Abstract
Pelvic venous disorders (PeVDs) can result in several different clinical presentations, but can be challenging to distinguish from other etiologies of chronic pelvic pain (CPP). Clinical evaluation of CPP patients optimally should be performed in a multidisciplinary fashion and patients who may have PeVD should be referred for consultation with a vascular interventionalist whose evaluation would utilize an imaging workup to search for pelvic varices. Additionally, it is critical to quantify the quality-of-life effects of all CPP to determine the impact on the patient's overall health. Diagnostic imaging, including transabdominal and transvaginal ultrasound, computed tomography, magnetic resonance imaging, and venography, can be utilized to identify pelvic varices, as well as venous reflux and obstruction leading to CPP. The use of the SVP tool is important to classify PeVD patients based on their clinical symptoms, varicose veins, and pathophysiology for precise clinical communication and for reporting clinical research. The goal of this publication is to delineate the clinical presentation, anatomy, pathophysiology, and imaging evaluation of patients with CPP suspected of having PeVD.
Keywords: pelvic venous disease, pelvic congestion, diagnostic imaging, interventional radiology
Chronic pelvic pain (CPP) represents a diagnostic and therapeutic challenge to all physicians who encounter it, but more importantly it is a burden to affected patients resulting in significantly decreased quality of life (QoL). Cost of evaluation and treatments for CPP are estimated per year at approximately $2.8 billion, and often fail to result in a definitive diagnosis. 1 Recent studies have shown that around 20% of diagnostic laparoscopies are performed to evaluate for unknown causes of CPP. 2 Diagnostic imaging modalities such ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) can result in improved diagnostic ability as a less invasive approach.
Pelvic venous disorder (PeVD) is an underappreciated cause of CPP. Favorable outcomes from ovarian vein embolization were first reported in the 1990s, 3 4 and although multiple large case series have demonstrated durable clinical improvement and safety, questions regarding its value and appropriate patient selection continue to exist resulting in inconsistent procedural insurance coverage and acceptance of intervention among the gynecology and CPP communities. PeVD should be considered in the spectrum of addressable pathologies resulting in CPP. An improved understanding of the various causes for PeVD, consensus diagnostic criteria, as well as outcomes data from interventional endovascular strategies will result in enhanced outcomes and broader acceptance in the CPP community.
The goal of this article is to provide an overview of clinical manifestations, pathophysiology, patient evaluation, and imaging assessment of patients with CPP presenting for the evaluation of PeVD.
Clinical Presentation
The diagnosis of PeVD should always begin with a thorough clinical investigation to assess for patient symptoms and quantify the duration, severity, extent, and modifying factors of CPP, which is reported in approximately one in six women. 5 American College of Obstetrics and Gynecology (ACOG) defines CPP as noncyclic pain lasting for at least 6 months, localized to the anatomic pelvis (anterior abdominal wall below the umbilicus, lumbosacral back, or buttocks) and associated with functional disability or need for medical care. CPP accounts for up to 50% of all office visits to an obstetrician/gynecologist (OB/GYN). Additionally, of those patients, 40% go on to diagnostic laparoscopy. 6 The incidence of PeVD increased with age greater than 20 years and only 26% of patients were nulliparous by history, suggesting an increased incidence with increasing number of pregnancies more than two and increasing age. 6 The typical presenting symptoms of pelvic venous disease that have been prevalent across all age groups include pelvic pain, dyspareunia, postcoital pain, dysmenorrhea, and less often vulvar varices. The VEIN-TERM Consensus document defined pelvic congestion syndrome as chronic symptoms, which may include pelvic pain, perineal heaviness, urgency of micturition, and post-coital pain, caused by ovarian and/or pelvic vein reflux and/or obstruction, and which may be associated with vulvar, perineal, and/or lower extremity varices. 7
To this end, a classification tool known as SVP (American Vein & Lymphatic Society) has been developed to define the clinical presentation and underlying pathophysiology in patients suspected of having PeVD. 8 The tool is similar to CEAP (Clinical-Etiology-Anatomy-Pathophysiology) classification for lower extremity venous disease utilizing three primary domains: S for symptoms, V for varicose vein location, and P for pathophysiology. The pathophysiology domain has three subdomains to classify the anatomical segment(s) involved, the associated pathophysiology (reflux or obstruction) and the etiology (thrombotic or nonthrombotic) for each segment. A calculator application for smartphones is available to assist in classifying patients using SVP (American Vein & Lymphatic Society, and available at the Apple and Google App stores). The use of this tool will be crucial in reporting patient populations in clinical trials and useful for physician communication and treatment decision-making. 9
Validated QoL assessment tools for PeVD are actively being developed by a multidisciplinary consensus panel. The currently published tools include a Visual Analog Scale pain scores that can be utilized generically or in subdomains of pain such as intensity while lying down, intensity while standing, intensity with menstruation, intensity with intercourse, and urge to urinate. 8 Secondary symptoms such as depression, central and peripheral sensitization, and pelvic floor dysfunction can also be encountered in this patient population. 10 A recent study has also proposed that patients with PeVD may suffer from postural orthostatic tachycardia syndrome demonstrating improved dysautonomia after intervention to relieve pelvic venous insufficiency. 11
When considering the pathophysiology of PeVD, it is important to include assessment for flank pain or renal colic in the evaluation of these patients prior to choosing intervention strategies as renal vein obstruction can occur. Lastly, evaluation for lower extremity swelling as well as lower extremity superficial and deep venous disease is also important as they can occur secondary to either reflux or obstructive pelvic venous pathology.
