Abstract
Pelvic venous disease (PeVD) in women encompasses a wide variety of entities all resulting in pelvic pain and varices. Successful treatment with percutaneous interventions is dependent on identifying underlying factors contributing to the disease and addressing them with either embolization of incompetent veins or stenting for venous stenoses. There are a multitude of embolization methods with marked practice heterogeneity. Moreover, with the ongoing development of dedicated venous stents in the treatment of chronic venous disease, there are more opportunities to consider this modality for the treatment of PeVD, as many patients present with combined vein reflux and central venous stenosis. The necessity to address both and the order of interventions in these patients is still to be elucidated. Here, we describe when to choose stenting or embolization for PeVD, their limitations, and our practice and identify further areas of research in this field.
Keywords: pelvic venous disease, stenting, embolization, interventional radiology
Pelvic venous disease (PeVD) is a cause of chronic pelvic pain in women and is associated with significant decrease in quality of life due to debilitating symptoms including chronic pelvic pain, perineal heaviness, urinary urgency, postcoital pain, and superficial nonsaphenous varices. The diagnosis is achieved following an extensive workup involving detailed history, examination, and imaging to confirm the diagnosis and exclude other etiologies. When diagnosed, there are multiple different treatment options ranging from medical treatment with medroxyprogesterone acetate to open surgical options including ligation of varices, and hysterectomy with oophorectomy. 1 2 3 4 Interventional radiology has increased its clinical role in the care of patients with PeVD, as percutaneous interventions are preferable or first line to open surgical treatments due to decreased morbidity, and the negative effects of hysterectomy on mental health and cardiovascular risks. 5
Percutaneous intervention for the treatment of PeVD has two fundamental treatment modalities: embolization and venous stenting. Additional adjunctive procedures such as sclerotherapy for the presence of escape points in the pelvis communicating with nonsaphenous superficial varices are also employed in addition to embolization and stenting. The selection of an effective treatment plan for patients with PeVD is highly dependent on identifying and addressing venous conditions contributing to each patient's disease.
Treatment Selection and Planning
Identification of Venous Conditions Contributing to PeVD
PeVD encompasses a heterogeneous group of entities that are all unified by the presence of pelvic varices with or without associated venous compression resulting in pelvic pain. Effective treatment defined by symptomatic improvement or resolution is dependent on the identification of the underlying venous conditions contributing to the patient's presentation.
The general clinical patterns that contribute to pelvic venous disease are as follows: (1) gonadal/internal iliac vein incompetence/reflux, (2) venous compression, (3) escape points, and (4) anatomical variants. 6 Each are summarized below and the presence or absence of each is important to consider when planning treatment in each patient. Most patients present with a combination of these entities; so, understanding their general treatment strategies is critical.
Gonadal/Internal Iliac Venous Incompetence/Reflux
The key feature is incompetent ovarian veins resulting in pelvic varices arising from the ovarian venous plexus which communicate with the broad ligament. This is often seen in younger multiparous patients. Valves are present in the ovarian veins 85% of the time, but 35 to 40% are incompetent. 7 Physiologic alterations and increased venous return during pregnancy in conjunction with hormonal influences of the veins is thought to result in varices and a further decrease in the number of competent venous valves. Reflux into multiple veins is common and the left ovarian vein and internal iliac vein are most often involved ( Fig. 1 ). 8
Fig. 1.
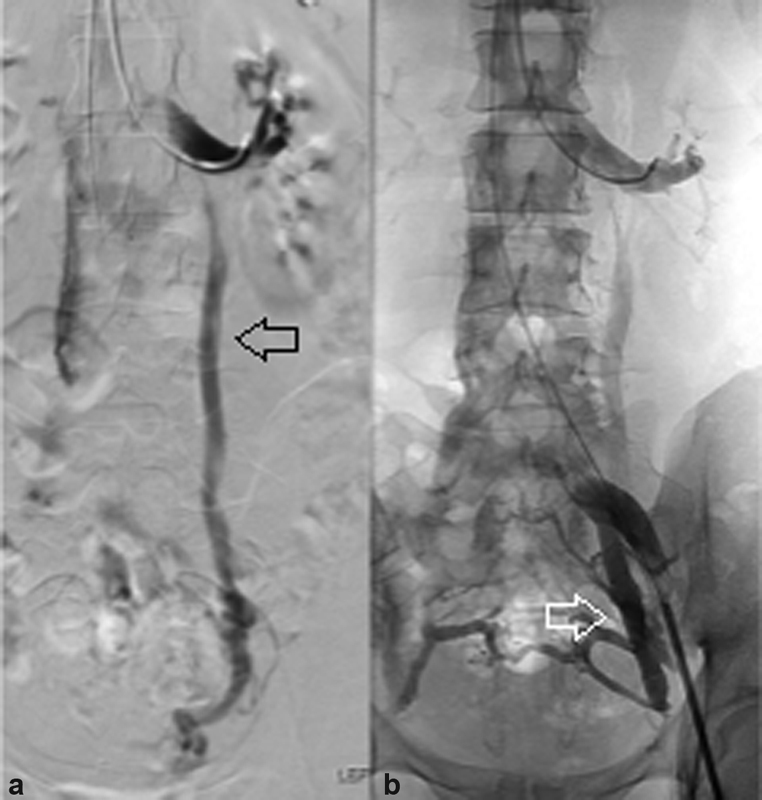
( a ) Venography demonstrates reflux into the left gonadal vein (arrow). ( b ) This patient's venogram additionally demonstrates reflux into the left internal iliac vein (arrow).
