INTRODUCTION
Vascular malformations are congenital disorders of vasculogenesis that affect approximately 0.5% of the population. Although benign, lesions cause significant morbidity, primarily because they enlarge over time. A common problem is psychosocial distress because most involve the integument resulting in a deformity. Other complications include: bleeding, pain, ulceration, loss of function, organ failure, and death.
Vascular malformations are differentiated from vascular tumors based on endothelial proliferation.1 Vascular malformations classically have been considered quiescent lesions with minimal cellular turnover. Vascular tumors, in contrast, have significant endothelial division. Because of this discrepancy in cellular proliferation, vascular tumors, similar to other neoplasms, have been able to be treated with drugs. For example, infantile hemangioma responds to beta-blockers and corticosteroids, while kaposiform hemangioendothelioma is managed with sirolimus or vincristine.
Physicians who focus on patients with vascular malformations recognize that these lesions are not static. Vascular malformations do exhibit cellular activity and growth, although less than vascular tumors. Drug development for vascular malformations has been limited because: (1) embryological structural problems are not believed to be amenable to pharmacotherapy, (2) drugs used for vascular tumors in the past were not effective for malformations (e.g., propranolol, prednisone), and (3) the mechanisms by which vascular malformations enlarge are unknown.
Over the past several years tremendous growth in our understanding of the pathophysiology of vascular malformations has occurred. Our group has focused on understanding the biology of vascular anomalies in order to develop improved therapies. The first step in solving the mystery of vascular malformations is to determine their genetic basis. Technological advances in next generation sequencing and bioinformatics has allowed the identification of low-level mutations in many somatic diseases, including vascular malformations (Table 1).
Table 1.
Genetic mutations associated with vascular malformations.
| Vascular Malformation | Mutation |
|---|---|
| Capillary Malformation (CM) | GNA11 GNAQ PIK3CA |
| Lymphatic Malformation (LM) | PIK3CA |
| Primary Lymphedema | CCBE1 FOXC2 SOX18 VEGFR3 |
| Venous Malformation (VM) | PIK3CA TIE2 |
| Cerebral Cavernous Malformation | CCM1/KRIT1 CCM2/Macalvernin CCM2/PDCD10 |
| Fibroadipose Vascular Anomaly | PIK3CA |
| Glomuvenous Malformation | Glomulin |
| Verrucous Venous Malformation | MAP3K3 |
| Arteriovenous Malformation (AVM) | BRAF HRAS KRAS MAP2K1 |
| Capillary Malformation-Arteriovenous Malformation | EPHB4 RASA1 |
| Hereditary Hemorrhagic Telangiectasia | ACVRL1/ALK1 ENG GDF2 SMAD4 |
| Parkes Weber Syndrome | EPHB4 RASA1 |
| PTEN Hamartoma-Tumor Syndrome | PTEN |
| Overgrowth Syndromes | |
| Maffucci | IDH1/IDH2 |
| PIK3CA-Related Overgrowth Spectrum (PROS) CLOVES Klippel-Trenaunay Facial Infiltrating Lipomatosis Megalencephaly-Capillary Malformation |
PIK3CA |
| Proteus | AKT1 |
Currently, the mutations responsible for most types of vascular malformations have been identified.2 Variants typically effect tyrosine kinase signaling along either the RAS or PIK3CA pathways. Mutations usually are enriched in the endothelial cells. Although the mutations and cell-type driving the pathophysiology of the malformations are known, the mechanisms by which the mutation causes the malformation and results in its growth are being studied.
Now that the mutations for vascular malformations have been identified, investigators can test potential drug therapies on cell function in-vitro as well as attempt to develop in-vivo animal models. Although numerous types of vascular malformations exist, almost all of them fall under 4 major categories (capillary malformation, lymphatic malformation, venous malformation, arteriovenous malformation [Figure 1]) that mirror the types of vasculature (capillaries, lymphatics, veins, arteries). Vascular malformations also contribute to overgrowth syndromes.
Figure 1.
