Abstract
Purpose
To report a French experience in patients admitted to Intensive Care Unit (ICU) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) requiring high fractional concentration of inspired oxygen supported by high flow nasal cannula (HFNC) as first-line therapy.
Methods
Retrospective cohort study conducted in two ICUs of a French university hospital. All consecutive patients admitted during 28-days after the first admission for SARS-CoV-2 pneumonia were screened. Demographic, clinical, respiratory support, specific therapeutics, ICU length-of-stay and survival data were collected.
Results
Data of 43 patients were analyzed: mainly men (72%), median age 61 (51–69) years, median body mass index of 28 (25–31) kg/m2, median simplified acute physiology score (SAPS II) of 29 (22–37) and median PaO2/fraction of inspired oxygen (FiO2) (P/F) ratio of 146 (100–189) mmHg. HFNC was initiated at ICU admission in 76% of patients. Median flow was 50 (45–50) L/min and median FiO2 was 0.6 (0.5–0.8). 79% of patients presented at least one comorbidity, mainly hypertension (58%). At day (D) 28, 32% of patients required invasive mechanical ventilation, 3 patients died in ICU. Risk factors for intubation were diabetes (10% vs. 43%, P = 0.04) and extensive lesions on chest computed tomography (CT) (P = 0.023). Patients with more than 25% of lesions on chest CT were more frequently intubated during ICU stay (P = 0.012). At ICU admission (D1), patients with higher SAPS II and Sequential Organ Failure Assessment (SOFA) scores (respectively 39 (28–50) vs. 27 (22–31), P = 0.0031 and 5 (2–8) vs. 2 (2–2.2), P = 0.0019), and a lower P/F ratio (98 (63–109) vs. 178 (126–206), P = 0.0005) were more frequently intubated. Among non-intubated patients, the median lowest P/F was 131 (85–180) mmHg. Four caregivers had to stop working following coronavirus 2 contamination, but did not require hospitalization.
Conclusion
Our clinical experience supports the use of HFNC as first line-therapy in patients with SARS-COV-2 pneumonia for whom face mask oxygen does not provide adequate respiratory support.
Keywords: SARS-COV-2 pneumonia, Hypoxemia, High flow oxygen therapy, Mechanical ventilation, Outcomes
1. Introduction
In December 2019, the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was diagnosed in China. SARS-CoV-2 is responsible for a rapidly spreading illness [1] known as Coronavirus Disease 2019 (COVID-19). Up to one third of patients hospitalized for SARS-CoV-2 infection require Intensive Care Unit (ICU) management. Severe COVID-19 may progress to acute hypoxemic respiratory failure requiring high fractional concentration of inspired oxygen (FiO2) and different ventilation strategies. High-flow nasal cannula (HFNC) is a non-invasive technique of respiratory support used in critically ill patients with acute hypoxemic respiratory failure [2]. However, HFNC may cause aerosol dispersion of infectious particles and therefore was not initially recommended as first-line respiratory therapy for SARS-CoV-2 infection by some experts [3].
In France, invasive ventilation was widely used from the start of the COVID-19 outbreak in the regions first affected, especially in the east of France, with a rapid saturation of ICU beds [4]. In our center in the north-west of France, we have used HFNC oxygen therapy for several decades to manage ICU patients with hypoxemic pneumonia [5]. We used this same technique of respiratory support to manage ICU patients with SARS-CoV-2 pneumonia. We implemented protective measures for all caregivers according to WHO and French recommendations [6], [7]. Later publications reported the use of HFNC oxygen therapy, either in connection with the use of non-invasive methods of oxygen therapy [8], [9], or in connection with physio-pathological considerations of these COVID-related pneumonias [10], [11].
The aim of this retrospective study was to report a French experience in patients admitted to ICU for SARS-CoV-2 pneumonia requiring high fractional concentration of inspired oxygen, supported by HFNC oxygen as systematic first-line respiratory therapy.