These patients are often treated with embolization of one or both ovarian veins with or without balloon-occlusion internal iliac vein sclerotherapy.
Venous Compression
Venous compression of the internal iliac or left renal vein can contribute to pelvic venous disease. Iliac vein stenosis due to compression of the left iliac vein by the crossing right iliac artery, known as May–Thurner anatomy, is present in a significant amount of people, but only results in symptoms in a minority of those with this anatomical variant. 9 10 Nutcracker syndrome is another entity which results in venous compression and may lead to pelvic venous disease. The compression of the left renal vein between the superior mesenteric artery anteriorly and the abdominal aorta posteriorly results in engorgement and incompetence of the valves in the left gonadal vein. Treatment for this is controversial, but endovascular treatment with stenting can be considered in young individuals who have symptomatic Nutcracker anatomy that is refractory to conservative management and are not surgical candidates. 11
Additional etiologies of venous compression include extrinsic compression from malignancy, lymphadenopathy, aneurysms, bladder distention, cysts, hematomas, and cross-fused kidney. 12 13 14 In such cases, the lesion causing the external compression should be addressed if possible.
Percutaneous treatment for venous compression is stenting to address stenoses and improve hemodynamics. The majority of data support stenting of iliac veins stenoses in advanced chronic venous disease. 15 However, more recent data suggest the effectiveness of venous stenting in a subset of patients with predominantly chronic pelvic pain as a major manifestation of PeVD. Daugherty and Gillespie demonstrated successful clinical outcomes in patients with PeVD and iliac vein stenting alone, while Gavrilov et al found that stent alone was not sufficient to completely improve symptoms in a patient population with predominant CPP and C1 venous disease and additional interventions were needed. 16 17
Escape Points
Venous compression resulting in obstruction in the common iliac vein can result in secondary reflux into the left internal iliac vein, the lower extremity, or vulvar veins via escape points which are summarized in Table 1 . This can result in symptoms including pain and swelling. 18
Table 1. Common escape points in PeVD (Pelvic Venous Disease).
| Escape point | Venous connection | Location of varices |
|---|---|---|
| Perineal | Internal to external pudendal veins | Perineum, posterior labia |
| Inguinal | Recanalized round ligament vein | Groin, labia |
| Gluteal | Inferior gluteal vein and femoral circumflex | Lower extremity |
| Obturator | Deep veins of medial thigh and obturator v | Lower extremity |
When escape points resulting in varices are present, they can be addressed in addition to venous compression with ultrasound or fluoroscopy-guided percutaneous sclerotherapy.
Anatomic Variations
While rare, it is important to consider anatomic variations in the inferior vena cava (IVC) such as a duplicated IVC, as they are present in up to 3% of patients. 19 Additionally, it is important to consider variant iliac venous anatomy which is present in 20 to 27% of individuals and can vary from the presence of extra iliac vessels, variant drainage patterns, and shortening or absence of the common iliac vein. 20 The presence of variations may affect compression points and the presence of additional collaterals or varices contributing to the patient's presentation. If variations are not identified, symptoms may not improve or may worsen following therapy. A published case of left common iliac vein stenting in the setting of unidentified variant venous anatomy highlights this concept. Moreland et al described a case in a patient with the left external iliac vein draining to the right common iliac vein resulting in worsening varices and symptoms following stenting. 21
Treatment Selection
The reality for many patients with PeVD is that a large proportion of patients have more than one of the aforementioned venous abnormalities contributing to their symptoms.
