Abstract
Zinc is an essential micronutrient with crucial roles in multiple facets of biological processes. Dysregulated zinc homeostasis impairs overall immune function and resultantly increases susceptibility to infection. Clinically, zinc supplementation is practiced for treatment of several infectious diseases, such as diarrhea and malaria. Recent focus on zinc as a beneficial element for immune system support has resulted in investigation of the immunomodulatory roles of zinc in a variety of immune cells. Besides its classical role as a cofactor that regulates the structural function of thousands of proteins, accumulating evidence suggests that zinc also acts, in a manner similar to calcium, as an ionic regulator of immune responses via participation as an intracellular messenger in signaling pathways. In this review, we focus on the role of zinc as a signaling molecule in major pathways such as those downstream of Toll-like receptors-, T cell receptor-, and cytokine-mediated signal transduction that regulate the activity and function of monocytes/macrophages and T cells, principal players in the innate and adaptive immune systems.
Keywords: monocytes/macrophages, phosphatase, signaling pathways, T cell receptor, T cells, Toll-like receptors, zinc, zinc transporter
INTRODUCTION
Zinc, the second most abundant dietary trace metal in organisms after iron, plays an essential role in multiple cellular processes (Wapnir, 1990). Zinc dyshomeostasis, such as zinc deficiency, impacts every organ of the body and has been implicated in clinical problems including growth retardation, neurological disorders, and immune dysfunction (Haase and Rink, 2009b; Prasad, 2013; Szewczyk, 2013). Therefore, intracellular zinc homeostasis is securely controlled by spatiotemporal and intricate regulation of zinc-specific transporters, zinc sensors, and zinc-binding proteins. There are two families of zinc-specific transporters; the solute-linked carrier 39 (SLC39A or Zip) family of zinc importers and solute-linked carrier 30 family (SLC30A or ZnT) of zinc exporters, which are expressed in a cell- or tissue-specific manner (Huang and Tepaamorndech, 2013; Jeong and Eide, 2013; Kambe et al., 2014). Metallothionein (MT) is a zinc-binding protein that functions as a reservoir of intracellular zinc with the ability to bind up to seven zinc ions per MT molecule. MT plays coordinated roles in the distribution, transport, and maintenance of intracellular zinc. Further, the induction of MT expression is dependent on the increase of intracellular zinc (Davis and Cousins, 2000). Human MT proteins consist of 11 functional isoforms, divided into four classes, with transcription directly induced by the zinc sensor, metal response element-binding transcription factor-1 (MTF-1), which is a zinc finger transcription factor (Kimura and Kambe, 2016). The total intracellular zinc concentration is in the micro-molar range (10-100 μM), but because zinc binds to several proteins in the cytoplasm the actual concentration of cytosolic, labile zinc ions is estimated to range between pico-molar and nano-molar (Hara et al., 2017). The intracellular zinc concentration is rapidly and markedly increased in immune cells upon stimulation, and this increase is enough to regulate the activity of signaling molecules during immune responses.
Although zinc is essential for major metabolic pathways in virtually every cellular system, accumulating evidence underscores the profound influence of zinc on the immune system (Wessels et al., 2017). Clinically, zinc deficiency caused by malnutrition, such as reduced dietary intake, inadequate absorption or dyshomeostasis, deteriorates overall immune functions contributing to increased host susceptibility to many viral and bacterial infections. Thus, zinc supplementation is considered beneficial for restoring immune function in infectious diseases. Besides its traditional role as a catalytic and structural cofactor, zinc has been known to play a cardinal role, similar to that of calcium, as a transducer of signals in immune responses (Haase and Rink, 2014; Hirano et al., 2008; Hojyo and Fukada, 2016; Maywald et al., 2017). Mechanistically, cytoplasmic zinc ions are recognized as inhibitors of protein tyrosine phosphatases (PTPs) and serine/threonine phosphatases that conserve phosphorylation of signaling proteins and generally sustain signaling activity (Bellomo et al., 2014; Haase and Maret, 2003), which mostly prolongs immune cell signaling and enhances immune responses (Anzilotti et al., 2019; Aydemir et al., 2009; Yu et al., 2011). However, mechanisms underlying zinc-mediated inhibition of phosphatases are still poorly understood. Although PTPs are generally not recognized as metalloenzymes (Singh and Maret, 2017), Bellomo et al. (2014) recently proposed that protein tyrosine phosphatase 1B (PTP1B) has Zn2+ binding sites. Of note, Zn2+ binding may occur when PTP1B is in a closed or phospho-intermediate form in which the zinc ion is in a coordination sphere surrounded by relevant amino acids (Bellomo et al., 2014).