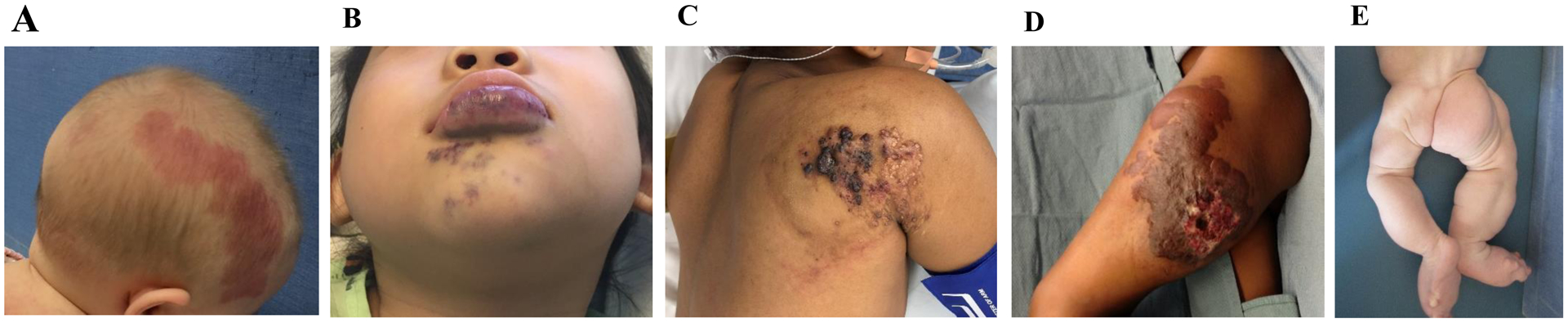
Major types of vascular malformations and an example of an overgrowth syndrome. (A) Capillary malformation. (B) Venous malformation. (C) Lymphatic malformation. (D) Arteriovenous malformation. (E) CLOVES syndrome.
Capillary Malformation (CM)
Capillary malformation is the most common type of vascular malformation, affecting approximately 1/300 individuals. Lesions are present at birth as pink cutaneous stains. CMs darken, the skin thickens, tissues beneath the lesion become enlarged, and pyogenic granulomas can develop. First-line therapy is pulsed-dye laser to lighten the malformation; overgrowth of tissues is managed with resection. Unfortunately, CMs re-darken and enlarge following treatment. Almost all CMs are sporadic and most are caused by mutations in GNAQ.3,4 Diffuse lesions involving an extremity may result from GNA11 or PIK3CA variants.5,6 The cell-type enriched for the somatic mutations is the endothelial cell lining the blood vessels which is thus driving the pathophysiology of this lesion.4 Although the mutations responsible for CMs are known, the biology by which the mutation causes the lesion and results in its growth remains unclear. Animal models of CM do not exist and targeted drug therapy has not been investigated experimentally or clinically.
Lymphatic Malformation (LM)
LMs result from abnormal lymphatic development and cause bleeding, infection, pain, ulceration, and destruction of tissues. Treatment includes sclerotherapy, laser, or resection. LMs are caused by mutations in PIK3CA.7 Primary lymphedema also is a type of LM and germline mutations in approximately 20 genes are known to cause this condition: VEGFR3, VEGFC, CCBE1, FOXC2, GJC2, GJA1, PTPN14, SOX18, HGF, KIF11, PTPN11, SOS1, GATA2, IKBKG, RASA1, 15q locus, ITGA9, KRAS, RAF1, HRAS.8 LM was the first vascular malformation to be successfully treated with targeted drug therapy using the mTOR inhibitor sirolimus (rapamycin) which blocks the PIK3CA signaling pathway.9–11
LMs currently are the most common type of vascular malformation treated with pharmacotherapy (Figure 2A). Initially, only patients with very problematic lesions that had failed traditional treatments received the drug. Because sirolimus has a favorable toxicity profile, however, the indications for treating patients with this medicine have expanded. Sirolimus is now considered as first-line therapy in some patients and is used to reduce recurrence following sclerotherapy and resection. Recently, a genetically engineered mouse model of LM was developed and PIK3CA inhibitors showed efficacy.12 Because PIK3CA mutations are the most prevalent variants in the field of vascular anomalies, pharmacotherapeutic development targeting this gene has received the most attention.