Anatomy and Pathophysiology
Knowledge of the interconnected venous anatomy in abdomen, pelvis, and proximal lower extremities is essential to understanding the various venous conditions that can lead to CPP. The venous drainage from the lower extremities and pelvis consolidates into the common iliac veins that join to become the inferior vena cava. The aforementioned drainage is accomplished via four interconnected systems: ovarian veins, iliac veins, the left renal vein, and superficial veins of the lower extremities. 10 When considering the symptomatology caused by PeVD, it is important to consider presentation in relation to the three venous beds where variceal dilation can occur: the visceral and parietal pelvic veins and their related venous plexuses, the renal and perihilar veins, and the superficial veins of the vulva and lower extremity ( Fig. 1 ).
Fig. 1.
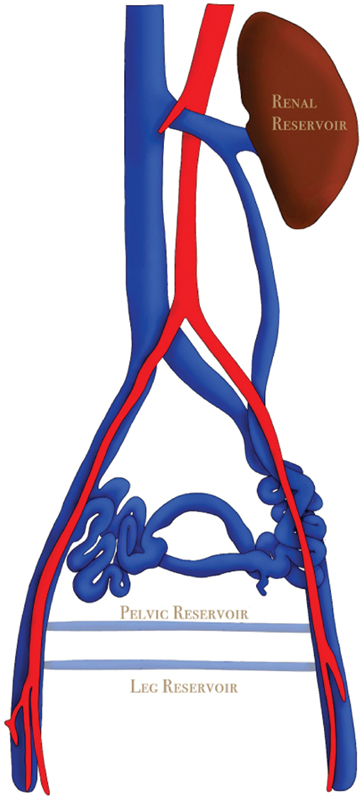
Demonstrates an artist rendering of pelvic venous anatomy showing the pelvic reservoir and leg reservoir in relation to the anatomic configuration of the iliac veins, inferior vena cava and ovarian veins.
Primary ovarian vein insufficiency is a cause of PeVD with a pathophysiology analogous to lower extremity varicosities. This etiology can be attributed to effects of estrogen and progesterone influences on venous dilation as well as venous obstruction during gestation with concomitant increased blood volume. 10 During gestation, blood volume and venous capacitance increase up to 50% in the pelvic veins, 12 which is strongly associated with gestational flux of estrogen and progesterone. Until recently, PeVD has been considered synonymous with ovarian vein reflux with ovarian vein embolization as the primary treatment strategy. However, clinical experience and research have identified iliac vin obstruction as another cause and has elucidated a benefit in women with CPP following iliac vein stenting. 6 13 Anatomic compression of the left common iliac vein can result in retrograde flow in the left internal iliac vein extending into pelvic venous reservoir. Similarly, compression of the left renal vein between the superior mesenteric artery and aorta (also known as nutcracker syndrome) can also impede venous outflow from the left kidney, which can result in flank pain and or hematuria. In a patient with an incompetent left gonadal vein, this pressure can be dissipated to the ovarian venous plexus through the left ovarian vein, and the freely communicating uterovaginal venous plexus.
