant adipokine activity, systemic inflammation, as well as lipotoxicity.32-34 In the current study, adjustment for HOMA-IR as a measure of insulin resistance did not alter the point estimates for NAFLD severity, suggesting that findings were not explained by this pathway. Interestingly, data from PCOS populations do highlight a direct role of androgens of increased systemic lipotoxicity, with hyperandrogenic PCOS patients having increased circulating levels of glycerophospholipids and lysoglycerophospholipids.35 These lipid species have been separately implicated in NASH-associated liver injury36, supporting the need for mechanistic studies to evaluate the relationship between androgens, visceral-fat, and liver injury in women with NASH.
There were some limitations and notable strengths of the current study. Our overall sample size was modest, and while findings were most pronounced in younger women, it is not clear whether larger sample size would have facilitated the detection of androgen-associated effects in older subgroups of pre-menopausal women. Likewise, women with PCOS represent a common, and established at-risk group for NAFLD/NASH, though limited numbers constrained our ability to separately study this population. As stated, visceral adiposity may be an important link between androgens and NAFLD in women, though imaging quantification of visceral fat was unavailable, thus waist circumference was used as a measure of abdominal adiposity. Despite these limitations, several strengths are noteworthy. Our study leverages the multi-center NASH CRN cohort which includes central, standardized review of liver biopsies, as well as comprehensive serologic and clinical measures of metabolic disease. Testosterone concentration was also measured using gold standard methodology of liquid chromatography tandem mass spectrometry, avoiding inaccuracy issues of commonly employed immunoassays.37
In conclusion, we identified a strong association of higher testosterone with the presence of NASH and NASH fibrosis in younger pre-menopausal women, and separately identified the association of testosterone with abdominal adiposity across all pre-menopausal women with NAFLD. These data indicate the importance of considering chronologic and reproductive-aging on the relationship between testosterone and NAFLD in women, and support the role of testosterone as an early, and potentially modifiable risk factor for NASH progression in young women. Our findings underscore the need for studies to evaluate testosterone as a potential novel therapeutic target in young women with NAFLD, with implications beyond young women with androgen excess.
Supplementary Material
ACKNOWLEDGMENTS:
We thank the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) investigators and the Ancillary Studies Committee for providing clinical samples and relevant data from the Nonalcoholic Fatty Liver Disease (NAFLD) Database, Nonalcoholic Fatty Liver Disease (NAFLD) Adult Database 2, PIVENS trial, and FLINT trial. Sex hormones measurements were supported by the National Institutes of Health, Award P51OD011106 to the Wisconsin National Primate Research Center, University of Wisconsin-Madison.
Financial Support:
This work was supported by a K23 from the NIDDK (DK111944 to MS). The NASH CRN is supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK).
Abbreviations (in order of appearance):
- NAFLD
Nonalcoholic Fatty Liver Disease
- NASH
Nonalcoholic Steatohepatitis
- NASH CRN
NASH Clinical Research Network
- Free T
Free Testosterone
- NAS
NAFLD Activity Score
- NAFL
nonalcoholic fatty liver
- BMI
Body Mass Index
- LDL
Low Density Lipoprotein
- HDL
High Density Lipoprotein
- HOMA-IR
Homeostatic Model of Assessment of Insulin Resistance
- DM2
Type 2 Diabetes Mellitus
- PCOS
Polycystic Ovary Syndrome
- SHBG
Sex Hormone Binding Globulin
Footnotes
Disclosures: Co-authors have no conflicts of interest or competing interests that are relevant to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 2.Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. J Clin Gastroenterol. 2018;52(4):339–346. [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women have Lower Risk of Nonalcoholic Fatty Liver Disease but Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 yearcommunity study. Hepatology. 2018;67(5):1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noureddin M, Vipani A, Bresee C, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. The American journal of gastroenterology. 2018;113(11):1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonardo A, Nascimbeni F, Ballestri S, et al. Sex Differences in NAFLD: State of the Art and Identification of Research Gaps. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. Jama. 2006;295(11):1288–1299. [DOI] [PubMed] [Google Scholar]
- 8.Blouin K, Despres JP, Couillard C, et al. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metabolism: clinical and experimental. 2005;54(8):1034–1040. [DOI] [PubMed] [Google Scholar]
- 9.Yassin A, Almehmadi Y, Saad F, Doros G, Gooren L. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clinical endocrinology. 2016;84(1):107–114. [DOI] [PubMed] [Google Scholar]