2. Methods
2.1. Design
This retrospective observational cohort study was conducted in two ICUs (one medical ICU of 27 beds, and one surgical ICU of 23 beds) of Rouen University Hospital, Normandy, France.
2.2. Patients
All consecutive patients admitted to these two ICUs during the 28 days following the first SARS-CoV-2 pneumonia admission on March 13th, 2020 were screened. The diagnosis of SARS-CoV-2 pneumonia was based on clinical characteristics, chest imaging and real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay.
Other patients with a diagnosis of SARS-CoV-2 pneumonia and hospitalized in a COVID ICU created de novo were excluded because of difficulty of data collection and possible influence on treatment specifically related to an unusual environment.
The study protocol was approved by the local ethics committee and the institutional review board (approval number E2020-31). Patients (or surrogates) were informed by letter of the study based on anonymous data from their medical file.
2.3. Data collection
Patients’ demographic characteristics: age, sex, body mass index (BMI), severity scores (Simplified Acute Physiology Score (SAPS) II score [12], Sequential Organ Failure Assessment (SOFA) [13] and comorbidities were recorded at baseline for all patients. Dates of first symptoms, hospital and ICU admissions and computed tomography (CT) results when performed within 24 hours of admission to ICU were reported. Daily respiratory support devices (oxygen mask, HFNC, non-invasive ventilation or invasive ventilation), fraction of inspired oxygen (FiO2) [14] and oxygen flow in case of high-flow oxygen therapy, respiratory rate, arterial blood gas, specific therapies for COVID 19, and adjuvant therapies for acute respiratory distress syndrome (ARDS) such as use of continuous neuromuscular blockers, nitric oxide or prone position until day 28 (D28) were also recorded. Data regarding hemodynamic failure, defined as the use of norepinephrine, and kidney failure, defined as a score ≥ 3 on “Kidney Disease: Improving Global Outcomes” (KDIGO) were recorded. The following outcomes were evaluated: dates of weaning from invasive mechanical ventilation or from HFNC, dates of ICU and hospital discharge, ICU mortality and D28 mortality.
2.4. Statistical analyses
Characteristics of patients are described as frequencies and percentages for categorical variables and medians and interquartile ranges (IQR) for continuous variables. Categorical variables were compared using chi-square or Fisher's exact test, and continuous variables were compared using Student's t-test or Wilcoxon's rank-sum test. We retained a P value of 0.05.
3. Results
3.1. Study population
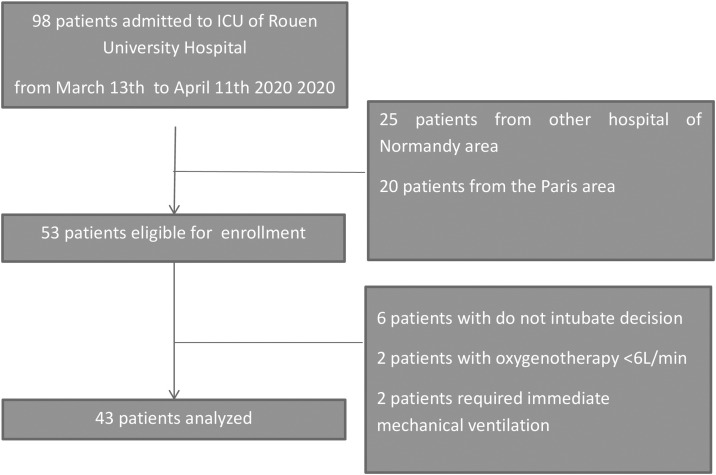
From March 13th to April 11th, 2020, 98 patients consecutively admitted to two ICUs for SARS-CoV-2 pneumonia with acute respiratory failure (ARF) were eligible for inclusion. Among them, 45 patients transferred from another region of France were not considered because of a lack of information on the initial severity of the disease. A majority of them had been initially intubated notably to secure medical transport. Fifty-three patients were eligible for enrollment and, finally, after exclusion of 10 patients for initial decision of palliative care or flow of standard oxygen below 6 L/min, 43 patients were analyzed (Fig. 1 ).
