Abstract
Venous leg ulcers (VLUs) affect as many as 20% of patients with advanced chronic venous insufficiency and are associated with significant morbidity and health care costs. VLUs are the most common cause of leg ulcers; however, other etiologies of lower extremity ulcerations should be investigated, most notably arterial insufficiency, to ensure appropriate therapy. Careful clinical examination, standardized documentation, and ultrasound evaluation are needed for diagnosis and treatment success. Reduction of edema and venous hypertension through compression therapy, local wound care, and treatment of venous reflux or obstruction is the foundation of therapy. As key providers in venous disease, interventional radiologists should be aware of current standardized disease classification and scoring systems as well as treatment and wound care guidelines for venous ulcers.
Keywords: venous leg ulcer, venous hypertension, wound care, interventional radiology
Chronic venous disease (CVD) is a common medical problem, affecting more than 25 million adults in the United States with a spectrum of presentation from reticular veins and asymptomatic varicose veins to edema, pain, hyperpigmentation, lipodermatosclerosis, and venous ulceration. 1 Venous ulcers are estimated to afflict 0.3% of the population, and as much as 20% in the subset that have advanced chronic venous insufficiency. 1 Prompt diagnosis and treatment is imperative, as ulceration confers a poor prognosis and can be a major source of disability. 2 Treatment involving diligent wound care and addressing the underlying venous hypertension are critical to healing and preventing recurrence. Although earlier series historically described average healing times on the order of 9 months with 20% of ulcers not being healed at 2 years, more recent series, using newer methodologies, report average healing time of 12 weeks. 2 3
This area is evolving with the advent of new devices and theories behind the etiology and persistence of venous ulcers. Predictive metrics involving wound area and perimeter and scoring systems to predict wound healing have aided physicians in predicting those patients who may be recalcitrant to therapy. 4 5 While there is heterogeneity of practice and scientific support for some commonly utilized devices and therapeutic techniques, herein we will describe the state of the science and practice with respect to the etiology and management of this vexing manifestation of chronic venous insufficiency.
Clinical Evaluation
The clinical evaluation of the patient with chronic venous insufficiency is a critical first-step in establishing a wound care plan. It is important to first confirm the etiology of the leg ulcer by taking a detailed medical history and risk factor assessment, performing a physical exam, and obtaining a sonographic evaluation. Other differential diagnoses of leg ulcers should be considered and identified prior to the initiation of therapy to ensure optimal treatment ( Table 1 ). Most commonly, leg ulcers are caused by venous disease alone (54%) related to venous reflux, outflow obstruction, or a combination of the two, though can be confounded with an additional disease process. 6 Arterial insufficiency is the second most common cause of leg ulcers (14.5%) and is often found to be combined with venous disease (17.6%). 7 8
Table 1. Differential diagnosis for lower extremity ulcerations 35 .
| Differential diagnosis for leg ulcers | |
|---|---|
| Vascular disease • Venous: postthrombotic syndrome, varicose veins, chronic venous reflux • Arterial: peripheral arterial occlusive disease, hypertension, arteriovenous fistulas, arterial thrombosis, embolism, dysplasia, thromboangiitis obliterans, aneurysm • Lymphatic: lymphedema • Microangiopathy: diabetes, livedoid vasculopathy • Vasculitis • Hypertensive arteriolopathy |
• Neuropathic • Peripheral neuropathy: diabetes, alcohol, medication, heredity • Central neuropathy: tabes dorsalis, myelodysplasia, syringomyelia, spina bifida, poliomyelitis, multiple sclerosis |
| Metabolic • Diabetes mellitus, gout, prolidase deficiency, Gaucher disease, amyloidosis, calciphylaxis, porphyria, hyperhomocysteinemia |