Initial data based on 19 patients suggested that in patients with both common iliac vein stenosis and left ovarian vein reflux just addressing the venous stenosis with a stent placement was effective in symptom improvement in the majority of patients. 16 However, a more recent study including 12 patients suggested that symptomatic relief of patients with pelvic venous disease was only 16.6% when only stenting was performed, and suggested that ovarian vein embolization should be performed 6 months after stenting. 17 These findings supported the data from a previous study involving a larger number of patients ( n = 277) published in 2018 which found that 80% of patients had both gonadal vein insufficiency and iliac vein compression, and advocated for the treatment of venous stenosis first followed by ovarian vein embolization subsequently if symptoms persist, but suggested that patients with large pelvic reservoirs receive simultaneous treatment. 22
Technique/How We Do It
Embolization
Indications
Diagnostic venographic criteria for PeVD are the following: (1) gonadal vein diameter more than 6 mm; (2) contrast retention more than 20 seconds; (3) congestion of the pelvic venous plexus and/or opacification of the ipsilateral (or contralateral) internal iliac vein; or (4) filling of vulvovaginal and thigh varicosities. 23
In the presence of documented venous reflux at venography, gonadal and internal iliac vein embolization is indicated for patients who are suffering from chronic pelvic pain secondary to PeVD, with a level 2B evidence. 24
Contraindications
There are few true contraindications to pelvic embolization. Active pelvic inflammatory disease and other significant infections should be treated prior to embolization. Iodinated contrast allergy is a secondary contraindication and patients should be premedicated prior to contrast administration.
Technique
Gonadal Vein Embolization
While the venous system can be accessed via the femoral, internal jugular, or arm veins, our preference is for the internal jugular vein as it results in a shorter and less tortuous pathway to the gonadal veins. Once venous access is achieved, a 6-Fr vascular sheath is advanced into the left renal vein. A hand injection venography is performed at this point to confirm positioning and to guide selection of the left gonadal vein usually via a 0.035-inch angled Glidewire. A 4- to 5-Fr multipurpose catheter is then advanced over the wire into left gonadal vein with a hand injection of contrast run to confirm positioning of the catheter and confirm venous reflux.
Since venous compression is often seen in conjunction with gonadal vein incompetence, we investigate the iliac venous system via intravascular ultrasound (IVUS) through femoral venous access to assess for compression that may need to be addressed concurrently. If found, it is treated with stenting following gonadal vein embolization at a later date, and the technique is described in detail in the following section.
Following IVUS interrogation of the iliac vessels, attention is redirected to the gonadal vein and at this point we elect to secure our access in the left renal vein with a 6-Fr curved angled sheath and then catheterize the gonadal vein with a 4-Fr glide catheter (Cobra-shaped or angled), which is then advanced into the inferior-most aspect of the left gonadal vein ( Fig. 2a ). For embolization, we use a combination of 0.035- or 0.018-inch detachable coils and 3% sodium tetradecyl sulfate (STS) solution (Sotradecol, Mylan Pharma Group Limited) mixed with air to create a 1:4 combination via the Tessari method. We begin with the injection of STS and air emulsion into the inferior segment of the vein during a Valsalva maneuver. The catheter is pulled back proximally, and the vein is subsequently packed with detachable coils to within approximately 2 cm of the confluence of the left gonadal vein and left renal vein or IVC in the case of right ovarian vein embolization. In some cases, coils are used alone and tight packing of the vein is preferred ( Fig. 2b, c ). The diameter of coils we use ranges from 8 to 20 mm in size and is dependent on the size of the vessel when measured on venography. A repeat venography is performed and any additional areas that need additional embolization such as duplicated segments of gonadal vein area addressed prior to withdrawing wires and sheaths ( Fig. 2d ).
Fig. 2.
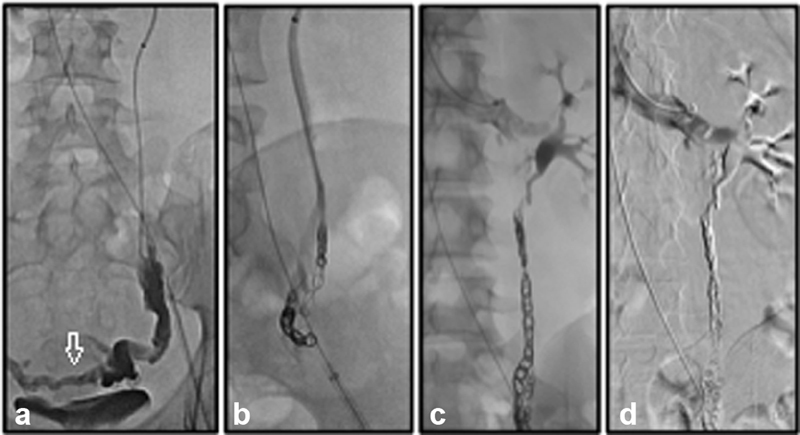
( a ) Venography in the distal aspect of the left gonadal vein demonstrates reflux as well as varices (arrow). ( b ) Following embolization of the varix, coil embolization of the proximal gonadal vein is performed. ( c ) Coils are placed within 2 cm of the confluence with the renal vein. ( d ) Following coil embolization, a final digital subtraction venography is performed to confirm adequate embolization and assess for any other sites that may need additional embolization such as duplications of the venous system.