The immune system provides two layers of defense against pathogens, innate and adaptive immunity. The frontline of host defense, innate immunity involves the recognition of pathogen-associated molecular patterns (PAMPs), conserved structures of invading pathogens, and the immediate initiation of immune responses. On the other hand, adaptive immunity is largely mediated by T and B cells, which allow for a robust antigen-specific immune response as well as immunological memory. Successful immune responses are guaranteed by coordinated and elaborate crosstalk between the adaptive and innate immune systems (Chaplin, 2010).
The role of zinc as a catalytic and structural cofactor has been extensively reviewed elsewhere (Auld, 2001; Vallee and Falchuk, 1993). Therefore, this review will focus on the effects of zinc on immune responses, and especially its function as an ionic signaling molecule, as well as recent advances in the understanding of the immunomodulatory role of zinc in monocytes/macrophages and T cells, major arms of the innate and adaptive immune system, respectively.
ZINC SIGNALS IN MONOCYTES AND MACROPHAGES
Innate immunity involves several different cell types including granulocytes, natural killer (NK) cells, and mononuclear phagocytes (monocytes, macrophages, and dendritic cells). Among these, mononuclear phagocytes are particularly important as a link between innate and adaptive immunity as they are directly or indirectly involved in the orchestration of immune responses. Zinc deficiency in the innate immune system is characterized by reduced chemotaxis of polymorphonuclear cells (PMNs) and phagocytosis of macrophages, with a resultant diminishment in production of pathogen-neutralizing reactive oxygen species (ROS) (Bonaventura et al., 2015). Moreover, the production of proinflammatory cytokines by monocytes is apparently impaired under zinc deficiency (Rink and Kirchner, 2000), suggesting an essential role of zinc in regulating, and potentially restoring, innate immune responses (Gao et al., 2018; Haase and Rink, 2007; Sapkota and Knoell, 2018).
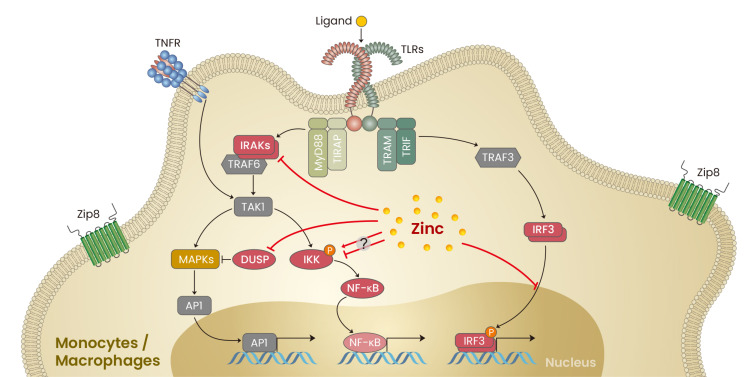
Mononuclear phagocytes rapidly recognize invading pathogens via sensing of PAMPs by pattern-recognition receptors (PRR), including Toll-like receptors (TLRs). Upon PAMP engagement, individual TLRs differentially recruit adaptor molecules, such as MyD88, TRIF, TIRAP, or TRAM (Kawasaki and Kawai, 2014). This leads to activation of several major signaling pathways, including MyD88/TIRAP-dependent NF-κB, MAPK, PI3K, and the TRIF/TRAM-dependent IRF3 pathway, and elicits a variety of monocyte and macrophage effector functions (Fig. 1) (Guha and Mackman, 2001).
Fig. 1. Roles of zinc in monocytes/macrophages signaling.
Black lines, signal transduction; red lines, the effect of zinc; arrows, activation; T bar, inhibition. TLRs, Toll-like receptors; TIRAP, TIR domain containing adaptor protein; MyD88, myeloid differentiation primary response 88; TRIF, TIR-domain-containing adaptor protein inducing interferon-β; TRAM, TRIF-related adaptor molecule; IRAK, interleukin-1 receptor-associated kinase; TRAF, tumor necrosis factor receptor–associated factor; TAK1, transforming growth factor-β-activated kinase 1; MAPKs, mitogen-activated protein kinase; AP1, activator protein 1; IKK, IκB kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; DUSP, dual-specificity phosphatase; IRF3, IFN regulatory factor 3; TNFR, tumor necrosis factor receptor; Zip8, Zrt- and Irt-like protein 8 (SLC39A8).