• Hematologic • Sickle cell anemia, thalassemia, polycythemia vera, leukemia, thrombocythemia, lymphoma, myeloplastic disorders, disorders of coagulation factors (factors I–XIII), coagulation inhibitors (antithrombin III, activated protein C resistance, protein C and S), or fibrinolysis factors (tissue plasminogen activator inhibitor, plasmin) |
| Autoimmune • Rheumatoid arthritis, leukocytoclastic vasculitis, polyarteritis nodosa, Wegener granulomatosis, Churg–Strauss syndrome, systemic lupus erythematosus, Sjogren syndrome, scleroderma, Behcet disease, cryoglobulinemia |
• Exogenous • Heat, cold pressure, ionizing radiation, chemical, allergens, trauma |
| Neoplasia • Basal cell carcinoma, squamous cell carcinoma (Marjolin ulcer), malignant melanoma, angiosarcoma, cutaneous lymphoma, papillomatosis cutis carcinoids, keratoacanthoma |
• Medication • Hydroxyurea, leflunomide, methotrexate, halogens, coumarin, vaccinations, ergotamine, infiltration cytostatic agents |
| Infection • Bacterial: furuncles, ecthyma, mycobacterioses, syphilis, erysipelas, anthrax, diphtheria, chronic vegetative pyodermia, tropical ulcer • Viral: herpes, variola virus, cytomegaly • Fungal: sporotrichosis, histoplasmosis, blastomycosis, coccidioidomycosis • Protozoal: leishmaniasis |
• Genetic defect • Klinefelter syndrome, Felty syndrome, TAP1 mutation, leukocyte adhesion deficiency, inherited hypercoagulable factors |
| Skin disorder • Pyoderma gangrenosum, necrobiosis lipoidica, sarcoidosis, perforating dermatosis, Langerhans cell histiocytosis, papulosis maligna atrophicans, bullous skin diseases |
Symptoms of CVD can include lower extremity pain, burning, aching, throbbing, cramps, heaviness, itching, tiredness, fatigue, and restless legs. These symptoms tend to be worse at the end of the day, exacerbated by limb dependence and relieved with limb rest and elevation. Common risk factors for CVD include advanced age, pregnancies, family history of venous disease, and elevated body mass index. A history of prior venous procedures, deep venous thrombosis (DVT), superficial thrombophlebitis, compression therapy use, or venotonic medications is also helpful in directing a venous diagnosis. Alternatively, common symptoms of arterial ulcerations include severe pain worse at night, relieved by limb dependency, with a past medical history of smoking, diabetes, hypertension, rest pain, or intermittent claudication.
The physical examination in a patient with leg ulcer includes lesion location and characteristics, evidence of bleeding and surrounding inflammation, and additional characteristics to suggest ulcer etiology. Venous leg ulcers (VLUs) are more common on the lower third of the leg, between the malleolus and lower calf with the majority at the medial malleolus ( Fig. 1 ). Venous ulcerations are often shallow with an irregular, shaggy shape and a granulating base, which may be flat or have step margins with moderate to heavy exudate, though exudate can vary greatly depending on the stage of healing. Other common indicators of CVD include telangiectasias, varicose veins, edema, chronic venous skin changes (such as skin discoloration, inflammation, eczema, hyperpigmentation, malleolar flair, corona phlebectatica, atrophie blanche, and lipodermatosclerosis), healed or active ulceration, intact pulses, normal capillary refill time, and ankle immobility. In comparison, arterial ulcerations are more common distally, on the dorsum of the foot or toes and over bony prominences and at pressure points. Arterial lesions often have irregular edges, poor granulation tissue, a dry necrotic base, and may be round or punched-out with sharp demarcation ( Fig. 2 ). There is little or no bleeding seen with manipulation of arterial ulcers and often no surrounding inflammation. Associated findings of arterial insufficiency may include hair loss, atrophic skin, cool feet, absence of pulses, prolonged capillary refill time, ankle-brachial index (ABI) less than 0.5, dependent rubor, and elevation pallor.
Fig. 1.
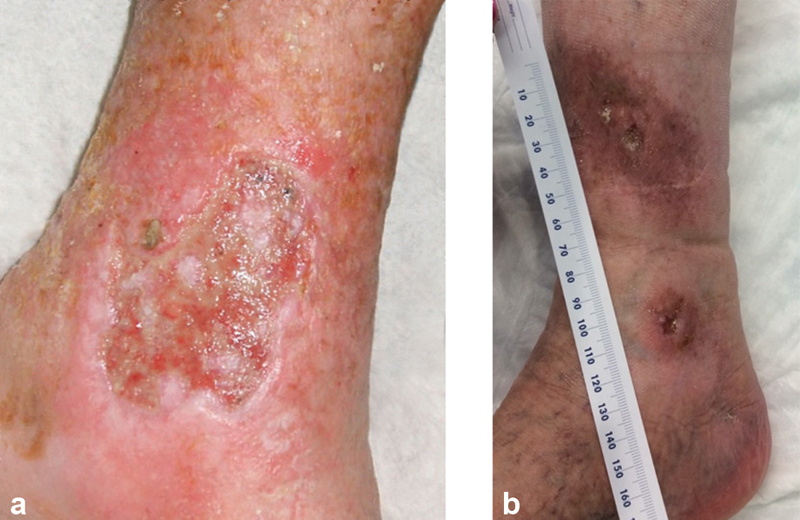
Venous ulcerations in typical locations overlying the medial malleolus with a flat wound bed, which can contain heavy exudate ( a ) or in later stages be more dry ( b ).
Fig. 2.