Fig. 1.
Study population: flow-chart.
The decision to intubate was based on the analysis of the clinician in charge of the patient regarding respiratory exhaustion, hemodynamic or neurological failure, but not on the sole criterion of oxygenation.
3.2. Patient's general characteristics
The characteristics of the 43 patients are presented in (Table 1 ). A majority were men (72%), with a median age of 61 (51.5 to 69.5) years, a median BMI of 28 (25.2 to 31) kg/m2. Patients were admitted to ICU in a median time of 9 (6 to 11) days after the onset of symptoms; 79% presented at least one comorbidity, mainly hypertension (58%).
Table 1.
General characteristics of population.
| Overall population | n = 43 | |
|---|---|---|
| Demographics | Male gender – n (%) | 31 (72) |
| Age (years) median (IQR) | 61 (51.5–69.5) | |
| Comorbidities, n (%) | 34 (79) | |
| Chronic liver disease | 0 | |
| Chronic kidney disease | 1 (2.3) | |
| Immunocompromised patients | 3 (7) | |
| Chronic heart failure | 5 (11.6) | |
| Diabetes mellitus | 9 (20.9) | |
| Respiratory disease | 13 (30.2) | |
| Hypertension | 25 (58.1) | |
| BMI, median (IQR) | 27.9 (25.2–31) | |
| Viral characteristics | Duration of symptoms before ICU admission (days), median (IQR) | 9 (6–11) |
| Length of hospital stay before ICU admission (days) median (IQR) | 1 (0–2) | |
| Chest computed tomographya, n (%) | ||
| Compatible | 34 (97.1) | |
| Crazy paving | 21 (61.8) | |
| Consolidation | 30 (88.2) | |
| Ground glass opacification | 33 (97) | |
| Extensive | ||
| < 25% | 13 (39.4) | |
| [25–50] % | 12 (36.3) | |
| [51–75] % | 6 (18.2) | |
| > 75% | 2 (6) | |
| One specific treatmentb, n (%) | 30 (69.7) | |
| Lopinavir/ritonavir | 27 (90) | |
| Hydroxychloroquine | 3 (10) | |
| Corticosteroids, n (%) | 9 (21) | |
| Length of ICU stay before corticosteroids (days) median (IQR) | 2 (0–3) |
BMI: body mass index.
Data are available for 35 of 43 included patients.
Patients admitted with one specific treatment: 27 (90%).
3.3. Patients’ characteristics at ICU admission and during ICU stay
The median SAPS II was 29 (22.5 to 37) and the median PaO2/FiO2 (P/F) ratio was 146 (100–189) mmHg (Table 2 ). HFNC was initiated at ICU admission in the majority of patients (33, 76%), with a median flow of 50 (45 to 50) L/min and a median FiO2 of 0.6 (0.5 to 0.8).
Table 2.
Characteristics of the population at ICU admission and during ICU stay.
| Overall population | n = 43 | |
|---|---|---|
| ICU admission | SAPS II, median (IQR) | 29 (22.5–37) |
| SOFA D1, median (IQR) | 2 (2–4) | |
| Respiratory ratea, median (IQR) | 24 (20–28) | |
| PaO2/FiO2, median (IQR) | 146 (100–189) | |
| At HFNC initiationb: Flow (L/min), median (IQR) | 50 (45–50) | |
| At HFNC initiationc: FiO2, median (IQR) | 0.6 (0.5–0.8) | |
| ICU stay | Hemodynamic failure, n (%) Kidney failure, n (%) |
13 (30) 7 (16) |
| Mechanical ventilation, n (%) | 14 (32) | |
| At HFNC initiation, after D2d: Flow (L/min), median (IQR) | 50 (50–50) | |
| At HFNC initiation, after D2d: FiO2, median (IQR) | 0.70 (0.5–0.75) |
ICU: Intensive Care Unit; SAPS II: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; HFNC: High Flow Nasal Cannula.