Irrespective of the mechanism by which the venous flow is affected, the pathophysiologic origins of a patient's clinical manifestations of CPP begin with preferential blood pooling in the pelvic venous plexuses. Increased stretch of veins from obstruction or vasodilation results in the recruitment of matrix metalloproteinases. These enzymes, when activated, cleave proteins responsible for cell-to-cell integrity in vein wall muscle layers and valvular structure. After enzymatic cleavage, and white cell infiltration secondary to endothelial dysfunction, increased vessel capacitance and worsening valvular incompetence occur. Through this cyclic mechanism, increased venous pressure (venous hypertension) develops in the pelvic viscera, particularly within the uterine walls and initiates activation of local nociceptors which may result in the clinical presentation of CPP. Additionally, in the pelvis, there are venous escape points (deep perforator veins) through which the pelvic venous plexus communicates with the superficial veins of the upper thighs, which themselves may become incompetent resulting in the lower extremity, vulvar, and perineal manifestations described earlier. 14
Imaging Assessment
Since the clinical presentation of CPP from a venous cause may have overlapping features with other etiologies of CPP, diagnostic imaging is an important component of patient evaluation and helps direct future treatment strategies. In many OB/GYN practices, PeVD is considered a diagnosis of exclusion based on the frequency of other pathologies such as endometriosis and pelvic floor dysfunction. Current gynecological US guidelines do not seek to identify pelvic varices. However, imaging demonstrating pelvic varices may be important to identify patients who may benefit from endovascular treatment. Although dilated veins in the pelvis are a common finding on imaging studies occurring in up to 15% of women aged 20 to 50 years, 15 not all of these patients will have CPP. No clear imaging criteria to define PeVD have been published.
Imaging definitions for pelvic venous disease based on transfundal venography performed by Beard and colleagues have been modified to be utilized in noninvasive imaging strategies in attempt to identify the pathophysiology of PeVD. 15 16 The predominant factors involved in imaging diagnosis include ovarian vein diameter, and the presence or absence of these findings: ovarian vein reflux, pelvic variceal reservoir, iliac vein obstruction, and renal vein compression. The optimal imaging tool may vary based on the availability of the modality and local expertise but can be extrapolated across modalities with the knowledge that the optimal treatment is not yet clearly defined. The SVP consensus document proposes a major criterion for establishing the diagnosis of PeVD as the presence of varices in the ovarian or uterovaginal plexuses ≥ 5 mm in diameter regardless of what imaging technique is utilized. 17
Ultrasound
Much of the abdominopelvic viscera can be evaluated accurately with transvaginal (TV) or transabdominal (TA) US techniques. Specifically, sonography can be used to look for uterine, ovarian, bowel, and vascular pathologies if performed with the appropriate protocols. Patients evaluated for CPP often undergo US examinations given its accessibility, cost-effectiveness, and convenience. However, most of these outpatient protocols do not include a vascular assessment. Additionally, limitations to venous evaluation are present, such as vein collapse from full bladder (required in TV OB/GYN protocol) and supine positioning. Although TVUS may depict the vessels more accurately, as mentioned few protocols include an assessment for the presence of varices and US technologists are directed by image acquisition protocols that include evaluation of uterus and ovaries only when ordered by primary care practitioners.