The left ovarian vein is embolized more frequently, but the right ovarian vein can be embolized as well. In cases where there is right ovarian vein reflux, the vein can be accessed via a Simmons 2 catheter as it arises directly off the IVC, if using a femoral approach, but otherwise an MPA catheter can be used to selectively catheterize the right gonadal vein. The embolization technique is same as described earlier.
Internal Iliac Vein Sclerotherapy
Further embolization of the varices from the internal iliac vein is thought to reduce the risk of recurrence due to the free communications between the ovarian varices and internal iliac vein tributaries.
While some publications support the use of coils and plugs to embolize the internal iliac veins, at our practice, we elect to use balloon-occlusion sclerotherapy for the treatment of the internal iliac veins. 25 The right femoral vein access is obtained via a 9-Fr short sheath and the contralateral internal iliac vein is selected with a 5-Fr Cobra catheter. A 5.5-Fr Fogarty occlusion balloon or 7-Fr Berman wedge catheter is exchanged over a 0.035-inch Rosen wire and is placed just above the true pelvis where the tributaries and ovarian vein join. This is repeated on the contralateral side to select both internal iliac veins. The volume of the pelvic varices venous plexus can be estimated by inflating the balloon and injecting contrast until normal veins are opacified. The volume of sclerosing agent should be 75% of the measured volume. We use a 3% STS solution mixed with air at a 1:4 ratio to create a sclerosant foam. Once delivered into the varices, the balloons remain inflated for 5 minutes to prevent nontarget sclerosis. The embolization can be repeated on the contralateral side if necessary. 6
Complications
Major complications from this procedure include pulmonary embolism, deep venous thrombosis, and embolic material in the lung vasculature. 26 Recurrence has recently been reported to be 5%. 25 The effect on fertility is not well studied, but successful pregnancies in patients have been reported following ovarian vein embolization. 27
Postprocedure and Follow-up Care
Postprocedure care largely focuses on pain management. After the pain is well controlled on oral medications, the patient is discharged. Initial follow-up in clinic is typically done at 1 month. Patients are instructed that symptomatic improvement may take more than 1 month. If the patient has only isolated gonadal vein reflux, they are additionally reevaluated again at 6 months. In patients with additional venous entities contributing to their disease such as venous stenoses, the patient is evaluated 1 and 3 months posttreatment with the second intervention, such as stenting, performed 6 months following the initial treatment.
Discussion
There have been numerous small studies dating back to 1993 that demonstrate that between 50 and 100% reported clinical success on gonadal vein embolization. 28 29 30 There are also debates as to the necessity of the bilateral ovarian vein embolization in addition to embolization of the internal iliac veins. 28 In one of the largest studies to date, De Gregorio et al followed up 520 patients who underwent embolization for pelvic venous disorders after embolization of both ovarian and internal iliac vein and found a 5% recurrence rate. 25
Furthermore, there is heterogeneity of embolic material used across studies. Embolic materials include coils, Gelfoam slurry, STS, and more recently ethylene vinyl alcohol copolymer or Onyx. 28 31 The combination of different techniques and utilization of various embolic materials across these studies begs the question of what is the optimal technique to employ based on patient characteristics and is an ongoing research question.
Stenting
Indications
Stenting of venous stenosis should be done in symptomatic patients whose symptoms are attributable to the stenosis. This is determined through a combination of history, medical workup, and imaging. Since there is a high prevalence of iliac vein stenosis in asymptomatic patients, it can be a challenge to determine the degree of stenosis that becomes clinically relevant and treatment would result in symptomatic relief in those with PeVD when there are multiple factors at play. 32
In the VIDIO study, Gagne et al demonstrated the superiority of IVUS for predicting when stenting of iliofemoral vein stenosis improves symptoms. They found that cross-sectional area reduction of more than 50% in thrombotic veins and more than 61% in nonthrombotic veins were clinically significant and predicted favorable outcomes after stenting in patients with symptomatic disease. 33 In addition to being superior to single-plane venography, IVUS is helpful in assessing response to the lesion from angioplasty, guides stent placement, and can identify in-stent stenosis. 34 35
Contraindications
Stenting should not be performed in asymptomatic patients. Additional contraindications to stenting include inadequate venous inflow from occlusion of the common femoral veins or femoral veins, and inadequate outflow such as occlusion of the IVC that is not amenable to proximal extension of the stent.