Data are available for 38 of 43 included patients.
Thirty-three (76%) of 43 included patients had HFNC at admission.
Five (12%) of 43 included patients had HFNC after D2.
At D28, 32% of patients required invasive mechanical ventilation, 30% had hemodynamic failure and 16% had renal failure. We did not use non-invasive ventilation or awake prone positioning in these patients.
3.4. Patients’ outcomes
Patients stayed respectively 8 (5 to 16) days in ICU and 28 (15 to 28) days in hospital; at D28, 4 (9%) patients were still in ICU and 19 (53%) were still in hospital (Table 3 ). Three (7%) patients died in ICU.
Table 3.
Outcomes of the population at day 28.
| Overall population | n = 43 | |
|---|---|---|
| Outcomes at D28 | Length of ICU stay (days)a median (IQR) Still in ICU, n (%) |
8 (5–16) 4 (9) |
| Length of hospital stay (days)a median (IQR) Still in hospital, n (%)b |
28 (15–28) 19 (52.7) |
|
| Mortality in ICU, n (%) | 3 (7) | |
| Mortality in hospital, n (%) | 3 (7) |
ICU: Intensive Care Unit.
Among 40 patients still alive: length of ICU stay (days) 11.2 (8.9); length of hospital stay (days) 23.3 (7.3).
Among 36 patients alive and discharged from ICU.
3.5. Comparison according to success or failure of HFNC
Twenty-nine (67%) patients were not intubated while 14 (33%) were intubated (Table 4 ). Intubated patients more frequently had diabetes (43% vs. 10%, P = 0.04). Patients with extensive lesions at chest computed tomography (≥ 25%) were more frequently intubated during ICU stay (P = 0.012).
Table 4.
Comparison between success and failure of HFNC (univariate analysis).
| Non intubated patients (n = 29) | Intubated patients (n = 14) | |||
|---|---|---|---|---|
| Demographic characteristics | Male gender, n (%) | 22 (76) | 9 (64.3) | |
| Age (years), median (IQR) | 61 (50–69) | 65 (56–73) | ||
| Comorbidities, n (%) | 18 (62) | 13 (92.9) | 0.23 | |
| Chronic liver disease | 0 | 0 | ||
| Chronic kidney disease | 1 (3.4) | 0 | ||
| Immunocompromised patients | 2 (6.9) | 1 (7) | ||
| Chronic heart failure | 2 (6.9) | 3 (21.4) | ||
| Diabetes mellitus | 3 (10.3) | 6 (42.8) | 0.04 | |
| Respiratory disease | 8 (27.6) | 5 (35.7) | ||
| Hypertension | 15 (51.7) | 10 (71.4) | ||
| BMI median (IQR) | 27.8 (24.7–30.8) | 28.4 (26.9–31.5) | ||
| ≥ 30, n (%) | 9 (31) | 5 (35.7) | ||
| < 30, n (%) | 20 (69) | 9 (64.3) | ||
| Viral characteristics | Duration of symptoms before ICU admission (days), median (IQR) | 9 (7–11) | 8 (5–15) | 0.73 |
| Length of hospital stay before ICU admission (days), median (IQR) | 1 (0–1) | 1 (0–3) | ||
| Chest computed tomographya | ||||
| Extensive lesions, n (%) | 0.023 | |||
| < 25% | 13(52) | 0 | 0.012 | |
| [25–50]% | 7 (28) | 5 (62) | ||
| [51–75]% | 4 (16) | 2 (25) | ||
| >75% | 1 (4) | 1 (12.5) | ||
| At ICU admission (D1) | SAPS II, median (IQR) | 27 (22–31) | 39 (28–50) | 0.0031 |
| SOFA score D1b, median (IQR) | 2 (2–2.2) | 5 (2–8) | 0.0019 | |
| Respiratory ratec, median (IQR) | 24 (20–27) | 24 (21–28) | ||
| Flow (L/min)§, median (IQR) | 50 (40–50) | |||
| FiO2, median (IQR) | 0.5 (0.4–0.6) | 0.8 (0.8–1) | 0.0001 | |
| PaO2 (mmHg), median (IQR) | 72.8 (65.3–84) | 63 (55–73) | 0.04 | |