Figure 2.

Current treatment of vascular malformations with pharmacotherapy. (A) Successful management of a tongue lymphatic malformation before (above) and after (below) oral sirolimus (photos courtesy of Cameron Trenor, MD). (B) Patient with an AVM treated with oral trametinib which improved her symptoms and slowed the progression of the lesion.
Venous Malformation (VM)
Several germline and somatic mutations result in VMs and many exhibit a genotype-phenotype relationship. Mutations in the tyrosine kinase receptor TIE2 cause both autosomal dominant cutaneomucosal VMs as well as most sporadic VMs.13–15 Cerebral cavernous malformation (CCM) involves the brain and may be sporadic or autosomal dominant. CCM can be associated with hyperkeratotic cutaneous lesions and are caused by mutations in CCM1/KRIT1, CCM2/macalvernin, or CCM2/PDCD10.16 A glomulin mutation leads to autosomal dominant glomuvenous malformation (GVM). GVMs are small, multifocal, typically cause pain, and do not respond well to sclerotherapy.17 A MAP3K3 variant causes somatic verrucous venous malformations (VVM) that is a hyperkeratotic lesion usually affecting an extremity.18 Somatic VMs also result from PIK3CA mutations which usually are located subcutaneously.19 Fibroadipose vascular anomaly (FAVA), which was previously thought to be an intramuscular VM, results from PIK3CA variants.7
VMs enlarge, especially during puberty, and are managed by sclerotherapy and resection. Like all vascular malformations, they can re-grow following treatment. A favorable cell-based animal model exists for VM that has been used for drug testing.20 Endothelial cells containing disease-causing mutations injected subcutaneously into immunodeficient mice create VMs that reasonably recapitulate the human phenotype. Sirolimus has shown efficacy against VMs in both animal models and humans.20–22
Arteriovenous Malformation (AVM)
Arteriovenous malformation is an abnormal connection between arteries and veins without a normal capillary bed. Oxygen delivery to tissues is reduced and patients suffer from pain, bleeding, ulceration, deformity, and rarely congestive heart failure. AVM is the most active “tumor-like” malformation and grows, especially during puberty. AVM can be the most challenging type of vascular malformation to treat. Management includes embolization and resection. Unfortunately, AVMs have a high recurrence rate following these interventions.
Autosomal dominant hereditary AVMs can occur. Hereditary hemorrhagic telangiectasia (HHT) causes epistaxis, telangiectasias, and visceral AVMs and results from mutations in ENG, ACVRL1/ALK1, SMAD4, or GDF2.23 Capillary malformation-arteriovenous malformation (CM-AVM) and Parkes Weber syndrome (PWS) are caused by germline variants in RASA1 and EPHB4.24–26 Germline PTEN mutations result in PTEN hamartoma-tumor syndrome; 50% of patients have AVMs that are typically multifocal, intramuscular, and have extra adipose tissue.27
Almost all AVMs are sporadic and most extracranial and intracranial lesions are caused by mutations in MAP2K1 and KRAS, respectively.28,29 Extracranial AVMs can result from HRAS, BRAF, and KRAS variants as well.30–32 Mutations in BRAF also have been found to cause intracranial AVMs.33 Although a genotype-phenotype association has not been present in intracranial AVMs, a relationship is emerging with extracranial AVMs. Lesions caused by HRAS variants have extra adipose tissue.30 KRAS mutations are more likely to cause intramuscular fast-flow vascular anomalies (IFVA) and lesions that appear similar to congenital hemangiomas.31,34 AVM MAP2K1 mutations are located in the endothelial cell and activate signaling causing tissue overgrowth by a cell non-autonomous mechanism.28,35,36 The activating effects of the mutation in endothelial cells can be mitigated by treating the cells with a MAP2K1 inhibitor.35 Consequently, patients in our center and others are being treated with a FDA-approved MAP2K1 inhibitor (trametinib) that was developed to treat melanoma and lung cancer (Figure 2B). Results in our experience as well as published data show that for the first time a drug can prevent the growth of AVM as well as cause it to become smaller.37,38
Vascular Malformation Overgrowth Syndromes
Capillary, venous, lymphatic, and arteriovenous malformations can be a major component of overgrowth syndromes. Proteus syndrome (progressive, asymmetrical overgrowth of the skeleton, cerebriform nevi of the hands or feet, epidermal nevi, vascular malformations, cerebral anomalies, skull hyperostosis, and megaspondylodysplasia) results from an AKT1 mutation.39 Maffucci syndrome (multiple enchondromas and soft-tissue VMs) is caused by variants in IDH1/IDH2.40
The most common overgrowth conditions result from PIK3CA mutations and are classified under the term PROS (PIK3CA-related overgrowth spectrum).41,42 CLOVES syndrome (congenital lipomatosis, overgrowth, vascular malformations, epidermal nevi, and skeletal anomalies), Klippel-Trenaunay syndrome (capillary-lymphatic-venous malformation of an extremity causing overgrowth), and facial infiltrating lipomatosis result from a PIK3CA mutation.7,43,44 The mutation is present throughout all structures and is enriched in non-endothelial cells.45 Megalencephaly-capillary malformation (MCM) causes neurologic abnormalities and patients typically have a CM involving the upper lip, trunk, or extremity.46 PROS conditions are being treated successfully in our center and others with sirolimus and PIK3CA inhibitors.47–49
Conclusions
It is a very exciting time in the field of vascular anomalies. Vascular malformations are at the crossroads of plastic surgery, basic research, innovation, and interdisciplinary collaboration. Molecular discoveries have been translated into clinical practice. We now use genetic testing to identify lesions with an unclear diagnosis, as well as to screen patients for potential drug treatment. We are at a point in time where we are transitioning from managing vascular malformations with resection, embolization, sclerotherapy, and laser to pharmacotherapy. Several FDA approved medications used off-label for vascular malformations have shown efficacy. In the future, drugs for vascular malformations will be a routine part of their management (Figure 3). Medications will be used to prevent these lesions from enlarging, as well as reducing their risk of recurrence following “traditional” interventions.
Figure 3.
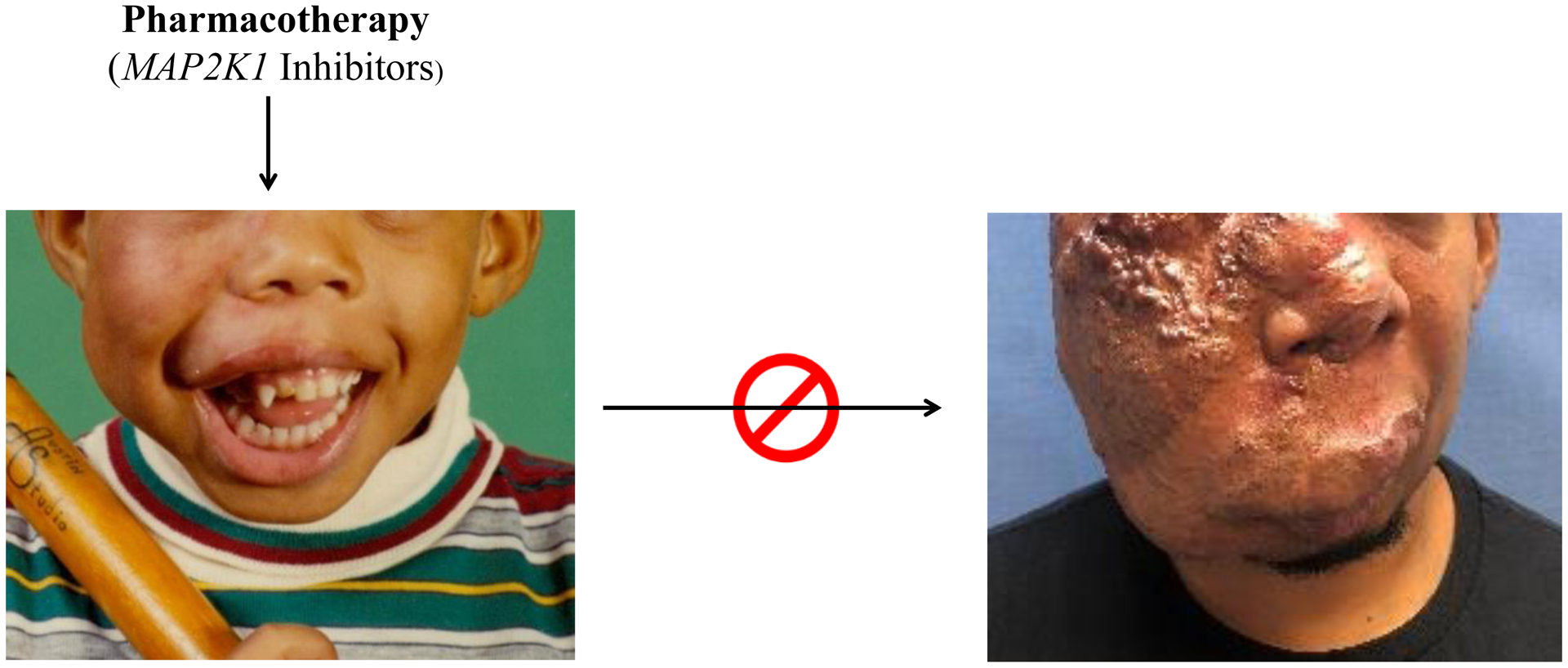
Future of drug treatment for vascular malformations. Our goal is to develop pharmacotherapy for patients that will prevent the enlargement of their lesion. In the future, this patient will receive medicine in childhood (left) that will stop his AVM from growing to cause significant morbidity in adulthood (right).
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD093735 (AKG), the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R01 HL127030-05 (AKG). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest exist.
REFERENCES