TRIF/TRAM-dependent pathway
Lipopolysaccharide (LPS) binding to TLR4 leads to rapid zinc influx into the cytoplasm of monocytes and macrophages, which triggers zinc-mediated regulation of major signaling pathways (Brieger et al., 2013; Feske et al., 2015). In fact, this finding is not limited to TLR4, as stimulation with tumor necrosis factor α (TNF-α) or various ligands for other TLRs (except TLR3) was also found to result in an increase in intracellular zinc concentrations (Brieger et al., 2013; Haase et al., 2008). Brieger et al. (2013) reported that increased labile zinc acts as a negative regulator of TRIF/TRAM-dependent signaling by attenuating IRF3 activation in murine macrophages. Pre-existing zinc deficiency increases levels of TLR4 or TLR7-induced nitrite, decreases production of NO, and augments LPS-induced interferon (IFN)-β, as well as CD80 and CD86, expression in murine macrophages. Mechanistically, zinc regulates TRIF signaling upstream of IRF3 and affects nuclear accumulation of phospho-IRF3. The IRF3-dependent enhanced production of IFN-β is responsible for NO release (Brieger et al., 2013). However, the potential molecular target(s) involved in zinc-medicated regulation of IRF3 activity have not yet been identified.
MyD88/TIRAP-dependent NF-κB pathway
The NF-κB transcription factor is a central regulator of proinflammatory gene induction and functions in a variety of immune responses. Thus, the impact of zinc on NF-κB signaling pathways has been investigated in detail. Zinc has multifaceted effects on immune responses in monocytes and macrophages, reflecting the complexity of the NF-κB pathway (Haase and Rink, 2014). Early studies demonstrated that zinc is indispensable for activation of the NF-κB pathway, as cytoplasmic zinc depletion with TPEN, a zinc-specific chelator, was found to reduce LPS-induced phosphorylation of IKKα/β in human monocytes (Haase et al., 2008) and also to inhibit NF-κB DNA binding activity in Mono Mac1 cells, a human monocyte line. On the other hand, a number of studies revealed an inhibitory effect of zinc on NF-κB signaling (Haase and Rink, 2009a; Prasad et al., 2011; von Bulow et al., 2007). Liu et al. (2013) recently reported that Zip8 (SLC39A8) is a direct transcriptional target of NF-κB and its expression is markedly induced by LPS or TNF-α stimulation of monocytes and macrophages. The Zip8-mediated increase of intracellular zinc negatively regulates proinflammatory responses via zinc-induced downmodulation of IκB kinase (IKK) activity by its direct binding to IKKβ. In vivo models of LPS and cecal ligation and puncture (CLP) challenge showed amplified inflammatory responses in mice fed a zinc-deficient diet compared to those fed a normal diet, along with increased proinflammatory cytokine levels and phosphorylated IκBα (Bao et al., 2010; Liu et al., 2013). In addition, another proposed mechanism for the inhibitory role of zinc in human monocytes is the repression of phosphodiesterase (PDE) activity, which leads to changes in intracellular cyclic nucleotides, particularly an increase of cGMP (Cyclic Guanosine monophosphate) levels. This in turn results in inhibition of the Raf-1/IKKβ/NF-κB pathway and downregulation of TNF-α (von Bulow et al., 2005). Thus, zinc has versatile, and occasionally diametrical, effects on TLR-mediated responses in monocytes and macrophages depending on the concentration of zinc and type of stimulation. Therefore, zinc appears to be involved in fine-tuning of signaling pathways for regulation of innate immunity.
MyD88/TIRAP-dependent MAPK pathway
Activation-induced rapid influx of zinc into the cytoplasm is involved in activation of MAPKs by TLR4, and zinc chelation by TPEN was shown to completely abate p38, MEK1/2, and ERK1/2 activation in response to LPS and to reduce release of pro-inflammatory cytokines, especially TNF-α (Haase et al., 2008; Wan et al., 2014). Moreover, the effect of zinc on MAPK activity is likely attributable to its inhibitory effect on MAP-kinase phosphatases, such as dual-specificity phosphatases (DUSPs). Another related mechanism is the zinc-mediated degradation of IRAK, which regulates NF-κB and MAPK pathways. TLR4 activation by LPS induces phosphorylation and degradation of IRAK1 in a MyD88-dependent manner. Degradation of IRAK1 occurs independently of the proteasome, but is dependent on zinc concentration. However, the exact mechanism and molecular target underlying zinc-dependent IRAK1 degradation remains unclear (Wan et al., 2014).
ZINC SIGNALS IN T CELLS
Adaptive immunity is mainly mediated by two types of lymphocytes, T and B cells, which have the ability to establish antigen specificity and immunological memory. Antigen-specific recognition is conferred by enormously diverse adaptive immune receptors expressed on the surface of T and B cells. Upon their recognition of specific antigens processed and presented by antigen-presenting cells (APCs), T cells undergo an intensive clonal expansion of cells expressing specific receptors for cognate antigens, which differentiate into memory and effector T cells. Thus, the adaptive immune system responds more slowly, but more robustly and precisely, than the innate immune system.