| PaO2/FiO2d, median (IQR) | 178 (126–206) | 98 (63–109) | 0.0005 | |
| Outcome at D28 | Hemodynamic failure, n (%) | 0 | 13 (93.9) | 0.002 |
| Kidney failure, n (%) | 2 (6.9) | 5 (36) | 0.026 | |
| Lowest PaO2/FiO2 (mmHg), median (IQR) | 131 (85–180) | |||
| Length of ICU stay (days) before lowest PaO2/FiO2, median (IQR) | 1 (0–2) | |||
| Flow (L/min) of HFNC at lowest PaO2/FiO2, median (IQR) | 50 (47–50) | |||
| FiO2 of HFNC at lowest PaO2/FiO2, median (IQR) | 0.55 (0.50–0.65) | |||
| PaO2/FiO2 before MV, median (IQR) | 85 (66–143) | |||
| Length of ICU stay and MV (days) median (IQR) | 0 (0–1) | |||
| Prone position, n (%) | 9 (64.3) | |||
| Nitric oxide, n (%) | 0 | 2 (15) | ||
| Duration of sedation (days) median (IQR) | 12 (11–15) | |||
| Duration of curare (days) median (IQR) | 5 (2–9) | |||
| Duration of MV (days)e, median (IQR) | 17 (14–26) | |||
| Length of ICU stay (days)e, median (IQR) | 6 (3–8) | 28 (19–28) | 0.00001 | |
| Still in ICU, n (%) | 0 | 4 (28) | ||
| Still in hospital, n No (%)f | 16 (55.2) | 7 (100) | ||
| Mortality in ICU, n (%) | 0 | 3 (21.4) | 0.013 | |
| Mortality in hospital, n (%) | 0 | 3 (21.4) |
BMI: body mass index; ICU: Intensive Care Unit; SAPS II: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; HFNC: High Flow Nasal Cannula; MV: Mechanical Ventilation.
Data are available for 25 of 29 patients in the non-intubated group and for 8 of 14 patients in the intubated group.
Data are available for 28 of 29 patients in the non-intubated group and for 13 of 14 patients in the intubated group.
Data are available for 26 of 29 patients in the non-intubated group and for 12 of 14 patients in the intubated group.
Data are available for 13 of 14 patients in the intubated group.
Among 11 patients still alive in the intubated group: length of ICU stay (days) 23.6 (5.3); length of hospital stay (days) 28; duration of mechanical ventilation (days) 19 (6.7).
Among 23 patients discharged from ICU in the non-intubated group and 7 patients alive and discharged from ICU.
Patients with higher median SAPS II and SOFA D1 scores (respectively, 39 (28 to 50) vs. 27 (22 to 31), P = 0.0031 and 5 (2 to 8) vs. 2 (2 to 2.2), P = 0.0019), and a lower median PaO2/FiO2 (P/F) ratio (98 (63 to 109) vs. 178 (126 to 206), P = 0.0005) were more frequently intubated. In addition, other additional factors, such as bacterial infection or pulmonary embolism that could have potentially contributed to intubation are shown in Table 5 .
Table 5.
Peri intubation events in intubated patients.
| Patient | Day of invasive mechanical ventilationa | Number of days between disease onset and ICU admission | Pulmonary embolism |
Bacterial co-infectionb | |
|---|---|---|---|---|---|
| Number of days between ICU admission and chest CT | Day of pulmonary embolism diagnosis | ||||
| 1 | D1 | 13 | No | ||
| 2 | D2 | 7 | No | ||
| 3 | D1 | 9 | No | ||
| 4 | D1 | 15 | No | ||
| 5 | D2 | 6 | No | ||
| 6 | D1 | 3 | No | ||
| 7 | D1 | 8 | No | ||
| 8 | D1 | 5 | No | ||
| 9 | D2 | 2 | No | ||
| 10 | D3 | 4 | 5 | No | |
| 11 | D15 | 15 | 10 | D11 | Yes |
| 12 | D1 | 15 | No | ||
| 13 | D1 | 16 | No | ||
| 14 | D6 | 2 | 6 | D7 | Yes |
D1: day of ICU admission.