- 10.Schiffer L, Kempegowda P, Arlt W, O'Reilly MW. MECHANISMS IN ENDOCRINOLOGY: The sexually dimorphic role of androgens in human metabolic disease. European journal of endocrinology / European Federation of Endocrine Societies. 2017;177(3):R125–R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaruvongvanich V, Sanguankeo A, Riangwiwat T, Upala S. Testosterone, Sex Hormone-Binding Globulin and Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-Analysis. Ann Hepatol. 2017;16(3):382–394. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar M, Yates K, Suzuki A, et al. Low Testosterone Is Associated With Nonalcoholic Steatohepatitis (NASH) and Severity of NASH Fibrosis in Men With NAFLD. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JP, Lee HS, Oh J, Lee Y. Serum testosterone level within normal range is positively associated with nonalcoholic fatty liver disease in premenopausal but not postmenopausal women. Journal of Women’s Health. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar M, Wellons M, Cedars MI, et al. Testosterone Levels in Pre-Menopausal Women are Associated With Nonalcoholic Fatty Liver Disease in Midlife. The American journal of gastroenterology. 2017;112(5):755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institutes of Health. https://www.nichd.nih.gov/health/topics/menopause/conditioninfo/default#f2 Web site. Accessed July 17th, 2020, 2020. [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 17.Mayo Clinical Laboratories. https://www.mayocliniclabs.com/testcatalog/Clinical+and+Interpretive/83686. Accessed July 17th, 2020. [Google Scholar]
- 18.Zawadski J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific; 1992:377–384. [Google Scholar]
- 19.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. The Journal of clinical endocrinology and metabolism. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 20.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. [DOI] [PubMed] [Google Scholar]
- 21.Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. The Journal of clinical endocrinology and metabolism. 1995;80(4):1429–1430. [DOI] [PubMed] [Google Scholar]
- 22.Hicks R, Tingly D. Causal mediation analysis. The Stata Journal.11(4):1–15. [Google Scholar]
- 23.Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. The Journal of clinical endocrinology and metabolism. 1997;82(7):2044–2047. [DOI] [PubMed] [Google Scholar]
- 24.Lovejoy JC, Bray GA, Bourgeois MO, et al. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women--a clinical research center study. The Journal of clinical endocrinology and metabolism. 1996;81(6):2198–2203. [DOI] [PubMed] [Google Scholar]
- 25.Jones H, Sprung VS, Pugh CJ, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. The Journal of clinical endocrinology and metabolism. 2012;97(10):3709–3716. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada T, Kenmochi H, Miyashita Y, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151(5):2040–2049. [DOI] [PubMed] [Google Scholar]
- 28.Polyzos SA, Kountouras J, Mantzoros CS, Polymerou V, Katsinelos P. Effects of combined low-dose spironolactone plus vitamin E vs vitamin E monotherapy on insulin resistance, non-invasive indices of steatosis and fibrosis, and adipokine levels in non-alcoholic fatty liver disease: a randomized controlled trial. Diabetes Obes Metab. 2017;19(12):1805–1809. [DOI] [PubMed] [Google Scholar]
- 29.Zulian E, Sartorato P, Benedini S, et al. Spironolactone in the treatment of polycystic ovary syndrome: effects on clinical features, insulin sensitivity and lipid profile. J Endocrinol Invest. 2005;28(1):49–53. [DOI] [PubMed] [Google Scholar]
- 30.Muneyyirci-Delale O, Kaplan J, Joulak I, Yang L, Von Gizycki H, Nacharaju VL. Serum free fatty acid levels in PCOS patients treated with glucophage, magnesium oxide and spironolactone. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2013;29(5):474–477. [DOI] [PubMed] [Google Scholar]
- 31.Gambineri A, Patton L, Vaccina A, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. The Journal of clinical endocrinology and metabolism. 2006;91(10):3970–3980. [DOI] [PubMed] [Google Scholar]
- 32.Choromanska B, Mysliwiec P, Razak Hady H, et al. Metabolic Syndrome is Associated with Ceramide Accumulation in Visceral Adipose Tissue of Women with Morbid Obesity. Obesity. 2019;27(3):444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagadala M, Kasumov T, McCullough AJ, Zein NN, Kirwan JP. Role of ceramides in nonalcoholic fatty liver disease. Trends in endocrinology and metabolism: TEM. 2012;23(8):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musso G, Cassader M, Paschetta E, Gambino R. Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155(2):282–302 e288. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly MW, Kempegowda P, Walsh M, et al. AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anjani K, Lhomme M, Sokolovska N, et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. Journal of hepatology. 2015;62(4):905–912. [DOI] [PubMed] [Google Scholar]
- 37.Kanakis GA, Tsametis CP, Goulis DG. Measuring testosterone in women and men. Maturitas. 2019;125:41–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