Technique
Nonthrombotic Iliac Vein Stenosis Treatment
The left femoral vein is accessed and a 9-Fr, 11-cm introducer sheath (Boston Scientific, Marlborough, MA) is placed to introduce the IVUS system. We use the IVUS system (Philips, Amsterdam, the Netherlands) to confirm venous compression and estimate the size of stent to be placed based on intraluminal measurements ( Fig. 3a, b ). Assessment begins at the IVC and continues into both common iliac veins, external iliac veins, common femoral veins, and femoral veins. We begin treating stenoses with balloon angioplasty with an appropriately sized balloon of 8 or 10 mm in diameter. Our current practice is to use the self-expanding Wallstent (Boston Scientific) and the diameter is determined by oversizing the reference lumen by 10 to 20% ( Fig. 3c ). Once the stent is deployed, a repeat venography is performed prior to the end of the procedure ( Fig. 3d ). 6 With newer dedicated venous stents in the U.S. market, this technique will substantially change, as these stents present with less foreshortening, more radial force, and therefore have more precise placement at the bifurcation as described by Murphy et al. 36
Fig. 3.
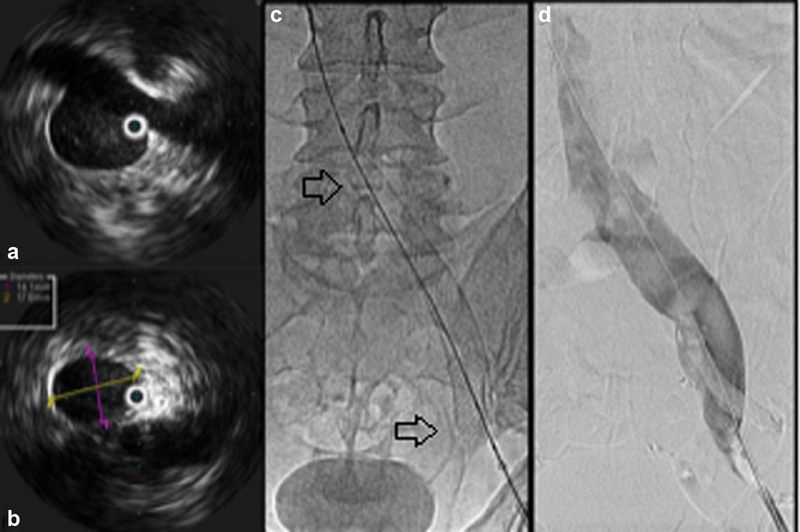
( a ) Intravascular ultrasound is used to assess for the presence of stenoses. ( b ) Once a venous stenosis is identified in the left common iliac vein, it is measured to guide selection of an appropriately sized Wallstent. ( c ) The stent (arrows) is deployed under fluoroscopy and/or ultrasound imaging guidance. ( d ) Once the stent is deployed, a final venography is performed prior to the end of the procedure.
Iliac Compression with Vulvar and Superficial Nonsaphenous Varices
Previous data have shown that a proportion (16.6%) of patients with pelvic venous disease and iliac vein stenosis have vulvar varices that required adjunctive procedures for treatment. 17 In our clinical practice, fluoroscopic and/or ultrasound guidance is used to directly access these veins, perform venography, and perform sclerotherapy with foam. Fluoroscopic guidance is advantageous in that it is more amenable to close drug dose titration and control of the injection to the level of the normal pelvic veins, which reduces the risk of nontarget sclerotherapy. Injection can be manipulated manually by compression and guiding sclerosant to desired target. Our preferred sclerosant is a mixture of 1% STS with air at a 1:5 ratio. A maximum volume of 10 mL can be safely injected per procedure.