At time of tracheal intubation (72 h before and after mechanical ventilation).
In patients with invasive ventilation, we observed more hemodynamic and kidney failure (respectively, 13 vs. 0, P = 0.002 and 5 vs. 2, P = 0.026), a longer median length of ICU stay (28 (19 to 28) vs. 6 (3 to 8) days, P = 0.00001) and more mortality (3 vs. 0, P = 0.013).
Among not-intubated patients, the lowest P/F was 131 mmHg.
3.6. Contamination of medical staff and caregivers
During the study period, four staff members were contaminated with Covid-19 leading to work disruption, but they did not require hospitalization. To our knowledge, they were not contaminated in the ICU.
Outcomes at three months are presented in supplementary Table 1.
4. Discussion
Based on the results of our clinical experience, HFNC could represent a safe and effective strategy of first-line oxygenation for patients with severe hypoxemic pneumonia due to SARS-COV-2. More than two thirds of our patients were not intubated, despite a PaO2/FiO2 ratio below 200 mmHg at admission and, a lowest PaO2/FiO2 ratio of 131 mmHg during ICU hospitalization, for non-intubated patients. Related to this strategy, we observed a mortality rate of only 7% at day 28 despite a severe SAPS II score of 29 at admission.
Intubating hypoxemic patients wisely remains a major challenge. The value of using HFNC is based on recent research, especially from Frat and colleagues [2]. A recent systematic review showed that HFNC, in patients with acute hypoxemic respiratory failure, was associated with similar outcomes compared to noninvasive ventilation or conventional oxygen therapy, regarding mortality and ICU length of stay [15]. In our study the decision to intubate was based on the analysis of the clinician in charge of the patient, in particular in view of the presence of signs of respiratory distress or associated visceral failure. We did not use the “ROX index”, as suggested in a letter published after our study period [16].
However, in the current pandemic context a possible contamination of caregivers was initially feared with HFNC, due to the high gas flow used which may increase bio-aerosol dispersion in the environment and favor transmission of viral agents. Faced with this specific risk of contamination of caregivers by aerosol dispersion, we worked with clinical hygienists to educate all caregivers about the classic preventive measures to adopt, based on data from the literature [17]: masks, gowns, and iterative use of hydro-alcoholic gel, in a context where the increased risk of aerosol dispersion via HFNC is not scientifically proven [18]. We observed only four COVID-19 cross contaminations among our ICU staff (more than 100 nurses and 20 physicians), without hospitalization and with a high probability of contamination outside the ICU. This result indicates that a good compliance with prevention measures, namely wearing protective clothing, glasses, gloves and extensive use of hydro-alcoholic solutions could be safe and effective.
The use of HFNC in patients with severe acute respiratory viral infection was previously described in a cohort of ICU patients hospitalized with H1N1 Influenza A [19]. HFNC appears to be an effective modality for the early respiratory support of adults with severe acute respiratory viral infection. A recent small study from China showed that among 17 patients with hypoxemic pneumonia due to SARS-COV-2 pneumonia treated with HFNC, 7 (41%) experienced treatment failure, and among patients with PaO2/FiO2 < 200 mmHg, failure rates reached 63% [20]. Other authors highlighted an association between excess mortality and a SAPS II score threshold of 17 [21]. It is interesting to note that our population was more severe in terms of SAPS II and hypoxemia than those recently analyzed [20], [21]. Moreover, an Italian observational study reported the success of HFNC in patients with a low PaO2/FiO2 ratio of 126 mmHg [22]. We hypothesize that our early use of HFNC, i.e., from a conventional oxygen flow rate of 6 L/min, contributed to the good results observed.
