Abstract
The advent of the major histocompatibility complex (MHC) multimer technology has led to a breakthrough in the quantification and analysis of antigen-specific T cells. In particular, this technology has dramatically advanced the measurement and analysis of CD8 T cells and is being applied more widely. In addition, the scope of application of MHC multimer technology is gradually expanding to other T cells such as CD4 T cells, natural killer T cells, and mucosal-associated invariant T cells. MHC multimer technology acts by complementing the T-cell receptor-MHC/peptide complex affinity, which is relatively low compared to antigen-antibody affinity, through a multivalent interaction. The application of MHC multimer technology has expanded to include various functions such as quantification and analysis of antigen-specific T cells, cell sorting, depletion, stimulation to replace antigen-presenting cells, and single-cell classification through DNA barcodes. This review aims to provide the latest knowledge of MHC multimer technology, which is constantly evolving, broaden understanding of this technology, and promote its widespread use.
Keywords: antigen-specific T cells, MHC multimer
INTRODUCTION
Recognition of peptide-major histocompatibility complex (pMHC) class I and II molecules by T cells plays a very important role in the control of intracellular pathogens and cancer cells through efficient T-cell stimulation. These interactions also play important roles in the development of autoimmune diseases. Almost all T cells express the T-cell receptor (TCR), a highly antigen-specific receptor unique to the cell surface. The TCR reacts by recognizing an antigenic peptide as a complex bound to MHC. This special interaction between the TCR and the MHC/peptide complex initiates the generation of general adaptive immunity, including cell-mediated and humoral immunity.
The overall low avidity of the TCR to the pMHC complex and the fast off-rate of this interaction make it difficult to detect antigen-specific T cells using the soluble monomeric pMHC complex. To overcome these shortcomings, MHC multimers have been prepared using antibody dimerization or tetravalent binding of biotin-streptavidin, and these MHC dimers or tetramers show increased avidity for their cognate TCR (Altman et al., 1996). To date, MHC multimers have been successfully used to visualize antigen-specific CD8 T cells ex vivo by flow cytometry. This technology enables precise detection and isolation of antigen-specific T cells, revealing a new dimension of T-cell study not only in animal model-based research but also in clinical settings.
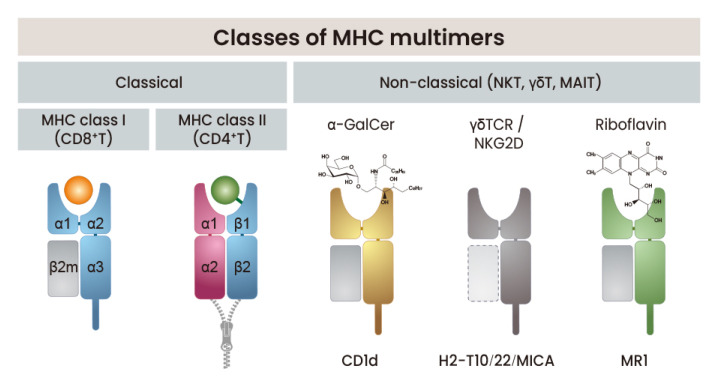
Basically, there are three classes of MHC multimers currently used for detection of various T cells (Fig. 1). MHC multimer technologies have mainly been used for the detection and analysis of CD8 T-cell responses. This is because the composition of the class I molecule is simpler to that of the MHC class II molecule; therefore, protein expression and folding can occur more easily, and the increased binding affinity and restricted length of the antigenic peptide allow the pMHC complex to form more efficiently and easily.
Fig. 1. Classes of MHC multimers.
Three classes of MHC multimers used in the detection of various T cells are schematically depicted. Classical MHC multimers include MHC class I for CD8 T cells and MHC class II for CD4 T cells. Non-classical MHC multimers have been also developed and used for the detection of NKT cells, γδ T cells, and MAIT cells.
Classical MHC multimers
Most of the MHC multimers currently used are dominated by the MHC I complex for CD8 T cells. MHC I multimers are easier to manufacture and use than MHC II multimers for the following reasons. MHC I molecules consist of heavy and light chains, both of which can refolded more easily in the presence of a peptide (Garboczi et al., 1992). In contrast, MHC II molecules consist of two polymorphic chains, both of which are noncovalently linked and form a more widely open peptide-binding groove. These complexes are structurally homogenous and can easily refold because the peptides bind to MHC I molecules in a restricted conformation. The simple and relatively efficient procedure devised in the early stage of development and refolding of denatured heavy and light chains derived from Escherichia coli with the desired peptide is still widely used.