Sonographic imaging protocols in PeVD are designed not only to identify pelvic varices but to evaluate for the pathophysiology described above. Labropoulos et al recommended a standard PeVD TA sonographic evaluation, as this is the best way to insonate all possible affected veins associated with PeVD. It begins in the supine position with head of bed at 30 degrees. The large abdominopelvic veins (inferior vena cava, left renal, left ovarian, left iliac) are examined with a curvilinear probe (1–5 MHz) in both transverse and longitudinal evaluation. A linear probe can be used at times, as most of these women are quite thin. When examining the central left iliac vein, it is important to scrutinize its caliber at the point of the right common iliac artery crossover to evaluate for nonthrombotic iliac vein compression. The use of Doppler may help find velocity acceleration at the point of narrowing. If the vein looks narrow, sit the patient up and reexamine the area, as the lumen may substantially improve the more upright you make the patient, usually demonstrating the lesion to be likely unimportant. Additionally, when evaluating the left ovarian vein, it is important to evaluate the left renal vein to assess for “nutcracker syndrome” by sweeping along the renal vein in short axis; the use of Doppler may help here as well. Protocols suggesting a significant iliac and renal vein stenosis are utilized to suggest a significant stenosis when the vein is narrowed, but at this point they are not based on robust data. These often include a diameter and velocity ratio for significance (1/5 the diameter and 5 × the velocity for the renal vein and >½ the diameter and 2.5 × the velocity of the iliac vein compared with a normal segment). 18 Attention is then turned to the periuterine and periovarian venous plexuses as well as the ovarian veins. Valsalva can be used in the supine position to help evaluate for reflux in the pelvic varicosities. The ovarian veins can be identified by initiating scanning over the psoas muscle where they cross anterior to the external iliac artery and vein. The ovarian vein can be followed centrally from this point over the psoas muscle where the vein diameter and presence of reflux can be examined. If the visualization of this phenomenon is difficult, it is recommended to have the patient stand, as gravitational force exposes and/or exacerbates these findings. Finally, if not already standing, some investigators recommend the patient does so to investigate for venous reflux into pelvic or upper thigh varicosities (vulvar, clitoral, gluteal, upper thigh).
Because of its ease of use and ability to perform dynamic/physiologic evaluations, in office US has become a mainstay of evaluation for PeVD prior to venography and/or embolization. There is significant added value of having a registered vascular technologist to perform these studies. Though no prospective analysis has been undergone to develop succinct imaging criteria, there have been many retrospective studies which compare US imaging findings to venographic observations and those with confirmed diagnoses and/or positive embolization outcomes. A literature review performed by Steenbeek and colleagues recorded features such as ovarian follicle number, diameter, as well as uterine volumes correlating to those with PeVD. 19 Additionally, the review portrayed results from several experiences detailing specific vascular positive predictors. In TVUS, the presence of dilated and tortuous parauterine veins (pelvic varicosities) yielded a sensitivity of 100%, and parametrial veins (those visualized crossing uterine body) with a diameter greater than 5 mm had a specificity of 91%. 20 21 In TAUS, increasing positive predictive value was noted to positively correlate with ovarian vein caliber (71.2% at >5 mm and 83.3% at >6 mm). Reversal of flow (caudal) in the ovarian vein was found to be a favorable screening characteristic with a negative predictive value of 100%. Specificity of this feature was 75%. 20
Filling a complementary role, duplex sonography of the lower extremities is also beneficial in evaluating for varicosities as a result of increased degree of reflux through the pelvic escape points. According to a study by Balian et al, in patients with pelvic veins measuring greater than 5 mm, approximately 70% of patients had lower limb varicosities, particularly in the proximal thigh. 22
Computed Tomography
CT scan is frequently used to evaluate abdominopelvic viscera for pathologies. CT scans provide high spatial resolution to evaluate organs and visceral parenchyma and can be further augmented using intravenous contrast for better visceral and vessel evaluation. Unfortunately, the supine positioning required in CT scanning prevents the recreation of flow dynamics which are normally seen in venous reflux (against gravity resulting in reflux in the setting of incompetence). In the setting of PeVD, it is easy to identify varices of periuterine and periovarian venous plexuses. Additionally, classification of vessel reflux was initially characterized with CT (Grade I: reflux in left renal vein, Grade II: reflux into ipsilateral paramedian veins, Grade III: reflux into contralateral parametrial/right ovarian vein) using early contrast filling from renal as a starting point. 23 Unfortunately, CT uses ionizing radiation which is even more of a concern in women with CPP who are typically of childbearing ages.
In a small cohort of 200 patients, Awad et al found that women with dilated left ovarian veins or parametrial veins had worsening dyspareunia and menstrual symptoms, thought to be more frequent with PeVD than women with other defined causes of CPP. 24 On the contrary, studies have also shown high incidence of incidental asymptomatic presence of venous congestion. Rozenblit et al recently evaluated asymptomatic women of reproductive age undergoing evaluation for renal donation. 25 Of those patients, nearly half were found to have dilated pelvic and/or ovarian veins ranging from 7 to 12 mm.