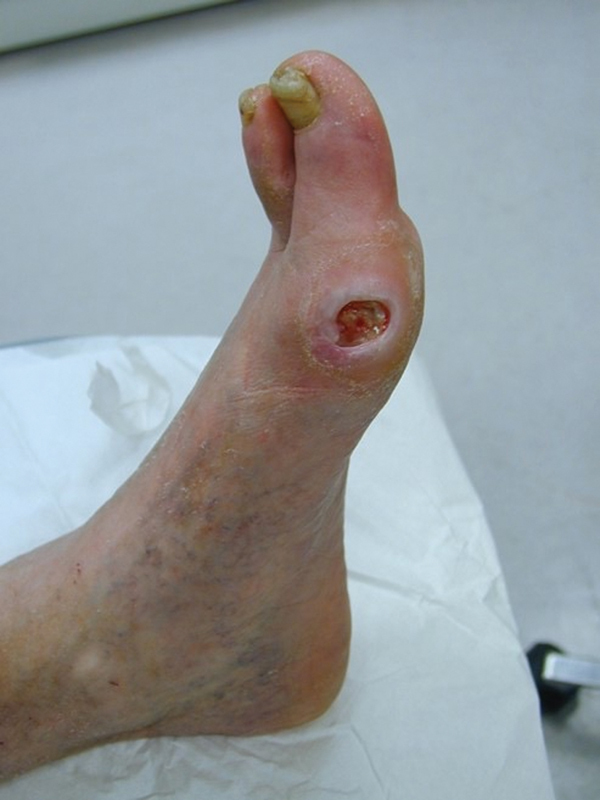
Typical arterial ulceration overlying a bony prominence with minimal exudate. Note that there can be overlap in appearance and so patients with ulcers should be evaluated for both venous and arterial pathology.
Sonographic evaluation of the ulcerated leg is imperative for objective documentation of the distribution and extent of vascular disease. Ultrasound is superb for identifying superficial and deep venous reflux, incompetent perforator veins, deep vein thrombosis, and venous outflow obstruction, which can lead to venous hypertension in the lower extremity. Ultrasound is also very effective in detecting arterial stenosis/obstruction and plaque. Close attention should be made on ultrasound evaluation for perforator veins beneath or leading to the healed or active VLU. A pathologic perforator vein has directional flow from deep to superficial, valve closure time ≥0.5 seconds, and vein diameter exceeding 3.5 mm. 9 10 Of note, ABI testing should be performed in all suspected cases of coexisting peripheral arterial disease (PAD) as the severity of PAD alters the recommended compression guidelines so as not to further compromise the arterial supply.
Clinical documentation using standardized venous disease classification is important for initial assessment and evaluation of treatment outcomes. The c linical class, e tiology, a natomy, and p athophysiology (CEAP) classification was most recently revised in 2020 and is widely used in documenting and staging CVD, though it is not particularly sensitive to response to treatment. The Venous Clinical Severity Score (VCSS), most recently revised in 2010, includes several descriptors, each rated 0 to 3. A total VCSS score of ≥8 indicates a patient with severe disease at risk for progression. 11 The VCSS system is more sensitive to assessing response to treatment. The Villalta score, which includes venous ulceration in its criteria, should be used to monitor and assess change in condition over time in all patients with prior DVT or postthrombotic syndrome.
Finally, thrombocytopenia laboratories are suggested on all patients with a history of recurrent venous thrombosis and chronic recurrent VLUs, as studies have shown patients with VLUs have an increased prevalence of thrombophilia (41%). 12
Treatment Strategies
Compression Therapy
Compression therapy is the mainstay for both treatment of existing ulcers and prevention of recurrence by reducing both edema and venous insufficiency. It has been shown that multilayer compression is more effective than single-layer compression; however, not all patients are candidates for compression. 13 Patients with evidence of severe PAD (ABI < 0.5) should not undergo compression therapy so as not to impede already reduced arterial supply to the lower extremity. Patients with mild or moderate PAD (ABI 0.5–0.79) should undergo single-layer compression therapy monitored by a trained professional for similar reasons. 14 Of note, successful arterial revascularization prior to ulcer treatment may improve the ABI enough to allow for adequate compression in mixed venous ulcers.
Compression can be elastic or inelastic and studies show conflicting superiority in ulcer healing times. 13 15 Inelastic systems, however, better reduce venous insufficiency and support the calf muscle pump by intermittently increasing peak pressures during walking. 16 The Unna boot is a three-layer inelastic system, consisting of an impregnated gauze layer, a wool padding layer, and a self-adherent compression layer (Coban, 3M, St. Paul, MN), which is intended to provide compression during muscle contraction (i.e., walking) but not at rest. The Unna boot is an affordable compression system that has been shown to allow venous ulcer wound healing in more than 70% of all patients, with 91% healing in first-time ulcers. 17 CircAid (San Diego, CA) is an inelastic compression garment providing graduated and adjustable compression that can be applied and adjusted by the patient. A traditional four-layer nonelastic wrap consists of a wool layer for padding, a crepe layer for support, a compression layer (light or high compression), and a self-adherent light compression layer. The compression layer strength is often based on ankle size, with larger ankles requiring higher compression.