On the other hand, efforts to develop soluble MHC II multimers for antigen-specific CD4 T-cell detection has only had limited success. Most MHC II complexes are produced by insect cell expression systems, while refolding from E. coli-expressing denatured proteins has resulted in very poor yields (Niemiec et al., 2006). The poor quality of MHC II multimer staining is because the TCR affinities of CD4 T cells are generally lower than those of CD8 T cells and because CD4 molecules stabilize MHC II binding to the TCR to a lesser degree than CD8 molecules (Huppa et al., 2010). There are also structural differences among the various MHC II molecules that can affect the expression and folding efficiencies. To increase the stability of the MHC II complex, the peptide could be covalently linked to the MHC molecule (Kozono et al., 1994).
Nonclassical MHC multimers
CD1d is a nonclassical MHC molecule that is restricted and recognized by natural killer T cells (NKT cells). CD1d binds to glycolipids of either microbial or self-origin and presents them to NKT cells. Recombinant CD1d molecules have been successfully produced in various expression systems and used in multimer forms to detect NKT cells (Sidobre and Kronenberg, 2002). Other nonclassical MHC molecules such as H2-T10, H2-T22, and MICA have also been explored for labeling specific γδ T cells (Crowley et al., 2000; Wu et al., 2002). Recently, mucosal-associated invariant T cells (MAIT cells), a special subset of T lymphocytes that recognize riboflavin (vitamin B2) metabolites as MHC class I-related protein-1 (MR1)-restricted antigens (Ags), have been identified and detected in a broad range of human and mouse tissues using MR1-ligand tetramers (Rahimpour et al., 2015; Reantragoon et al., 2013).
EVOLUTION OF MHC MULTIMERS
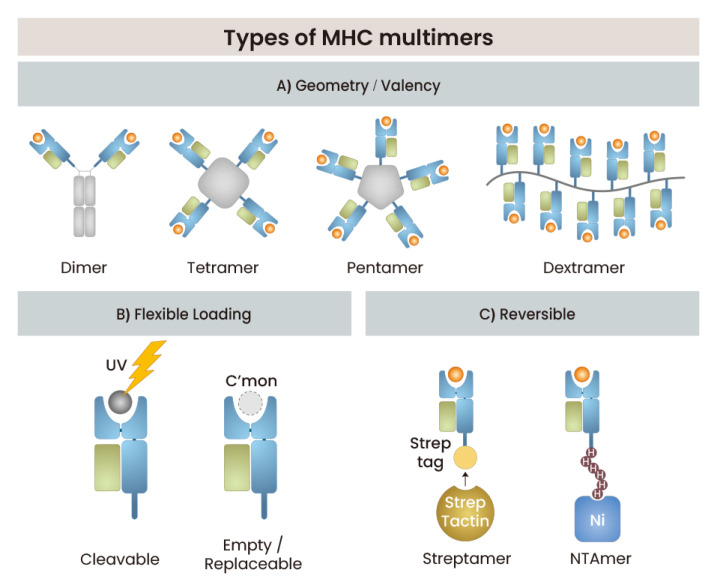
Geometry and valency
Conventional MHC tetramers are constructed by tetramerization of C-terminal biotinylated pMHC monomers with streptavidin molecules (Fig. 2A). MHC tetramers have been proven to be versatile and useful for detecting αβ T cells through flow cytometry and in situ staining. The well-established characteristics of MHC tetramers and their public (US NIH Tetramer Core Facility; https://tetramer.yerkes.emory.edu/) and commercial availability have led to their widespread usage in T-cell research. MHC tetramer-staining technology has been expanding to include new research areas such as enrichment of rare T cells, construction of large libraries and high-throughput epitope screening, cytometry by time-of-flight mass spectrometry (CyTOF), and combinatorial staining for multiple detection (Davis et al., 2011).
Fig. 2. Evolution of MHC multimers.
A schematic illustration is shown for the evolution of MHC multimer technology. It includes a variety of multimers in different geometry and valency (A), multimers with flexible loading capability (B), and variants for reversible binding (C).