Additional benefits of the use of CT in diagnosing PeVD include their ability to identity obstruction points. Kuo et al compared left iliac vein cross-sectional area ratios (area of narrowing:area of most peripheral segment) to the degree of pelvic reflux and reflux start time on digital subtraction venography with a significant correlation indicating its potential utilities. 26
Magnetic Resonance Imaging/Magnetic Resonance Venography
MRI and magnetic resonance venography (MRV) have been utilized as a nonionizing radiation alternative providing reliable anatomic and reflux analysis. Given the often high cost and minimal data demonstrating its value, MR exams can be challenging to obtain insurance authorization. Also, modalities such as US and CT are more easily accessible in the setting of pelvic pain. With regard to its utility, MRI is a favorable alternative in those where US cannot successfully image or evaluate the pelvic venous structures (such as in patients with large body habitus). Just as in US or CT, the main parameters of evaluation for PeVD would be pelvic vessel caliber, the presence of reflux, obstruction points, or distant varices.
Using three-dimensional flow-dependent imaging (TRICKS-MRV), and normal T2 sequence parameters, Dick et al demonstrated that MRI/MRV correlated well with venography and US findings with respect to caliber and reflux grading. This study focused on a small patient cohort ( n = 13), but the findings were promising. 27 These findings were also reproduced by Yang et al using time-resolved magnetic resonance angiography (MRA) showing a positive correlation in reflux grading with that of conventional angiography. 28 Additional studies were able to match decreased or reversal of ovarian vein flow velocities (with objective velocity evaluation) on time-resolved MRA. 29 The related parameters of congestive appearance including diameter were measured by Asciutto and colleagues. This investigation yielded high sensitivities for ovarian (88%), hypogastric (100%), or pelvic plexus (91%) insufficiency when compared with that of venography. 30
Catheter Venography
The use of conventional venography to evaluate PeVD has been employed since at least 1953, when Topolanski-Sierra evaluated the pelvic veins using X-ray via intrauterine and intravenous injection. 31 As venography has become more advanced and as interventionalists have become more facile at its performance, it has become the standard of care in diagnosing PeVD. 7 As it is performed today, diagnostic catheters are navigated to the high-frequency obstruction points and iodinated contrast is directly injected to image across potential areas of compression, and within pelvic veins to scale the magnitude of distension, reflux, and pooling. Additionally, measuring pressure gradients or intravascular US across obstructions offers additional data on the severity of obstructions. The normal pressure gradient across and iliac or renal vein is less than 1 mm Hg. 5 Some have used a pressure gradient of greater than 3 mm Hg to be considered significant. 5 32 There are data to support this parameter for renal vein compression to define a significant stenosis when there is no significant reflux in the ovarian vein. However, with ovarian vein reflux, a pressure gradient may not appear, as the renal venous bed is decompressed through the refluxing gonadal vein. As one of the primary methods of vascular analysis in pelvic venous disease, venography also affords proceduralists the ability to intervene as well if significant pathology is encountered during the study. Based on scant literature, the findings associated with inclination to treat (not validated) were pelvic contrast retention of more than 20 seconds, ovarian vein diameter of 6 mm or greater, reflux into the iliac vein, or appearance of contrast through pelvic escape points into the perineal or lower extremity varicosities. 9
Conclusion
CPP is a challenge to all involved providers attempting to manage these patients. Based on a growing literature which consistently documents clinical improvements in QoL after embolization and stenting, it is important to consider PeVD as a contributor to this problem. Without robust investigations providing clear-cut criteria for diagnosis and therapeutic trials with comparison groups, the morbidity of PeVD will continue to be ignored. Based on the affordability and accessibility of sonography in most outpatient offices, particularly interventional radiology offices, those who intend on treatment of this entity should consider utilizing the SVP clinical evaluation tool and US evaluation to guide further diagnostic and treatment choices. To ultimately gain acceptance, the treatment of venous etiologies of CPP will require that we improve the quality of the literature in a prospective manner using a clinical approach with evidence-based diagnostic criteria, and a randomized controlled trial to establish the role of embolization and/or stenting. 10 33
Footnotes
Conflict of Interest None declared.