Iliac Vein Compression with Gonadal Vein Insufficiency
The technique for gonadal vein embolization is described in the “Gonadal Vein Embolization” section. In clinical practice, our preference is to perform one intervention and see if there is clinical improvement. If not, the second procedure could be performed 6 months after the first procedure, or simultaneously as described by Santoshi et al. 22
Complications
Technical success of stenting is achieved in 94 to 100% of cases. Major complications include major bleeding (0.3–1.1%), pulmonary embolism (0.2–0.9%), periprocedural mortality (0.1–0.7%), and early restenosis or occlusion of the treated lesion (1.0–6.8%). 37 Stent migration has been previously described with Wallstent and this complication was addressed with stabilization via a second stent. 17 Thrombotic complications are rare in patients who do not have a prior history of venous thrombosis at initial presentation. 22 Additionally, when patients become pregnant, there are no specific guidelines for anticoagulation, but there is a theoretical risk of stent compression due to the growing fetus. Treatment of prophylactic anticoagulation with low-molecular-weight heparin was shown to be associated with no episodes of deep-venous thromboses or thrombotic events in a series by Hartung et al. 38
Postprocedure and Follow-up Care
There is marked variation in anticoagulation and antiplatelet management after stenting for nonthrombotic central venous stenosis. A Delphi consensus was performed to generate consensus statements among venous stenting experts. They stated that anticoagulation is preferred to antiplatelet therapy for the first 6 to 12 months after stenting for nonthrombotic venous stenosis. Low-molecular-weight heparin is the first-choice anticoagulant in the first 2 to 6 weeks poststenting. In the case of postthrombotic venous stenting, anticoagulation can be discontinued 6 to 12 months following stent placements if the following criteria are met: (1) negative thrombophilia screen, (2) thrombotic event was the first for the patient, and (3) stent patency is demonstrated on ultrasound. In patients who have multiple deep venous thromboses and iliac vein stenting, anticoagulation should be continued indefinitely barring contraindications. 39
In our practice, patients who are in a hypercoagulable state or have a history of prior DVT are placed on anticoagulation for 6 months only. All other patients are treated on an antiplatelet agent (clopidogrel: 75 mg) for 6 to 8 weeks after the procedure. Our rationale is that in general, our patient population is young and healthy and presents with nonthrombotic disease. The risks of both bleeding and thrombosis are discussed with patients prior to making the decision of antiplatelet versus anticoagulation.
If superficial nonsaphenous vein ablation was performed, patients are also instructed to wear compression stockings. Stent patency is evaluated with either ultrasound or computed tomography venography at 1 month and then follow-up imaging with ultrasound is performed prior to the second visit at 6 months.
Discussion
Stenting is primarily used for iliac vein compression as angioplasty alone is not an effective therapy with high rate of patency loss and stenting can improve long-term patency. 40
Wallstents have been shown to improve patency and symptoms when compared with angioplasty alone but have drawbacks which include recoil and foreshortening after deployment, posing a challenge for accurate stent placement, particularly when considering the longevity of patients undergoing stent placement in PeVD population, who are younger and relatively healthy. An alternative stent is the Nitinol stent which does not foreshorten, but comparative data between the two stents are not yet available. 40
There is ongoing development and testing of dedicated venous stents such as the VICI (Boston Scientific) and the Venovo (BD Interventional, Temp, AZ), which thus far have demonstrated high patency rates and improved precision of placement at the time of deployment. 41 42 These have been studied in patients with chronic venous disease, but current studies demonstrating the efficacy in the pelvic venous disease patient population have yet to be published.
Conclusion
PeVD encompasses a wide array of clinical entities which makes it difficult to study. Additional studies are needed to establish guidelines for treatment in these patients, but a few factors have made this difficult. First, there are no patient-reported outcome instruments that have been specifically developed for PeVD which hinders more objective analysis of symptom improvement. This has been recognized as a research priority by a multidisciplinary research consensus panel for PeVD. 43 Second, there is a wide variety in the management of these patients clinically especially in those who have venous Stenosis and ovarian reflux. While some studies evaluated the outcomes in treating patients with both stenosis and reflux, more data is needed in addition to developing standardized patient-reported outcome tool.
Footnotes
Conflict of Interest None declared.