Furthermore, our cohort of patients exhibited similar characteristics to those of European cohorts: male, over 50 years old, with cardiovascular diseases, most often resuscitated due to an initial isolated episode of ARF [23], [24]. The subgroup of intubated patients in our study could be distinguished by greater severity at admission (parenchymal involvement assessed on CT, severity scores, severity of hypoxemia) and a clinical course marked by more organ failures and a prolonged hospital stay. These data are consistent with those in the literature which highlight a benefit of HFNC in the event of hypoxemic ARF without other organ failure [2].
In addition, the good outcome of our patients treated with HFNC was associated with a decreased risk of subsequent intubation. This point is crucial in the current pandemic as medical resources worldwide are limited, particularly ICU capacity and specific drug supplies in the first stages of any invasive mechanical ventilation, namely powerful sedatives and neuroblockers. After our data collection, Demoule et al. [9] published a retrospective study of 379 patients among whom 146 (39%) received HFNC within the first 24 hours following ICU admission. A propensity score matched analysis was performed: results suggest that HFNC significantly reduces intubation and subsequent invasive mechanical ventilation, without effect on mortality. In our study, D28 ICU mortality was found notably low (7%). The specificities of our population (both early and systematic use of first-line HFNC) do not allow a direct comparison between this result and that of the recent French multicenter “Covid ICU” study [25] as well as that of Contou et al. [26]. Our interesting result, however, should encourage ICU physicians to confirm the benefit of an early and systematic use of first-line HFNC in this setting based on a prospective multicenter study.
Our study presents several limitations. First it is a retrospective study, including a small number of patients. Due to this small number, we did not search for predictive factors by multivariate analysis. However, the population was relatively homogeneous exhibiting a similar respiratory pathology due to Covid-19. Also, we only included patients from the local area, who were consecutively admitted to our two ICUs during a one-month period. These patients were all deemed eligible to receive specific care in a skilled environment for the application of this technique of ventilatory support. Our results may not be generalizable, therefore, to other centers or in other conditions. Nevertheless, the early and systematic use of this technique allowed us to progressively increase the number of ICU beds when needed, thus making it possible to maintain our high level of usual care, and vice versa. Moreover, we did not compare our respiratory strategy based on HFNC as first choice with the common strategy used in other French centers, based on systematic early intubation despite a relative standard level of oxygen. Such a comparative analysis has to be carried out.
5. Conclusion
This French clinical experience supports the use of HFNC as first line management in patients with acute respiratory failure due to SARS-COV-2 pneumonia for whom standard face mask oxygen does not provide adequate respiratory support. The use of HFNC may reduce the overall rate of intubation in ICU and improve patient outcomes without any major risk of contamination among caregivers, providing that adequate protection measures are strictly observed. The COVID-19 pandemic still requires further comprehensive risk-benefit analysis to consider HFNC as an alternative technique of respiratory support in patients presenting with acute respiratory failure.
Ethical approval and consent to participate
The study protocol was approved by the local ethics committee and the institutional review board (approval number E2020-31). Patients (or surrogates) were informed by letter of the study based on anonymous data from their medical file.
Consent for publication
Not applicable.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
Not applicable.
Authors’ contributions
G.B., D.B., C.G. and F.T. conceived the study, and were involved in the study design.
G.B., D.B., P.G., C.G. and F.T. performed data analysis and drafted the manuscript.
D.B., G.B. and P.G.G. participated in data collection.
G.B., D.B., P.G.G., P.G., D.C., S.G., C.G., and F.T. analysed results.
All authors read and approved the final manuscript.
Acknowledgements
We thank all the staff who participated in the management of patients and data collection.
The authors are grateful to Nikki Sabourin-Gibbs, Rouen University Hospital, for her precious help in editing the manuscript.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.resmer.2021.100834.