References
- 1.Stones R W, Price C. Health services for women with chronic pelvic pain. J R Soc Med. 2002;95(11):531–535. doi: 10.1258/jrsm.95.11.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latthe P, Mignini L, Gray R, Hills R, Khan K.Factors predisposing women to chronic pelvic pain: systematic review BMJ 2006332(7544):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards R D, Robertson I R, MacLean A B, Hemingway A P. Case report: pelvic pain syndrome--successful treatment of a case by ovarian vein embolization. Clin Radiol. 1993;47(06):429–431. doi: 10.1016/s0009-9260(05)81067-0. [DOI] [PubMed] [Google Scholar]
- 4.Cordts P R, Eclavea A, Buckley P J, DeMaioribus C A, Cockerill M L, Yeager T D. Pelvic congestion syndrome: early clinical results after transcatheter ovarian vein embolization. J Vasc Surg. 1998;28(05):862–868. doi: 10.1016/s0741-5214(98)70062-x. [DOI] [PubMed] [Google Scholar]
- 5.Beinart C, Sniderman K W, Tamura S, Vaughan E D, Jr, Sos T A. Left renal vein to inferior vena cava pressure relationship in humans. J Urol. 1982;127(06):1070–1071. doi: 10.1016/s0022-5347(17)54230-5. [DOI] [PubMed] [Google Scholar]
- 6.Sulakvelidze L, Tran M, Kennedy R, Lakhanpal S, Pappas P J. Presentations in women with pelvic venous disorders differ based on age of presentation. Phlebology. 2020;36(02):135–144. doi: 10.1177/0268355520954688. [DOI] [PubMed] [Google Scholar]
- 7.American Venous Forum ; European Venous Forum ; International Union of Phlebology ; American College of Phlebology ; International Union of Angiology . Eklof B, Perrin M, Delis K T, Rutherford R B, Gloviczki P. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49(02):498–501. doi: 10.1016/j.jvs.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Lurie F, Passman M, Meisner M. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(03):342–352. doi: 10.1016/j.jvsv.2019.12.075. [DOI] [PubMed] [Google Scholar]
- 9.Black C M, Thorpe K, Venrbux A. Research reporting standards for endovascular treatment of pelvic venous insufficiency. J Vasc Interv Radiol. 2010;21(06):796–803. doi: 10.1016/j.jvir.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Khilnani N M, Meissner M H, Learman L A. Research priorities in pelvic venous disorders in women: recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2019;30(06):781–789. doi: 10.1016/j.jvir.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Knuttinen M G, Zurcher K S, Khurana N. Imaging findings of pelvic venous insufficiency in patients with postural orthostatic tachycardia syndrome. Phlebology. 2021;36(01):32–37. doi: 10.1177/0268355520947610. [DOI] [PubMed] [Google Scholar]
- 12.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14(03):601–612. [PubMed] [Google Scholar]
- 13.Santoshi R KN, Lakhanpal S, Satwah V, Lakhanpal G, Malone M, Pappas P J. Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2018;6(02):202–211. doi: 10.1016/j.jvsv.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Antignani P L, Lazarashvili Z, Monedero J L. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int Angiol. 2019;38(04):265–283. doi: 10.23736/S0392-9590.19.04237-8. [DOI] [PubMed] [Google Scholar]
- 15.Cura M, Cura A. What is the significance of ovarian vein reflux detected by computed tomography in patients with pelvic pain? Clin Imaging. 2009;33(04):306–310. doi: 10.1016/j.clinimag.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Beard R W, Reginald P W, Wadsworth J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol. 1988;95(02):153–161. doi: 10.1111/j.1471-0528.1988.tb06845.x. [DOI] [PubMed] [Google Scholar]
- 17.Meissner M H, Khilnani N M, Labropoulos N, Gasparis A P, Gibson K, Greiner M, Learman L A, Atashroo D, Lurie F, Passman M A, Basile A, Lazarshvilli Z, Lohr J, Kim M D, Nicolini P H, Pabon-Ramos W M, Greenblatt M.The Symptoms-Varices-Pathophysiology classification of pelvic venous disorders: A report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders J Vasc Surg Venous Lymphat Disorders 2021903568–584.. Doi: 10.1016/j.jvsv.2020.12.084. Epub 2021 Jan 30. PMID 33539720 [DOI] [PubMed] [Google Scholar]