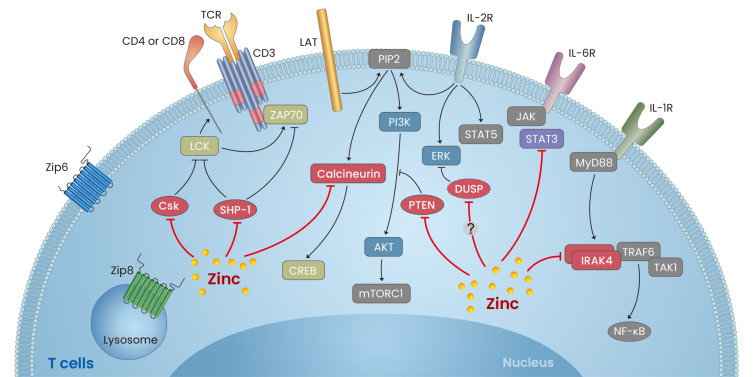
A large body of evidence suggests that zinc contributes to the regulation of a broad spectrum of adaptive immune cellular functions including their development, differentiation, proliferation, survival, and cytokine production (Gruber et al., 2013; Hirano et al., 2008; King et al., 2005; Prasad, 2000). Here, we discuss recent understanding of the effect of zinc on the signaling pathways initiated upon T cell receptor (TCR) or cytokine stimulation and the resultant effects on the alteration of immune function (Fig. 2).
Fig. 2. A vital role of zinc in T-cell signaling.
Schematic overview of major T cell signaling pathways, inculding TCR- (T cell receptor), IL-1R- (interleukin-1-receptor), IL-2R- and IL-6R-mediated pathways. Explanations are illustrated in the text. Black lines, signal transduction; red lines, the effect of zinc; arrows, activation; T bars, inhibition. LCK, lymphocyte-specific protein tyrosine kinase; ZAP70, zeta-chain-associated protein kinase 70; SHP-1, Src homology region 2 domain-containing phosphatase-1; Csk, C-terminal Src kinase; LAT, linker for activation of T cells; PIP2, phosphatidylinositol 4,5-bisphosphate; CREB, cAMP response element-binding protein; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTORC1, mammalian target of rapamycin complex 1; ERK, extracellular-signal-regulated kinase; STAT, signal transducer and activator of transcription; JAK, Janus kinase; MyD88, myeloid differentiation primary response 88; Zip, Zrt- and Irt-like protein.
TCR activation-meditated signaling pathway
The TCR complex is comprised of one TCRαβ heterodimer with an antigen-binding site and three CD3 subunits (CD3ζζ, CD3δε, and CD3γε) containing cytoplasmic signaling domains. Ligation of TCRαβ by cognate peptide presented by major histocompatibility (MHC) molecules on APCs induces phosphorylation of tyrosine residues in immunoreceptor tyrosine-based activation motifs (ITAMs) of the CD3 subunits by the Src-family kinase Lck. Phosphotyrosines of the CD3ζ chain provide docking sites for the tandem SH2 domains of ZAP-70 kinase, which is then phosphorylated by Lck. Recruitment of ZAP-70 to the activated TCR complex is an essential step for cooperative assembly of the immunological synapse (IS). Formation of the IS allows recruitment of additional adaptor proteins through phosphorylation or activation, and ultimately leads to activation of multiple downstream signaling cascades such as Ras/Rac/MAPK and PLC-γ pathways (Hwang et al., 2020).
Zinc has been shown to play a critical role in regulation of Lck activity. Lck associates with the cytoplasmic tail of the CD4 and CD8 coreceptors and upon TCR stimulation they bind to invariant regions of MHC molecules stabilizing the interaction between TCR and MHC-peptide. As a result, Lck is recruited into close proximity with the ITAMs of CD3. Early studies demonstrated that zinc facilitates the binding of Lck to CD4 and CD8α by acting as a molecular adhesive to bridge the two proteins (Lin et al., 1998), which is important for the initiation of TCR signaling required for T-cell development and activation (Huse et al., 1998; Kim et al., 2003; Lin et al., 1998; Romir et al., 2007). In addition to its structural role, zinc also influences the regulation of Lck activity by regulating the balance between phosphorylation of inhibitory tyrosine Y505, regulated by the PTP CD45 and the kinase Csk, and activating tyrosine Y394, which is dephosphorylated by PTPN22. While Csk activity depends on magnesium, it is inhibited by nanomolar quantities of zinc (Sun and Budde, 1999). Given that PTPs are particularly sensitive to the inhibitory action of zinc ions (Haase and Maret, 2003), PTPN22 activity may also be influenced by zinc. Inhibition of Csk and PTPN22 could contribute to retention of the active status of Lck thereby sustaining its signaling activity.