Local Wound Care
To accomplish wound healing, the main tenets of venous ulcer wound care center around reduction of edema and venous hypertension. Chronic wounds are frequently managed using the TIME framework: tissue, infection/inflammation, moisture balance, and edge of wound. The tissue component addresses wound bed preparation with debridement. Infection and inflammation are managed with antimicrobial therapy or anti-inflammatory therapies. Moisture balance is achieved with adequate dressing care for exudate reduction and maintaining an optimal moist healing environment. The edge of wound must be assessed to prevent skin maceration and to encourage advancing wound margin and healing, often through debridement. 18
At each dressing change, wounds should be inspected for healing and signs of infection. Serial wound measurements and documentation should be performed to establish a baseline and monitor treatment effect. This should include number and location of ulcers on the leg as well as area, perimeter and depth, wound base quality, drainage, and infection. Traditional signs of wound infection including warmth, erythema, pain, and swelling are similar to noninfectious inflammatory response and therefore additional criteria may be necessary to suspect infection. Delayed healing, discoloration, odor, wound breakdown, and friable granulation tissue can all be signs of infection. Routine wound culture is not recommended as colonization is very common and yet has not been associated with delayed wound healing. There is no evidence to support systemic antibiotics in the routine care of venous ulceration. 19 However, if there is concern for wound infection, culture can be obtained from the wound surface or drainage with swabs. Deep wound tissue cultures can be obtained for multiorganism infections when the surface swab is unrevealing or for recurrent/persistent infection despite antimicrobial therapy. 21 22 23 24 25 Biofilms can form and are a bacterial layer that demonstrates increased tolerance to both topical and systemic antimicrobial therapies. 20 Biofilms respond best to mechanical removal via sharp debridement or mechanical debridement.
If there is no sign of improvement after 4 weeks of dedicated wound care, the ulceration should undergo biopsy as malignancy is in the differential for a nonhealing wound. Specialized specimen evaluation by a dermatopathologist may be necessary. If there is no evidence of malignancy, patients with nonhealing wound may require hospitalization to assist with a concentrated period of wound care, compression therapy, and possible evaluation of skin grafting once edema and venous hypertension are controlled. 15 Skin grafting should be considered early in treatment if a dressing is larger than 25 cm 2 . 21 Patient compliance may prevent wound healing and depression has been shown to be a risk factor in the nonhealing wound, requiring that a comprehensive assessment of the patient be made during routine examination.
Wound Bed Preparation
Wound bed preparation is essential to wound healing and is accomplished via cleansing, debridement, infection control, and appropriate dressing choice. Wounds should be regularly cleaned with water and dried. Small trials suggest a benefit in cleansing with hydrogen peroxide; however, there are various options for wound cleansers, including Dakin's solution, chlorhexidine gluconate, hypochlorous acid, and polyhexamethylene biguanide. Debridement is intended to assist the wound in overcoming the inflammatory stage to promote productive healing. Removal of necrotic material and sloughing tissue can be either office-based or performed in an operating room depending on the degree of debridement needed and patient tolerance. EMLA cream has been shown to improve pain control during wound debridement. 22 23 Debridement is most commonly performed using sharp techniques with a curette or scissors but can also be performed via hydrosurgery (VersaJet; Smith & Nephew, Largo, FL). Hydrosurgery allows rapid debridement times by both cutting and aspirating tissue simultaneously; however, it has not been shown to improve wound healing time compared with sharp debridement. 24 Other means of wound debridement are enzymatic, mechanical, biologic, or autolytic. Enzymatic debridement is achieved with the use of collagenase, which can be applied as frequently as daily. Mechanical debridement consists of dry gauze dressings, wet-to-dry gauze dressings, or monofilament fiber pads. 20 In patients with associated severe arterial insufficiency, mechanical debridement is not recommended. 20 Larval therapy as biologic debridement has been shown to provide faster debridement times but did not provide improved ulcer healing times or reduced infection. 25 The skin surrounding an ulcer should be closely monitored and barrier ointment is recommended to avoid further skin breakdown prior to dressing application. 26
Dressings
In addition to compression therapy, dressings allow control of wound exudate to promote healing and can improve patient tolerance of compression therapy. Depending on the wound characteristics, different dressings may be needed. If part of a multilayer compression system, no single dressing has been shown to be superior to another for venous ulcers. 27 28 29 30 31 For example, if a wound has significant exudate, a highly absorbent dressing would be preferred, whereas a wound with low exudate but possible superimposed infection may benefit more from an antimicrobial soft polymer dressing ( Table 2 ). A moist environment is optimal for epithelialization and avoiding too dry or wet of a wound bed is necessary. Exudate control and wound moisture will dictate frequency of dressing changes (ranging from daily changes to one or twice weekly). Dressings should cover the entire wound with a 1- to 2-inch border and are applied under a usual three- or four-layer wrap system. A patient's allergies and skin sensitivities should always be taken into consideration when deciding on dressing choices.