- 18.Labropoulos N, Jasinski P T, Adrahtas D, Gasparis A P, Meissner M H. A standardized ultrasound approach to pelvic congestion syndrome. Phlebology. 2017;32(09):608–619. doi: 10.1177/0268355516677135. [DOI] [PubMed] [Google Scholar]
- 19.Steenbeek M P, van der Vleuten C JM, Schultze Kool L J, Nieboer T E. Noninvasive diagnostic tools for pelvic congestion syndrome: a systematic review. Acta Obstet Gynecol Scand. 2018;97(07):776–786. doi: 10.1111/aogs.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S J, Lim J W, Ko Y T, Lee D H, Yoon Y, Oh J H. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182:683–688. doi: 10.2214/ajr.182.3.1820683. [DOI] [PubMed] [Google Scholar]
- 21.Giacchetto C, Cotroneo G B, Marincolo F, Cammisuli F, Caruso G, Catizone F. Ovarian varicocele: ultrasonic and phlebographic evaluation. J Clin Ultrasound. 1990;18(07):551–555. doi: 10.1002/jcu.1870180705. [DOI] [PubMed] [Google Scholar]
- 22.Balian E, Lasry J L, Coppé G. Pelviperineal venous insufficiency and varicose veins of the lower limbs. Phlebolymphology. 2008;5:17–26. doi: 10.1016/j.jmv.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Hiromura T, Nishioka T, Nishioka S, Ikeda H, Tomita K. Reflux in the left ovarian vein: analysis of MDCT findings in asymptomatic women. AJR Am J Roentgenol. 2004;183(05):1411–1415. doi: 10.2214/ajr.183.5.1831411. [DOI] [PubMed] [Google Scholar]
- 24.Awad A S, Taha M MM, Manaf M HA. Role of multi-detector CT venography in evaluation of pelvic congestion syndrome. Egypt J Radiol Nucl Med. 2020;51:159. [Google Scholar]
- 25.Rozenblit A M, Ricci Z J, Tuvia J, Amis E S., Jr Incompetent and dilated ovarian veins: a common CT finding in asymptomatic parous women. AJR Am J Roentgenol. 2001;176(01):119–122. doi: 10.2214/ajr.176.1.1760119. [DOI] [PubMed] [Google Scholar]
- 26.Kuo Y S, Chen C J, Chen J J. May-Thurner syndrome: correlation between digital subtraction and computed tomography venography. J Formos Med Assoc. 2015;114(04):363–368. doi: 10.1016/j.jfma.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Dick E A, Burnett C, Anstee A, Hamady M, Black D, Gedroyc W M. Time-resolved imaging of contrast kinetics three-dimensional (3D) magnetic resonance venography in patients with pelvic congestion syndrome. Br J Radiol. 2010;83(994):882–887. doi: 10.1259/bjr/82417499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang D M, Kim H C, Nam D H, Jahng G H, Huh C Y, Lim J W.Time-resolved MR angiography for detecting and grading ovarian venous reflux: comparison with conventional venography Br J Radiol 201285(1014):e117–e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meneses L Q, Uribe S, Tejos C, Andía M E, Fava M, Irarrazaval P. Using magnetic resonance phase-contrast velocity mapping for diagnosing pelvic congestion syndrome. Phlebology. 2011;26(04):157–161. doi: 10.1258/phleb.2010.010049. [DOI] [PubMed] [Google Scholar]
- 30.Asciutto G, Mumme A, Marpe B, Köster O, Asciutto K C, Geier B. MR venography in the detection of pelvic venous congestion. Eur J Vasc Endovasc Surg. 2008;36(04):491–496. doi: 10.1016/j.ejvs.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Topolanski-Sierra R. Pelvic phlebography. Am J Obstet Gynecol. 1958;76(01):44–52. doi: 10.1016/s0002-9378(16)36864-8. [DOI] [PubMed] [Google Scholar]
- 32.Nishimura Y, Fushiki M, Yoshida M. Left renal vein hypertension in patients with left renal bleeding of unknown origin. Radiology. 1986;160(03):663–667. doi: 10.1148/radiology.160.3.3737903. [DOI] [PubMed] [Google Scholar]
- 33.Daniels J P, Champaneria R, Shah L, Gupta J K, Birch J, Moss J G. Effectiveness of embolization or sclerotherapy for pelvic veins for reducing chronic pelvic pain. Syst Rev. 2016;27(10):1478–1486. doi: 10.1016/j.jvir.2016.04.016. [DOI] [PubMed] [Google Scholar]