Although many zinc-specific transporters exist, certain transporters, such as Zip6 and Zip8, are highly expressed in T cells and are directly involved in T-cell activation via zinc influx (Aydemir et al., 2009; Lee et al., 2008; Yu et al., 2011). Previous reports by our group and others have revealed that Zip6, which is mainly expressed on the plasma membrane of T cells, contributes to activation-induced zinc influx from extracellular sources. Zip6-mediated zinc influx occurs very rapidly (~1 min) after TCR triggering. Of note, the accumulation of cytoplasmic zinc ions is not diffuse throughout T cells. Rather, zinc is compartmentalized in the subsynaptic area of the IS, which serves as a platform for TCR signaling transduction (Yu et al., 2011). Zinc influx into the subsynaptic area inhibits the recruitment of phosphatase SHP-1 to Lck and consequently enhances ZAP70 phosphorylation due to its hindrance of SHP-1-mediated dephosphorylation of Y394 of Lck (Chiang and Sefton, 2001; Stefanova et al., 2003). Zip6 is constitutively expressed in human and murine T cells and is further upregulated following TCR stimulation. Recently the CRISPR/Cas9 system was used to construct a Zip6 knockout (KO) human T-cell line, and studies in these cells suggest that Zip6 is a critical component of the T cell activation machinery. Reduced zinc influx in Zip6 KO T cells leads to decreased expression of T cell activation markers such as CD25 and CD69 as well as IL-2 production (Colomar-Carando et al., 2019).
A regulatory role of Zip8 has been suggested in many type of cells including T cells, monocytes/macrophages, and chondrocytes (Aydemir et al., 2009; Kim et al., 2014; Liu et al., 2013). Unlike Zip6, which is mainly expressed on the surface of cells, Zip8 is predominantly localized to lysosomes and its expression is upregulated following TCR stimulation. Activation-induced upregulation of Zip8 is responsible for the translocation of lysosomal labile zinc ions into the cytoplasm, resulting in increased intracellular zinc levels. Cytoplasmic labile zinc serves as a signaling molecule that regulates the phosphorylation of CREB (cAMP response element-binding protein), a transcription factor for IFN-γ production, via inhibition of calcineurin phosphatase activity. Sustained phosphorylation of CREB leads to production of IFN-γ and perforin, major soluble factors produced by T cells for host defense. This finding was confirmed by treatment with zinc supplements, calcineurin inhibitor FK506, and overexpression of Zip8, suggesting a potent role of Zip8 in T-cell activation and function (Aydemir et al., 2009).
Cytokine-mediated signaling pathway
Cytokines have a fundamental role in activation, proliferation, and differentiation of target cells, including immune cells, via induction of intracellular signaling cascades downstream of their specific receptors. Zinc has also been shown to modulate many signaling pathways following cytokine stimulation. Interleukin-2 (IL-2), a key T-cell growth factor produced by activated CD4+ and CD8+ T cells, elicits its effect by binding the IL-2 receptor complex mainly expressed on T cells and NK cells. IL-2 signal leads to three different intracellular signal transduction pathways, JAK1/3-STAT5, ERK1/2, and PI3K/Akt. Cytoplasmic zinc released from lysosomes is essential for ERK and PI3K/Akt activation, but not activation of STAT5 (Kaltenberg et al., 2010; Plum et al., 2014; Tanaka et al., 1990). Upon IL-2 stimulation, murine and human T-cell lines supplemented with zinc exhibit intensified phosphorylation of ERK1/2 and MEK1/2 downstream of Raf-1. This is likely due to inhibition of a phosphatase, possibly PP2A, and results in increased cell proliferation (Kaltenberg et al., 2010; Tanaka et al., 1990). Phosphorylation of Akt is also triggered by zinc and markedly abrogated by zinc depletion with TPEN in IL-2-stimulated T cells. Cell cycle progression is mediated through several signaling pathways, including PI3K/Akt, and IL-2-driven cell cycle entry into the S and G2/M phases is strictly dependent on zinc. Mechanistically, zinc inhibits the activity of a PI3K-antagonizing lipid phosphatase, PTEN, a phosphatase that negatively controls Akt activity. This occurs presumably through binding of zinc ions to cysteine thiols, which are essential for the catalytic activity of PTEN (Plum et al., 2014).
IL-6 and IL-1β are major proinflammatory cytokines abundantly secreted by activated innate immune cells such as monocytes and macrophages. Both cytokines critically contribute to differentiation of Th17 cells, a key player in the pathogenesis of many autoimmune diseases. Recently, studies have shown that zinc plays a negative role in Th17 responses by inhibiting IL-6 and IL-1β-mediated signaling pathways. In a murine model, attenuation of IL-6-mediated STAT3 activation by zinc was found to negatively regulate Th17 development in vivo and in vitro (Kitabayashi et al., 2010). Zinc enhances the IL-6-induced activation of JAK proteins and ERK1/2, whereas IL-6-induced binding of STAT3 to target DNA is restrained by zinc. This study demonstrates that zinc binding leads to an alteration of the α-helical secondary structure of STAT3, blocking the association of STAT3 with JAK2 kinase and with a phospho-peptide containing a STAT3-binding motif from gp130, a critical transducer of the IL-6 signal (Kitabayashi et al., 2010). We recently reported that increased cytoplasmic zinc in T cells suppresses IL-1β signaling via repression of IL-1 receptor-associated kinase 4 (IRAK4) phosphorylation. Thus, this has an inhibitory effect on Th17 responses facilitated by monocyte-derived IL-1β in humans (Lee et al., 2015).