Table 2. Multiple dressings are available for the treatment of venous ulcers, some with antimicrobial properties, to provide exudative control, patient comfort, and optimal healing environment.
| Dressing type | Exudate level | Notes |
| Alginates | High | • Derived from seaweed to form a soft gel when exposed to exudate; can be hemostatic after biopsy or debridement • Promotes autolytic debridement • Preferred for deep cavities or sinus tracts |
| Foam | Moderate | • Provide cushioning with polyurethane foam • Preferred for flat wounds |
| Hydrocolloid sheet | Variable a | • Allows a moist environment and promotes autolytic debridement • Preferred for flat wounds |
| Hydrocolloid fibrous | High | • Useful for both flat wounds and deep cavities or sinuses |
| Hydrogel sheet | Dry/Low | • High water content polymer that provides wound moisture and promotes autolytic debridement • High moisture can lead to skin maceration |
| Low adherence | Dry/Low | • Protective layer impregnated with paraffin to reduce damage during dressing changes • Preferred for flat wounds |
| Soft polymer | Low/Moderate | • Nonadherent silicone polymer • Preferred for fragile or macerated skin |
| Protease modulating matrix | Variable a | • Collagen based to remove protease from the wound bed to promote tissue granulation and epithelialization |
| Semipermeable | Dry/Low | • Permeable to oxygen and water vapor but barrier for liquid and bacteria • Preferred for flat wounds |
| Antimicrobial options | Notes | Evidence |
| Iodine | • Free iodine is antiseptic and released when exposed to wound exudate • Contraindicated in pregnancy, breast feeding, concomitant lithium use, or in patients with thyroid disorders |
Cadexomer-iodine has also been shown to absorb exudate, promote debridement, and has improved healing outcomes compared with standard dressings 19 |
| Silver | • Many dressing types impregnated with silver ions, which are antimicrobial | Has not been shown to improve wound healing over standard dressings and may not be cost-effective 26 36 |
| Manuka honey | • Contains natural antimicrobial and anti-inflammatory properties and its osmotic behavior allows autolytic debridement • Wound deodorizer |
Has not been shown to improve wound healing over standard dressings 37 38 |
| Chlorhexidine | • Low adherence and must be combined with absorbent dressing for higher exudate wounds | Single RCT did not show improved wound healing compared with usual care and no RCT exists for infected ulcers 39 |
| DACC | • DACC binds bacteria with high cell surface hydrophobicity (i.e., Staphylococcus aureus , Pseudomonas aeruginosa , Enterococcus faecalis, Candida albicans ), allowing bacterial removal with each dressing change 40 | Biofilms have been shown to be removed with DACC-coated fibers 41 |
Abbreviations: DACC, dialkylcarbamoylchloride; RCT, randomized control trial.
Absorbency is dressing-specific.
Procedural Treatment
Operative and endovascular management of VLUs is classified anatomically by superficial venous disease (great saphenous vein, small saphenous vein), perforator disease, deep infrainguinal venous disease, and iliocaval disease ( Table 3 ). Closure of the incompetent superficial veins can be done in a variety of ways including endovenous thermal ablation (laser or radiofrequency ablation); cyanoacrylate embolization; mechanochemical ablation; and foam sclerotherapy depending on the anatomical characteristics of the vessels (diameter, depth, tortuosity, length), location, and the presence of deep vein communication. Closure of pathologic perforator veins can be accomplished with percutaneous thermal ablation or foam sclerotherapy and more recently described percutaneous glue closure. The approach to treating incompetent infrainguinal deep veins is generally surgical to restore valve competency, though it is usually considered only after exhausting other treatment options. Finally, proximal chronic venous stenosis or occlusion is treated with venous angioplasty and stent reconstruction to relieve venous hypertension. If endovascular reconstruction fails, surgical bypass and possible arteriovenous fistula creation to increase flow can be considered. Compressive therapy is an important treatment adjunct to improve ulcer healing and prevent ulcer recurrence no matter the procedural undertaking.