Appendix A. Supplementary data
References
- 1.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frat J.-P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 3.Darmon M. 2020. Recommandations d’experts portant sur la prise en charge en réanimation des patients en période d’épidémie à SARS-CoV2. SRLF-SFAR-SFMU-GFRUP-SPILF-SPLF. [Google Scholar]
- 4.Kuteifan K., Pasquier P., Meyer C., Escarment J., Theissen O. The outbreak of COVID-19 in Mulhouse: hospital crisis management and deployment of military hospital during the outbreak of COVID-19 in Mulhouse, France. Ann Intensive Care. 2020;10:59. doi: 10.1186/s13613-020-00677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besnier E., NSeir S., Lambiotte F., Du Cheyron D., Sauneuf B., Misset B., et al. High-flow nasal cannula therapy: clinical practice in intensive care units. Ann Intensive Care. 2019;9:98. doi: 10.1186/s13613-019-0569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World health organisation . 2020. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) [Google Scholar]
- 7.Société française d’hygiène hospitalière SF2H . 2020. Avis relatif aux indications du port des masques chirurgicaux et des appareils de protection respiratoire de type FFP2 pour les professionnels de santé. [Google Scholar]
- 8.Arulkumaran N., Brealey D., Howell D., Singer M. Use of non-invasive ventilation for patients with COVID-19: a cause for concern? Lancet Respir Med. 2020;8:e45. doi: 10.1016/S2213-2600(20)30181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demoule A., Vieillard Baron A., Darmon M., Beurton A., Géri G., Voiriot G., et al. High-flow nasal cannula in critically III patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L., Marini J.J., Busana M., Chiumello D., Camporota L. Spontaneous breathing, transpulmonary pressure and mathematical trickery. Ann Intensive Care. 2020;10:88. doi: 10.1186/s13613-020-00708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tobin M.J. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201:1319–1320. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 13.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 14.Coudroy R., Frat J.-P., Girault C., Thille A.W. Reliability of methods to estimate the fraction of inspired oxygen in patients with acute respiratory failure breathing through non-rebreather reservoir bag oxygen mask. Thorax. 2020;75(9):805–807. doi: 10.1136/thoraxjnl-2020-214863. [DOI] [PubMed] [Google Scholar]
- 15.Rochwerg B., Granton D., Wang D.X., Helviz Y., Einav S., Frat J.P., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45:563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 16.Zucman N., Mullaert J., Roca O., Ricard J.D. Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med. 2020;46:1924–1926. doi: 10.1007/s00134-020-06177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, surface environmental, and personal protective equipment contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J., Fink J.B., Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020;55:2000892. doi: 10.1183/13993003.00892-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rello J., Pérez M., Roca O., Poulakou G., Souto J., Laborda C., et al. High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care. 2012;27:434–439. doi: 10.1016/j.jcrc.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang K., Zhao W., Li J., Shu W., Duan J. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care. 2020;10:37. doi: 10.1186/s13613-020-00653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou X., Li S., Fang M., Hu M., Bian Y., Ling J., et al. Acute Physiology and Chronic Health Evaluation II Score as a Predictor of Hospital Mortality in Patients of Coronavirus Disease 2019. Crit Care Med. 2020;48:e657–e665. doi: 10.1097/CCM.0000000000004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vianello A., Arcaro G., Molena B., Turato C., Sukthi A., Guarnieri G., et al. High-flow nasal cannula oxygen therapy to treat patients with hypoxemic acute respiratory failure consequent to SARS-CoV-2 infection. Thorax. 2020;75(11):998–1000. doi: 10.1136/thoraxjnl-2020-214993. [DOI] [PubMed] [Google Scholar]
- 23.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;36:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.COVID Group on behalf of the REVA Network, COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contou D., Fraissé M., Pajot O., Tirolien J.-A., Mentec H., Plantefève G. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021;25:3. doi: 10.1186/s13054-020-03449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.