CONCLUSION AND PERSPECTIVES
It is well established that zinc is a catalytic and structural cofactor for a number of proteins. However, the regulatory role of zinc in signaling transduction has only been demonstrated relatively recently, and the importance of this micronutrient has attracted attention in various research fields, including immunology. Upon activation with subset-specific stimuli, immune cells undergo a change of zinc homeostasis in the cytoplasm through zinc transporters. Cytoplasmic, bioavailable zinc actively participates in signaling pathways, mainly by inhibiting different phosphatases, to help direct robust and appropriate immune responses. However, there are still many questions to be answered. Despite their importance for regulating cytoplasmic zinc homeostasis in immune cells, the mechanism by which zinc transporters facilitate the movement of zinc ions across the cell membrane is still poorly understood. Recent studies have suggested that zinc influx is attributable to activation-induced upregulation of Zip expression in a cell-specific manner and to regulation of Zip activity through its protein phosphorylation by signaling molecules (Colomar-Carando et al., 2019; Liu et al., 2013). There is also a growing body of evidence suggesting that signaling networks mediate metabolic reprogramming in order to meet the demands of participating in immune responses. However, the effect of zinc on metabolic reprogramming in activated immune cells remains unclear. Clinically, zinc deficiency is known to be common in children in developing countries, and reports of a correlation between zinc levels and the incidence of several infectious diseases suggest zinc plays an important role for function of both the innate and adaptive immune systems. In addition, immune function declines with age and elderly people are at increased risk for zinc deficiency (Haase et al., 2006). Therefore, further research is needed to determine how zinc modulates the molecular mechanisms leading to an effective immune response in order to improve immune function and for development of potential therapeutics.
ACKNOWLEDGMENTS
The authors sincerely apologize many colleagues whose work they were unable to cite owing to space limitations. This work was supported by grants (grant No. 2018R1A2B2006310 to W.W.L.) from the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (MSIT), Republic of Korea.
Footnotes
AUTHOR CONTRIBUTIONS
B.K. and W.W.L. wrote the manuscript and B.K. created the figures.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Anzilotti C., Swan D.J., Boisson B., Deobagkar-Lele M., Oliveira C., Chabosseau P., Engelhardt K.R., Xu X., Chen R., Alvarez L., et al. An essential role for the Zn2+ transporter ZIP7 in B cell development. Nat. Immunol. 2019;20:350–361. doi: 10.1038/s41590-018-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld D.S. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:271–313. doi: 10.1023/a:1012976615056. [DOI] [PubMed] [Google Scholar]
- Aydemir T.B., Liuzzi J.P., McClellan S., Cousins R.J. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J. Leukoc. Biol. 2009;86:337–348. doi: 10.1189/jlb.1208759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S., Liu M.J., Lee B., Besecker B., Lai J.P., Guttridge D.C., Knoell D.L. Zinc modulates the innate immune response in vivo to polymicrobial sepsis through regulation of NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L744–L754. doi: 10.1152/ajplung.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo E., Massarotti A., Hogstrand C., Maret W. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics. 2014;6:1229–1239. doi: 10.1039/c4mt00086b. [DOI] [PubMed] [Google Scholar]
- Bonaventura P., Benedetti G., Albarede F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Brieger A., Rink L., Haase H. Differential regulation of TLR-dependent MyD88 and TRIF signaling pathways by free zinc ions. J. Immunol. 2013;191:1808–1817. doi: 10.4049/jimmunol.1301261. [DOI] [PubMed] [Google Scholar]
- Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125(2 Suppl 2):S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang G.G., Sefton B.M. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr-394 by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem. 2001;276:23173–23178. doi: 10.1074/jbc.M101219200. [DOI] [PubMed] [Google Scholar]
- Colomar-Carando N., Meseguer A., Company-Garrido I., Jutz S., Herrera-Fernandez V., Olvera A., Kiefer K., Brander C., Steinberger P., Vicente R. Zip6 transporter is an essential component of the lymphocyte activation machinery. J. Immunol. 2019;202:441–450. doi: 10.4049/jimmunol.1800689. [DOI] [PubMed] [Google Scholar]