Table 3. Procedural therapy recommendations for venous insufficiency associated with ulceration 42 .
| Anatomic disease classification | Management guidelines |
|---|---|
| Superficial venous reflux and active or healed venous leg ulcer | • Closure of the axial incompetent veins directed to the ulcer |
| Combined superficial and perforator venous reflux with or without deep venous reflux and active venous leg ulcer | • Closure of both the incompetent superficial veins directed to the ulcer and pathologic perforator vein if it is beneath or associated with the ulcer bed |
| Combined superficial and perforator venous reflux with or without deep venous reflux and healed venous leg ulcer or at risk for venous leg ulcer | • Staged treatment with reevaluation of the perforator after correction of axial reflux |
| Pathologic perforator in the absence of superficial venous disease, with or without deep venous reflux and a healed or active ulcer | • Closure by ablation or sclerotherapy of the perforator or open venous perforator surgery |
| Infrainguinal deep venous obstruction and skin changes at risk for venous leg ulcer or healed or active venous leg ulcer | • Autogenous venous bypass or endophlebectomy |
| Infrainguinal deep venous reflux with skin changes at risk for venous leg ulcer, or healed or active venous leg ulcer | • Deep vein ligation of the femoral or popliteal veins (if collateral pathways exist), primary valve repair (external banding or valvuloplasty), valve transposition/transplantation, or autogenous valve substitute |
| Proximal chronic total venous occlusion/severe stenosis (inferior vena cava or iliac veins) with or without deep venous reflux with skin changes at risk for venous leg ulcer, healed or active venous leg ulcer | • Endovascular repair with venous angioplasty and stent recanalization • If failure of endovascular reconstruction, open surgical bypass |
Medications and Adjunctive Therapies
Few systemic medications have been shown in randomized controlled trials to improve venous ulcer healing; however, pentoxifylline, a hemorheologic agent that reduces viscosity, has been shown both alone and in conjunction with compression to improve healing. 32 Up to 6 months of 400 mg three times daily can help improve microcirculation and healing. Micronized purified flavonoid fraction has minimal adverse effects and has been shown to reduce venous insufficiency leg symptoms, including pain, heaviness, swelling and pruritus, and reduced objective measurements of ankle circumference and leg erythema. 33 Iron is deposited in the tissues of venous ulcers and has been shown to perpetuate the inflammatory cycle of the chronic wound. It has been suggested that iron chelators (i.e., deferoxamine) may help reduce iron's inflammatory effects to allow wound healing to commence; however, further investigation is needed. Tumor necrosis factor-α (TNF-α) plays a key role in the inflammatory cycle of chronic wounds and etanercept, a TNF-α blocker, may help reduce this proinflammatory component in patients with chronic wounds. 34 There is currently insufficient evidence to support hyperbaric oxygen therapy and intermittent pneumatic compression.
Conclusion
Ulceration related to significant CVD represents an advanced manifestation of this common condition. Focus on correct classification of the ulcer etiology, identification of coexisting conditions, and discerning the underlying venous issue are key to guiding treatment. While compression therapy and appropriate local wound care remain the mainstays of treatment, now more than ever, there are tools at the interventional radiologist's disposal to treat this condition. Interventional radiologists who treat venous disease should be prepared to treat these lesions and keep abreast of the evidence behind this evolving frontier.
Take-Home Points
Venous disease is the most common cause of lower extremity ulcers.
All patients with ulcers should have a workup for venous and arterial insufficiency.
Compression therapy is the mainstay of therapy for venous ulcers but should not be used in patients with significant arterial insufficiency.
Routine wound culture is not recommended due to significant contamination with skin flora.
Nonhealing ulcers should be biopsied to rule out malignancy.
Biofilm and exudate can be removed with debridement. There is presently no evidence that shows hydrosurgical, enzymatic, or biologic debridement techniques are superior to sharp surgical debridement.
Treatment of incompetent perforators and venous reflux can improve the underlying cause for venous ulceration.
Acknowledgments
The authors thank Stephen Leschak, MD, for providing Figures 1 and 2 .
Footnotes
Conflict of Interest None declared.