- 1.Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: Recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136(1):e203–e214. doi: 10.1542/peds.2014-3673 [DOI] [PubMed] [Google Scholar]
- 2.Greene AK, Goss JA. Vascular Anomalies: From a Clinicohistologic to a Genetic Framework. Plast Reconstr Surg. 2018;141(5):709e–717e. doi: 10.1097/PRS.0000000000004294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirley MD, Tang H, Gallione CJ, et al. Sturge–Weber Syndrome and Port-Wine Stains Caused by Somatic Mutation in GNAQ. N Engl J Med. 2013;368(21):1971–1979. doi: 10.1056/nejmoa1213507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto JA, Huang L, Vivero MP, et al. Endothelial cells from capillary malformations are enriched for somatic GNAQ mutations. Plast Reconstr Surg. 2016;137(1):77e–82e. doi: 10.1097/PRS.0000000000001868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couto JA, Ayturk UM, Konczyk DJ, et al. A somatic GNA11 mutation is associated with extremity capillary malformation and overgrowth. Angiogenesis. 2017;20(3):303–306. doi: 10.1007/s10456-016-9538-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss JA, Konczyk DJ, Smits P, et al. Diffuse capillary malformation with overgrowth contains somatic PIK3CA variants. Clin Genet. January 2020: 10.1111/cge.13702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr. 2015;166(4):1048–1054.e5. doi: 10.1016/j.jpeds.2014.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest. 2014;124(3):898–904. doi: 10.1172/JCI71614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammill AM, Wentzel M, Gupta A, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57(6):1018–1024. doi: 10.1002/pbc.23124 [DOI] [PubMed] [Google Scholar]
- 10.Adams DM, Trenor CC, Hammill AM, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137(2). doi: 10.1542/peds.2015-3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rössler J, Geiger J, Földi E, Adams DM, Niemeyer CM. Sirolimus is highly effective for lymph leakage in microcystic lymphatic malformations with skin involvement. Int J Dermatol. 2017;56(4):e72–e75. doi: 10.1111/ijd.13419 [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Corral I, Zhang Y, Petkova M, et al. Blockade of VEGF-C signaling inhibits lymphatic malformations driven by oncogenic PIK3CA mutation. Nat Commun. 2020;11(1). doi: 10.1038/s41467-020-16496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vikkula M, Boon LM, Carraway KL, et al. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87(7):1181–1190. doi: 10.1016/S0092-8674(00)81814-0 [DOI] [PubMed] [Google Scholar]
- 14.Limaye N, Wouters V, Uebelhoer M, et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009;41(1):118–124. doi: 10.1038/ng.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soblet J, Limaye N, Uebelhoer M, Boon LM, Vikkula M. Variable somatic TIE2 mutations in half of sporadic venous malformations. Mol Syndromol. 2013;4(4):179–183. doi: 10.1159/000348327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laberge-le Couteulx S, Jung HH, Labauge P, et al. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat Genet. 1999;23(2):189–193. doi: 10.1038/13815 [DOI] [PubMed] [Google Scholar]