- Davis S.R., Cousins R.J. Metallothionein expression in animals: a physiological perspective on function. J. Nutr. 2000;130:1085–1088. doi: 10.1093/jn/130.5.1085. [DOI] [PubMed] [Google Scholar]
- Feske S., Wulff H., Skolnik E.Y. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Dai W., Zhao L., Min J., Wang F. The role of zinc and zinc homeostasis in macrophage function. J. Immunol. Res. 2018;2018:6872621. doi: 10.1155/2018/6872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber K., Maywald M., Rosenkranz E., Haase H., Plumakers B., Rink L. Zinc deficiency adversely influences interleukin-4 and interleukin-6 signaling. J. Biol. Regul. Homeost. Agents. 2013;27:661–671. [PubMed] [Google Scholar]
- Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Haase H., Maret W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp. Cell Res. 2003;291:289–298. doi: 10.1016/s0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- Haase H., Mocchegiani E., Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7:421–428. doi: 10.1007/s10522-006-9057-3. [DOI] [PubMed] [Google Scholar]
- Haase H., Ober-Blobaum J.L., Engelhardt G., Hebel S., Heit A., Heine H., Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J. Immunol. 2008;181:6491–6502. doi: 10.4049/jimmunol.181.9.6491. [DOI] [PubMed] [Google Scholar]
- Haase H., Rink L. Signal transduction in monocytes: the role of zinc ions. Biometals. 2007;20:579–585. doi: 10.1007/s10534-006-9029-8. [DOI] [PubMed] [Google Scholar]
- Haase H., Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu. Rev. Nutr. 2009a;29:133–152. doi: 10.1146/annurev-nutr-080508-141119. [DOI] [PubMed] [Google Scholar]
- Haase H., Rink L. The immune system and the impact of zinc during aging. Immun. Ageing. 2009b;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase H., Rink L. Zinc signals and immune function. Biofactors. 2014;40:27–40. doi: 10.1002/biof.1114. [DOI] [PubMed] [Google Scholar]
- Hara T., Takeda T.A., Takagishi T., Fukue K., Kambe T., Fukada T. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017;67:283–301. doi: 10.1007/s12576-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Murakami M., Fukada T., Nishida K., Yamasaki S., Suzuki T. Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv. Immunol. 2008;97:149–176. doi: 10.1016/S0065-2776(08)00003-5. [DOI] [PubMed] [Google Scholar]
- Hojyo S., Fukada T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016;2016:6762343. doi: 10.1155/2016/6762343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Tepaamorndech S. The SLC30 family of zinc transporters - a review of current understanding of their biological and pathophysiological roles. Mol. Aspects Med. 2013;34:548–560. doi: 10.1016/j.mam.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Huse M., Eck M.J., Harrison S.C. A Zn2+ ion links the cytoplasmic tail of CD4 and the N-terminal region of Lck. J. Biol. Chem. 1998;273:18729–18733. doi: 10.1074/jbc.273.30.18729. [DOI] [PubMed] [Google Scholar]
- Hwang J.R., Byeon Y., Kim D., Park S.G. Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 2020;52:750–761. doi: 10.1038/s12276-020-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Eide D.J. The SLC39 family of zinc transporters. Mol. Aspects Med. 2013;34:612–619. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenberg J., Plum L.M., Ober-Blobaum J.L., Honscheid A., Rink L., Haase H. Zinc signals promote IL-2-dependent proliferation of T cells. Eur. J. Immunol. 2010;40:1496–1503. doi: 10.1002/eji.200939574. [DOI] [PubMed] [Google Scholar]
- Kambe T., Hashimoto A., Fujimoto S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 2014;71:3281–3295. doi: 10.1007/s00018-014-1617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Jeon J., Shin M., Won Y., Lee M., Kwak J.S., Lee G., Rhee J., Ryu J.H., Chun C.H., et al. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Kim P.W., Sun Z.Y., Blacklow S.C., Wagner G., Eck M.J. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science. 2003;301:1725–1728. doi: 10.1126/science.1085643. [DOI] [PubMed] [Google Scholar]
- Kimura T., Kambe T. The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int. J. Mol. Sci. 2016;17:336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.E., Frentzel J.W., Mann J.J., Fraker P.J. Chronic zinc deficiency in mice disrupted T cell lymphopoiesis and erythropoiesis while B cell lymphopoiesis and myelopoiesis were maintained. J. Am. Coll. Nutr. 2005;24:494–502. doi: 10.1080/07315724.2005.10719495. [DOI] [PubMed] [Google Scholar]