References
- 1.Eberhardt R T, Raffetto J D. Chronic venous insufficiency. Circulation. 2014;130(04):333–346. doi: 10.1161/CIRCULATIONAHA.113.006898. [DOI] [PubMed] [Google Scholar]
- 2.Callam M J, Harper D R, Dale J J, Ruckley C V.Chronic ulcer of the leg: clinical history Br Med J (Clin Res Ed) 1987294(6584):1389–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gethin G, Cowman S, Kolbach D N. Debridement for venous leg ulcers. Cochrane Database Syst Rev. 2015;(09):CD008599. doi: 10.1002/14651858.CD008599.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fife C E, Horn S D. The wound healing index for predicting venous leg ulcer outcome. Adv Wound Care (New Rochelle) 2020;9(02):68–77. doi: 10.1089/wound.2019.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill D P, Poore S, Wilson J, Robson M C, Cherry G W.Initial healing rates of venous ulcers: Are they useful as predictors of healing? Am J Surg 2004188(1A, Suppl):22–25. [DOI] [PubMed] [Google Scholar]
- 6.Nelzén O, Bergqvist D, Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg. 1994;81(02):182–187. doi: 10.1002/bjs.1800810206. [DOI] [PubMed] [Google Scholar]
- 7.Adam D J, Naik J, Hartshorne T, Bello M, London N JM. The diagnosis and management of 689 chronic leg ulcers in a single-visit assessment clinic. Eur J Vasc Endovasc Surg. 2003;25(05):462–468. doi: 10.1053/ejvs.2002.1906. [DOI] [PubMed] [Google Scholar]
- 8.Körber A, Klode J, Al-Benna S. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J Dtsch Dermatol Ges. 2011;9(02):116–121. doi: 10.1111/j.1610-0387.2010.07535.x. [DOI] [PubMed] [Google Scholar]
- 9.Labropoulos N, Mansour M A, Kang S S, Gloviczki P, Baker W H. New insights into perforator vein incompetence. Eur J Vasc Endovasc Surg. 1999;18(03):228–234. doi: 10.1053/ejvs.1999.0812. [DOI] [PubMed] [Google Scholar]
- 10.Sandri J L, Barros F S, Pontes S, Jacques C, Salles-Cunha S X. Diameter-reflux relationship in perforating veins of patients with varicose veins. J Vasc Surg. 1999;30(05):867–874. doi: 10.1016/s0741-5214(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 11.Jayaraj A, Meissner M H. A comparison of Villalta-Prandoni scale and venous clinical severity score in the assessment of post thrombotic syndrome. Ann Vasc Surg. 2014;28(02):313–317. doi: 10.1016/j.avsg.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Mackenzie R K, Ludlam C A, Ruckley C V, Allan P L, Burns P, Bradbury A W. The prevalence of thrombophilia in patients with chronic venous leg ulceration. J Vasc Surg. 2002;35(04):718–722. doi: 10.1067/mva.2002.121749. [DOI] [PubMed] [Google Scholar]
- 13.Mauck K F, Asi N, Elraiyah T A.Comparative systematic review and meta-analysis of compression modalities for the promotion of venous ulcer healing and reducing ulcer recurrence J Vasc Surg 201460(2 Suppl):71S–90S.e1–2. [DOI] [PubMed] [Google Scholar]
- 14.Franks P J, Barker J, Collier M. Management of patients with venous leg ulcers: challenges and current best practice. J Wound Care. 2016;25 06:S1–S67. doi: 10.12968/jowc.2016.25.Sup6.S1. [DOI] [PubMed] [Google Scholar]
- 15.Patel N P, Labropoulos N, Pappas P J.Current management of venous ulceration Plast Reconstr Surg 2006117(7, Suppl):254S–260S. [DOI] [PubMed] [Google Scholar]
- 16.Partsch H, Menzinger G, Mostbeck A. Inelastic leg compression is more effective to reduce deep venous refluxes than elastic bandages. Dermatol Surg. 1999;25(09):695–700. doi: 10.1046/j.1524-4725.1999.98040.x. [DOI] [PubMed] [Google Scholar]
- 17.Lippmann H I, Fishman L M, Farrar R H, Bernstein R K, Zybert P A. Edema control in the management of disabling chronic venous insufficiency. Arch Phys Med Rehabil. 1994;75(04):436–441. doi: 10.1016/0003-9993(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 18.Leaper D J, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years?(*) Int Wound J. 2012;9 02:1–19. doi: 10.1111/j.1742-481X.2012.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Meara S, Al-Kurdi D, Ologun Y, Ovington L G, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev. 2014;(01):CD003557. doi: 10.1002/14651858.CD003557.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi T, Wolcott R D, Peghetti A. Recommendations for the management of biofilm: a consensus document. J Wound Care. 2016;25(06):305–317. doi: 10.12968/jowc.2016.25.6.305. [DOI] [PubMed] [Google Scholar]
- 21.Jankunas V, Bagdonas R, Samsanavicius D, Rimdeika R. An analysis of the effectiveness of skin grafting to treat chronic venous leg ulcers. Wounds. 2007;19(05):128–137. [PubMed] [Google Scholar]