- 17.Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866–874. doi: 10.1086/339492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couto JA, Vivero MP, Kozakewich HPW, et al. A somatic MAP3K3 mutation is associated with verrucous venous malformation. Am J Hum Genet. 2015;96(3):480–486. doi: 10.1016/j.ajhg.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limaye N, Kangas J, Mendola A, et al. Somatic Activating PIK3CA Mutations Cause Venous Malformation. Am J Hum Genet. 2015;97(6):914–921. doi: 10.1016/j.ajhg.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boscolo E, Limaye N, Huang L, et al. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015;125(9):3491–3504. doi: 10.1172/JCI76004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammer J, Seront E, Duez S, et al. Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: A monocentric prospective phase II study. Orphanet J Rare Dis. 2018;13(1). doi: 10.1186/s13023-018-0934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salloum R, Fox CE, Alvarez-Allende CR, et al. Response of Blue Rubber Bleb Nevus Syndrome to Sirolimus Treatment. Pediatr Blood Cancer. 2016;63(11):1911–1914. doi: 10.1002/pbc.26049 [DOI] [PubMed] [Google Scholar]
- 23.McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8(4):345–351. doi: 10.1038/ng1294-345 [DOI] [PubMed] [Google Scholar]
- 24.Eerola I, Boon LM, Mulliken JB, et al. Capillary Malformation–Arteriovenous Malformation, a New Clinical and Genetic Disorder Caused by RASA1 Mutations. Am J Hum Genet. 2003;73(6):1240–1249. doi: 10.1086/379793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revencu N, Boon LM, Mulliken JB, et al. Parkes Weber syndrome, vein of galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008;29(7):959–965. doi: 10.1002/humu.20746 [DOI] [PubMed] [Google Scholar]
- 26.Amyere M, Revencu N, Helaers R, et al. Germline loss-of-function mutations in EPHB4 cause a second form of capillary malformation-arteriovenous malformation (CM-AVM2) deregulating RAS-MAPK signaling. Circulation. 2017;136(11):1037–1048. doi: 10.1161/CIRCULATIONAHA.116.026886 [DOI] [PubMed] [Google Scholar]
- 27.Tan WH, Baris HN, Burrows PE, et al. The spectrum of vascular anomalies in patients with PTEN mutations: Implications for diagnosis and management. J Med Genet. 2007;44(9):594–602. doi: 10.1136/jmg.2007.048934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Couto JA, Huang AY, Konczyk DJ, et al. Somatic MAP2K1 Mutations Are Associated with Extracranial Arteriovenous Malformation. Am J Hum Genet. 2017;100(3):546–554. doi: 10.1016/j.ajhg.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolaev SI, Vetiska S, Bonilla X, et al. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N Engl J Med. 2018;378(3):250–261. doi: 10.1056/NEJMoa1709449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konczyk DJ, Goss JA, Smits PJ, et al. Arteriovenous malformation associated with a HRAS mutation. Hum Genet. 2019;138(11–12):1419–1421. doi: 10.1007/s00439-019-02072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudduth CL, McGuire AM, Smits PJ, et al. Arteriovenous Malformation Phenotype Resembling Congenital Hemangioma Contains KRAS Mutations. Clin Genet. August 2020. doi: 10.1111/cge.13833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Olabi L, Polubothu S, Dowsett K, et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J Clin Invest. 2018;128(4):1496–1508. doi: 10.1172/JCI98589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goss JA, Huang AY, Smith E, et al. Somatic mutations in intracranial arteriovenous malformations. Thomas AJ, ed. PLoS One. 2019;14(12):e0226852. doi: 10.1371/journal.pone.0226852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goss JA, Konczyk DJ, Smits PJ, et al. Intramuscular fast-flow vascular anomaly contains somatic MAP2K1 and KRAS mutations. Angiogenesis. 