- Kitabayashi C., Fukada T., Kanamoto M., Ohashi W., Hojyo S., Atsumi T., Ueda N., Azuma I., Hirota H., Murakami M., et al. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int. Immunol. 2010;22:375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- Lee H., Kim B., Choi Y.H., Hwang Y., Kim D.H., Cho S., Hong S.J., Lee W.W. Inhibition of interleukin-1β-mediated interleukin-1 receptor-associated kinase 4 phosphorylation by zinc leads to repression of memory T helper type 17 response in humans. Immunology. 2015;146:645–656. doi: 10.1111/imm.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.W., Cui D., Czesnikiewicz-Guzik M., Vencio R.Z., Shmulevich I., Aderem A., Weyand C.M., Goronzy J.J. Age-dependent signature of metallothionein expression in primary CD4 T cell responses is due to sustained zinc signaling. Rejuvenation Res. 2008;11:1001–1011. doi: 10.1089/rej.2008.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.S., Rodriguez C., Veillette A., Lodish H.F. Zinc is essential for binding of p56(lck) to CD4 and CD8alpha. J. Biol. Chem. 1998;273:32878–32882. doi: 10.1074/jbc.273.49.32878. [DOI] [PubMed] [Google Scholar]
- Liu M.J., Bao S., Galvez-Peralta M., Pyle C.J., Rudawsky A.C., Pavlovicz R.E., Killilea D.W., Li C., Nebert D.W., Wewers M.D., et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep. 2013;3:386–400. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywald M., Wessels I., Rink L. Zinc signals and immunity. Int. J. Mol. Sci. 2017;18:2222. doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L.M., Brieger A., Engelhardt G., Hebel S., Nessel A., Arlt M., Kaltenberg J., Schwaneberg U., Huber M., Rink L., et al. PTEN-inhibition by zinc ions augments interleukin-2-mediated Akt phosphorylation. Metallomics. 2014;6:1277–1287. doi: 10.1039/c3mt00197k. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000;182(Suppl 1):S62–SS68. doi: 10.1086/315916. [DOI] [PubMed] [Google Scholar]
- Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A.S., Bao B., Beck F.W., Sarkar F.H. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-kappaB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Rink L., Kirchner H. Zinc-altered immune function and cytokine production. J. Nutr. 2000;130(5S Suppl):1407S–1411S. doi: 10.1093/jn/130.5.1407S. [DOI] [PubMed] [Google Scholar]
- Romir J., Lilie H., Egerer-Sieber C., Bauer F., Sticht H., Muller Y.A. Crystal structure analysis and solution studies of human Lck-SH3; zinc-induced homodimerization competes with the binding of proline-rich motifs. J. Mol. Biol. 2007;365:1417–1428. doi: 10.1016/j.jmb.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Sapkota M., Knoell D.L. Essential role of zinc and zinc transporters in myeloid cell function and host defense against infection. J. Immunol. Res. 2018;2018:4315140. doi: 10.1155/2018/4315140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.B., Maret W. The interactions of metal cations and oxyanions with protein tyrosine phosphatase 1B. Biometals. 2017;30:517–527. doi: 10.1007/s10534-017-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I., Hemmer B., Vergelli M., Martin R., Biddison W.E., Germain R.N. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat. Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- Sun G., Budde R.J. Substitution studies of the second divalent metal cation requirement of protein tyrosine kinase CSK. Biochemistry. 1999;38:5659–5665. doi: 10.1021/bi982793w. [DOI] [PubMed] [Google Scholar]
- Szewczyk B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013;5:33. doi: 10.3389/fnagi.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Shiozawa S., Morimoto I., Fujita T. Role of zinc in interleukin 2 (IL-2)-mediated T-cell activation. Scand. J. Immunol. 1990;31:547–552. doi: 10.1111/j.1365-3083.1990.tb02805.x. [DOI] [PubMed] [Google Scholar]
- Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- von Bulow V., Dubben S., Engelhardt G., Hebel S., Plumakers B., Heine H., Rink L., Haase H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J. Immunol. 2007;179:4180–4186. doi: 10.4049/jimmunol.179.6.4180. [DOI] [PubMed] [Google Scholar]
- von Bulow V., Rink L., Haase H. Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in monocytes by elevation of guanosine 3',5'-cyclic monophosphate. J. Immunol. 2005;175:4697–4705. doi: 10.4049/jimmunol.175.7.4697. [DOI] [PubMed] [Google Scholar]
- Wan Y., Petris M.J., Peck S.C. Separation of zinc-dependent and zinc-independent events during early LPS-stimulated TLR4 signaling in macrophage cells. FEBS Lett. 2014;588:2928–2935. doi: 10.1016/j.febslet.2014.05.043. [DOI] [PubMed] [Google Scholar]
- Wapnir R.A. Protein Nutrition and Mineral Absorption. CRC Press; Florida: 1990. [Google Scholar]
- Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9:1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Lee W.W., Tomar D., Pryshchep S., Czesnikiewicz-Guzik M., Lamar D.L., Li G., Singh K., Tian L., Weyand C.M., et al. Regulation of T cell receptor signaling by activation-induced zinc influx. J. Exp. Med. 2011;208:775–785. doi: 10.1084/jem.20100031. [DOI] [PMC free article] [PubMed] [Google Scholar]