- 22.Lok C, Paul C, Amblard P.EMLA cream as a topical anesthetic for the repeated mechanical debridement of venous leg ulcers: a double-blind, placebo-controlled study J Am Acad Dermatol 199940(2, Pt 1):208–213. [DOI] [PubMed] [Google Scholar]
- 23.Briggs M, Nelson E A, Martyn-St James M. Topical agents or dressings for pain in venous leg ulcers. Cochrane Database Syst Rev. 2012;11(11):CD001177. doi: 10.1002/14651858.CD001177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caputo W J, Beggs D J, DeFede J L, Simm L, Dharma H. A prospective randomised controlled clinical trial comparing hydrosurgery debridement with conventional surgical debridement in lower extremity ulcers. Int Wound J. 2008;5(02):288–294. doi: 10.1111/j.1742-481X.2007.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VenUS II Team . Dumville J C, Worthy G, Bland J M. Larval therapy for leg ulcers (VenUS II): randomised controlled trial. BMJ. 2009;338:b773. doi: 10.1136/bmj.b773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scottish Intercollegiate Guidelines Network . Scottish Intercollegiate Guidelines Network; 2010. Management of Chronic Venous Leg Ulcers: A National Clinical Guideline. [Google Scholar]
- 27.Tate S, Price A, Harding K. Dressings for venous leg ulcers. BMJ. 2018;361:k1604. doi: 10.1136/bmj.k1604. [DOI] [PubMed] [Google Scholar]
- 28.O'Meara S, Martyn-St James M. Foam dressings for venous leg ulcers. Cochrane Database Syst Rev. 2013;(05):CD009907. doi: 10.1002/14651858.CD009907.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Meara S, Martyn-St James M, Adderley U J. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev. 2015;(08):CD010182. doi: 10.1002/14651858.CD010182.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westby M J, Norman G, Dumville J C, Stubbs N, Cullum N. Protease-modulating matrix treatments for healing venous leg ulcers. Cochrane Database Syst Rev. 2016;12(12):CD011918. doi: 10.1002/14651858.CD011918.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norman G, Westby M J, Rithalia A D, Stubbs N, Soares M O, Dumville J C. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev. 2018;6(06):CD012583. doi: 10.1002/14651858.CD012583.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jull A B, Arroll B, Parag V, Waters J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst Rev. 2012;12:CD001733. doi: 10.1002/14651858.CD001733.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Kakkos S K, Nicolaides A N. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018;37(02):143–154. doi: 10.23736/S0392-9590.18.03975-5. [DOI] [PubMed] [Google Scholar]
- 34.Cowin A J, Hatzirodos N, Rigden J, Fitridge R, Belford D A. Etanercept decreases tumor necrosis factor-alpha activity in chronic wound fluid. Wound Repair Regen. 2006;14(04):421–426. doi: 10.1111/j.1743-6109.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 35.Dissemond J, Körber A, Grabbe S. [Differential diagnoses in leg ulcers] J Dtsch Dermatol Ges. 2006;4(08):627–634. doi: 10.1111/j.1610-0387.2006.06052.x. [DOI] [PubMed] [Google Scholar]
- 36.Michaels J A, Campbell B, King B, Palfreyman S J, Shackley P, Stevenson M. Randomized controlled trial and cost-effectiveness analysis of silver-donating antimicrobial dressings for venous leg ulcers (VULCAN trial) Br J Surg. 2009;96(10):1147–1156. doi: 10.1002/bjs.6786. [DOI] [PubMed] [Google Scholar]
- 37.Honey as Adjuvant Leg Ulcer Therapy Trial Collaborators . Jull A, Walker N, Parag V, Molan P, Rodgers A. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br J Surg. 2008;95(02):175–182. doi: 10.1002/bjs.6059. [DOI] [PubMed] [Google Scholar]
- 38.Jull A B, Cullum N, Dumville J C, Westby M J, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2015;(03):CD005083. doi: 10.1002/14651858.CD005083.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fumal I, Braham C, Paquet P, Piérard-Franchimont C, Piérard G E. The beneficial toxicity paradox of antimicrobials in leg ulcer healing impaired by a polymicrobial flora: a proof-of-concept study. Dermatology. 2002;204 01:70–74. doi: 10.1159/000057729. [DOI] [PubMed] [Google Scholar]
- 40.Mosti G, Magliaro A, Mattaliano V, Picerni P, Angelotti N.Comparative study of two antimicrobial dressings in infected leg ulcers: a pilot study J Wound Care 20152403121–122., 124–127 [DOI] [PubMed] [Google Scholar]
- 41.Cooper R, Jenkins L.Binding of two bacterial biofilms to dialkyl carbamoyl chloride (DACC)-coated dressings in vitro J Wound Care 2016250276, 78–82 [DOI] [PubMed] [Google Scholar]
- 42.Society for Vascular Surgery American Venous Forum O'Donnell T F, Jr, Passman M A, Marston W A.Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum J Vasc Surg 201460(2, Suppl):3S–59S. [DOI] [PubMed] [Google Scholar]