References
- 1.Farquhar C M, Rogers V, Franks S. A randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. BJOG An Int J Obstet Gynaecol. 1989;96(10):1153–1162. doi: 10.1111/j.1471-0528.1989.tb03190.x. [DOI] [PubMed] [Google Scholar]
- 2.Beard R W, Kennedy R G, Gangar K F. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. BJOG An Int J Obstet Gynaecol. 1991;98(10):988–992. doi: 10.1111/j.1471-0528.1991.tb15336.x. [DOI] [PubMed] [Google Scholar]
- 3.Behera M, Vilos G A, Hollett-Caines J, Abu-Rafea B, Ahmad R. Laparoscopic findings, histopathologic evaluation, and clinical outcomes in women with chronic pelvic pain after hysterectomy and bilateral salpingo-oophorectomy. J Minim Invasive Gynecol. 2006;13(05):431–435. doi: 10.1016/j.jmig.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Cheong Y C, Smotra G, Williams A CDC. Non-surgical interventions for the management of chronic pelvic pain. Cochrane Database Syst Rev. 2014;2014(03):CD008797. doi: 10.1002/14651858.CD008797.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harnod T, Chen W, Wang J H, Lin S Z, Ding D C. Clinical medicine hysterectomies are associated with an increased risk of depression: a population-based cohort study. J Clin Med. 2018;7(10):366. doi: 10.3390/jcm7100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutsenko O, Salazar G.Treatment strategies for varying patterns and presentations of pelvic venous disorderThere is not a one-size-fits-all solution! Endovascular Today 2020;19(04):
- 7.Kaufman J A. Philadelphia: Elsevier-Mosby; 2014. Inferior vena cava and tributaries; pp. 304–306. [Google Scholar]
- 8.Asciutto G, Asciutto K C, Mumme A, Geier B. Pelvic venous incompetence: reflux patterns and treatment results. Eur J Vasc Endovasc Surg. 2009;38(03):381–386. doi: 10.1016/j.ejvs.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 9.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(05):419–427. doi: 10.1177/000331975700800505. [DOI] [PubMed] [Google Scholar]
- 10.Kibbe M R, Ujiki M, Goodwin A L, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(05):937–943. doi: 10.1016/j.jvs.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 11.Lourenço De Macedo G, Alves M, Santos D, Sarris A B, Gomes R Z. Diagnosis and treatment of the Nutcracker syndrome: a review of the last 10 years. Jul-Set. 2018;17(03):220–228. doi: 10.1590/1677-5449.012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung J B, Hsu C W, Tsai S H. Prostatism and May-Thurner syndrome. Am J Emerg Med. 2013;31(02):4450–44500. doi: 10.1016/j.ajem.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Crowner J, Marston W, Almeida J, McLafferty R, Passman M. Classification of anatomic involvement of the iliocaval venous outflow tract and its relationship to outcomes after iliocaval venous stenting. J Vasc Surg Venous Lymphat Disord. 2014;2(03):241–245. doi: 10.1016/j.jvsv.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Elsharawy M A, Moghazy K M, Alsaif H S, Al-Asiri M M. Unusual case of left iliac vein compression secondary to May-Thurner syndrome and crossed fused renal ectopia. Saudi Med J. 2008;29(04):603–605. [PubMed] [Google Scholar]
- 15.Mahnken A H, Thomson K, de Haan M, O'Sullivan G J. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37(04):889–897. doi: 10.1007/s00270-014-0875-4. [DOI] [PubMed] [Google Scholar]
- 16.Daugherty S F, Gillespie D L. Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outflow obstruction. J Vasc Surg Venous Lymphat Disord. 2015;3(03):283–289. doi: 10.1016/j.jvsv.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Gavrilov S G, Vasilyev A V, Krasavin G V, Moskalenko Y P, Mishakina N Y. Endovascular interventions in the treatment of pelvic congestion syndrome caused by May-Thurner syndrome. J Vasc Surg Venous Lymphat Disord. 2020;8(06):1049–1057. doi: 10.1016/j.jvsv.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Greiner M, Dadon M, Lemasle P, Cluzel P. How does the patho-physiology influence the treatment of pelvic congestion syndrome and is the result long-lasting? Phlebology. 2012;27 01:58–64. doi: 10.1258/phleb.2011.012s07. [DOI] [PubMed] [Google Scholar]
- 19.Sürücü H S, Erbil K M, Tastan C, Yener N. Anomalous veins of the retroperitoneum: clinical considerations. Surg Radiol Anat. 2001;23(06):443–445. doi: 10.1007/s00276-001-0443-x. [DOI] [PubMed] [Google Scholar]
- 20.DePietro D M, Carlon T, Trerotola S O, Sudheendra D. Endovascular treatment of an anatomically complex iliac lesion and a review of variant iliac venous anatomy. J Vasc Interv Radiol. 2020;31(02):260–264. doi: 10.1016/j.jvir.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Moreland A J, Holly B P, AAssar O S, Lessne M L. Variant drainage of left internal iliac vein to right common iliac vein potentiating pelvic congestion syndrome. J Vasc Interv Radiol. 2019;30(04):622–625. doi: 10.1016/j.jvir.2018.12.731. [DOI] [PubMed] [Google Scholar]