2019;22(4):547–552. doi: 10.1007/s10456-019-09678-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smits PJ, Konczyk DJ, Sudduth CL, Goss JA, Greene AK. Endothelial MAP2K1 mutations in arteriovenous malformation activate the RAS/MAPK pathway. Biochem Biophys Res Commun. 2020;529(2):450–454. doi: 10.1016/j.bbrc.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konczyk DJ, Goss JA, Smits PJ, Sudduth CL, Al-Ibraheemi A, Greene AK. Arteriovenous Malformation MAP2K1 Mutation Causes Local Cartilage Overgrowth by a Cell-Non Autonomous Mechanism. Sci Rep. 2020;10(1):4428. doi: 10.1038/s41598-020-61444-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edwards E, Phelps A, Cooke D. Monitoring arteriovenous malformation response to genotype-targeted therapy. Pediatrics. 2020;146(3):e20193206. doi: 10.1542/peds.2019-3206 [DOI] [PubMed] [Google Scholar]
- 38.Lekwuttikarn R, Lim YH, Admani S, Choate KA, Teng JMC. Genotype-Guided Medical Treatment of an Arteriovenous Malformation in a Child. JAMA Dermatology. 2019;155(2):256–257. doi: 10.1001/jamadermatol.2018.4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the proteus syndrome. N Engl J Med. 2011;365(7):611–619. doi: 10.1056/NEJMoa1104017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amary MF, Damato S, Halai D, et al. Ollier disease and Maffucci syndrome are caused by somatic mosaic mutations of IDH1 and IDH2. Nat Genet. 2011;43(12):1262–1265. doi: 10.1038/ng.994 [DOI] [PubMed] [Google Scholar]
- 41.Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am J Med Genet Part A. 2014;164(7):1713–1733. doi: 10.1002/ajmg.a.36552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keppler-Noreuil KM, Rios JJ, Parker VER, et al. PIK3CA-related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet Part A. 2015;167(2):287–295. doi: 10.1002/ajmg.a.36836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurek KC, Luks VL, Ayturk UM, et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet. 2012;90(6):1108–1115. doi: 10.1016/j.ajhg.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maclellan RA, Luks VL, Vivero MP, et al. PIK3CA activating mutations in facial infiltrating lipomatosis. Plast Reconstr Surg. 2014;133(1):12–19. doi: 10.1097/01.prs.0000436822.26709.7c [DOI] [PubMed] [Google Scholar]
- 45.Couto JA, Konczyk DJ, Vivero MP, et al. Somatic PIK3CA mutations are present in multiple tissues of facial infiltrating lipomatosis. Pediatr Res. 2017;82(5):850–854. doi: 10.1038/pr.2017.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirzaa GM, Rivière JB, Dobyns WB. Megalencephaly syndromes and activating mutations in the PI3K-AKT pathway: MPPH and MCAP. Am J Med Genet Part C Semin Med Genet. 2013;163(2):122–130. doi: 10.1002/ajmg.c.31361 [DOI] [PubMed] [Google Scholar]
- 47.Parker VER, Keppler-Noreuil KM, Faivre L, et al. Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum. Genet Med. 2019;21(5):1189–1198. doi: 10.1038/s41436-018-0297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venot Q, Blanc T, Rabia SH, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature. 2018;558(7711):540–546. doi: 10.1038/s41586-018-0217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.López Gutiérrez JC, Lizarraga R, Delgado C, et al. Alpelisib Treatment for Genital Vascular Malformation in a Patient with Congenital Lipomatous Overgrowth, Vascular Malformations, Epidermal Nevi, and Spinal/Skeletal Anomalies and/or Scoliosis (CLOVES) Syndrome. J Pediatr Adolesc Gynecol. 2019;32(6):648–650. doi: 10.1016/j.jpag.2019.07.003 [DOI] [PubMed] [Google Scholar]