- 22.Santoshi R KN, Lakhanpal S, Satwah V, Lakhanpal G, Malone M, Pappas P J. Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2018;6(02):202–211. doi: 10.1016/j.jvsv.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Arnoldussen C WKP, de Wolf M AF, Wittens C HA.Diagnostic imaging of pelvic congestive syndrome Phlebology 201530(1, Suppl):67–72. [DOI] [PubMed] [Google Scholar]
- 24.Society for Vascular Surgery American Venous Forum Gloviczki P, Comerota A J, Dalsing M C.The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum J Vasc Surg 201153(5, Suppl):2S–48S. [DOI] [PubMed] [Google Scholar]
- 25.De Gregorio M A, Guirola J A, Alvarez-Arranz E, Anchez-Ballestin M S, Urbano J, Sierre S. Pelvic venous disorders in women due to pelvic varices: treatment by embolization: experience in 520 patients. J Vasc Interv Radiol. 2020;31(10):1560–1569. doi: 10.1016/j.jvir.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero A, Theophanous R G. A case report of a migrated pelvic coil causing pulmonary infarct in an adult female. Clin Pract Cases Emerg Med. 2020;4(03):436–439. doi: 10.5811/cpcem.2020.5.47463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Han L, Han X. The effect of a subsequent pregnancy after ovarian vein embolization in patients with infertility caused by pelvic congestion syndrome. Acad Radiol. 2019;26(10):1373–1377. doi: 10.1016/j.acra.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Kies D D, Lee J M, Venbrux A C, Kim H S. Philadelphia: Elsevier - Saunders; 2014. Management of female venous congestion syndrome; pp. 563–568. [Google Scholar]
- 29.Daniels J P, Champaneria R, Shah L, Gupta J K, Birch J, Moss J G. Effectiveness of embolization or sclerotherapy of pelvic veins for reducing chronic pelvic pain: a systematic review. J Vasc Interv Radiol. 2016;27(10):1478–1.486E11. doi: 10.1016/j.jvir.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoud O, Vikatmaa P, Aho P. Efficacy of endovascular treatment for pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4(03):355–370. doi: 10.1016/j.jvsv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Marcelin C, Izaaryene J, Castelli M. Embolization of ovarian vein for pelvic congestion syndrome with ethylene vinyl alcohol copolymer (Onyx ® ) . Diagn Interv Imaging. 2017;98(12):843–848. doi: 10.1016/j.diii.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Esposito A, Charisis N, Kantarovsky A, Uhl J F, Labropoulos N. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur J Vasc Endovasc Surg. 2020;60(01):118–125. doi: 10.1016/j.ejvs.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Gagne P J, Gasparis A, Black S. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6(01):48–560. doi: 10.1016/j.jvsv.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35(04):694–700. doi: 10.1067/mva.2002.121127. [DOI] [PubMed] [Google Scholar]
- 35.Thorpe P E. Identification and treatment of restenosis in failing venous stents: the role of intravascular ultrasound. J Vasc Surg Venous Lymphat Disord. 2014;2(01):109–110. doi: 10.1016/j.jvsv.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Murphy E. Surveying the 2019 venous stent landscape. Endovasc Today. 2019;18(07):53–64. [Google Scholar]
- 37.Razavi M K, Jaff M R, Miller L E. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8(10):e002772. doi: 10.1161/CIRCINTERVENTIONS.115.002772. [DOI] [PubMed] [Google Scholar]
- 38.Hartung O, Barthelemy P, Arnoux D, Boufi M, Alimi Y S. Management of pregnancy in women with previous left ilio-caval stenting. J Vasc Surg. 2009;50(02):355–359. doi: 10.1016/j.jvs.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 39.Milinis K, Thapar A, Shalhoub J, Davies A H. Antithrombotic therapy following venous stenting: International Delphi Consensus. Eur J Vasc Endovasc Surg. 2018;55(04):537–544. doi: 10.1016/j.ejvs.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Radaideh Q, Patel N M, Shammas N W. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag. 2019;15:115–122. doi: 10.2147/VHRM.S203349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lichtenberg M KW, de Graaf R, Stahlhoff W F, Özkapi A, Rassaf T, Breuckmann F. Venovo venous stent in the treatment of non-thrombotic or post-thrombotic iliac vein lesions - short-term results from the Arnsberg venous registry. Vasa. 2019;48(02):175–180. doi: 10.1024/0301-1526/a000763. [DOI] [PubMed] [Google Scholar]
- 42.Lichtenberg M, Breuckmann F, Stahlhoff W F, Neglén P, Rick G. Placement of closed-cell designed venous stents in a mixed cohort of patients with chronic venous outflow obstructions - short-term safety, patency, and clinical outcomes. Vasa. 2018;47(06):475–481. doi: 10.1024/0301-1526/a000731. [DOI] [PubMed] [Google Scholar]
- 43.Khilnani N M, Meissner M H, Learman L A. Research priorities in pelvic venous disorders in women: recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2019;30(06):781–789. doi: 10.1016/j.jvir.2018.10.008. [DOI] [PubMed] [Google Scholar]