An pMHC dimer was produced in the form of a fusion protein made by fusing the pMHC monomer with an IgG backbone (Greten et al., 1998). To date, various pMHC class I and II dimers, including synthetic linkers with various lengths and flexibility, have been produced and used to study TCR interactions and T-cell activation properties (Cebecauer et al., 2005; Schneck et al., 2001). The basic Ig structure of their flexible tether might allow pMHC dimers to have a high degree of accessibility to TCRs and high avidity binding.
An MHC pentamer consisting of five pMHC complexes arranged in a planar configuration is commercially produced and has increased sensitivity for staining T cells with the low-affinity TCR because of its higher valency than that of dimers or tetramers. More recently, another commercially available pMHC complex linked to a dextran backbone, a pMHC dextramer, has also been found to be useful for the detection of T cells with low-affinity TCRs because of its increased valency (Scholler et al., 2010).
An MHC NTAmer built on reversible chelate complexes of Ni2+-nitrilotriacetic acid (NTA) with a His-tag, can be dissociated into monomeric complex in the presence of free imidazole, allowing accurate measurement of dissociation rates (Schmidt et al., 2011). This MHC NTAmer is also a flexible platform in which the number of Ni2+-NTA moiety and His-tag sequences can be controlled for specific purposes to build higher-order multimers. This technique has been successfully applied for detection and sorting of antigen-specific T cells and measurement of dissociation kinetics of pMHC and TCR interactions.
Flexible loading system
MHC molecules are structurally very unstable when the peptide binding cleft is empty. For this reason, only when the antigenic peptide binding to a specific MHC allele is first determined can an MHC multimer with the peptide be produced. In addition, even if the MHC alleles are the same, individual folding and purification must be performed separately for different antigenic peptides. This disadvantage makes high-throughput T-cell screening difficult using large libraries of pMHC complexes.
Several methods have been devised to solve this problem. The first is a method of exchanging any peptide ligand of interest as a rescue peptide using a conditional pMHC complex loaded with a peptide that is degraded by a specific stimulus such as UV light (Fig. 2B) (Rodenko et al., 2006; Toebes et al., 2006). Through this UV-cleavable ligand strategy, libraries with many different types of pMHC complexes that can be used in high-throughput manner can be produced (Frosig et al., 2015). The second strategy employs preferential folding of oxidized and pre-biotinylated MHC class I heavy chain molecules, allowing efficient folding of complexes in a shorter time; this strategy be used to generate large libraries of pMHC complexes (Leisner et al., 2008). Recently, temperature-induced exchange technology has been developed; with this technology, MHC I-peptide complexes are formed at low temperatures with conditional peptides and are loaded with the peptides of choice at a defined elevated temperature (Luimstra et al., 2018). However, these exchange technologies still have disadvantages related to the inefficiency and time constraints of exchange process and the prerequisite for MHC allele-specific design and optimization.
More recently, disulfide-stabilized variants of murine and human MHC I molecules with an empty pocket have been used to make peptide-loadable MHC I tetramers (Saini et al., 2019), and the specificities of these peptide-receptive empty MHC I molecules can be determined by direct incubation with the peptide of interest. This technology might be advantageous for generating flexible and do-it-yourself reagents for T-cell analysis.
Reversible MHC multimers
The multivalent binding of an MHC multimer and the TCR is relatively stabilized, the original function of the T cell to which the MHC multimer is bound seems to be degraded (Maile et al., 2001; O'Herrin et al., 2001). However, since a monovalent interaction has a fast off-rate, isolating the untouched T cells by reversing the combined MHC multimer into a monomer may be possible. The method called “streptamer” makes use of tandem Strep-tags, which are linear peptide sequences that mimic biotins, and Strep-Tactin, which is a streptavidin mutant that has higher avidity for Strep-tag than streptavidin (Fig. 2C) (Knabel et al., 2002). The other type of reversible multimer employs desthiobiotin, a biotin derivative with a much lower binding affinity for streptavidin (Hirsch et al., 2002). These reversible tetramers can be readily dissociated in the presence of high concentrations of free biotin. Recently, a third type of reversible multimer, NTAmer that makes use of Ni2+-NTA and His-tag chelation and dissociation with free imidazole, has been reported (Schmidt et al., 2011). Sorting of antigen-specific CD8 cytotoxic T lymphocytes (CTLs) using streptamers or NTAmers produces fully functional CTLs, whereas using conventional tetramers impairs cytotoxicity and proliferation functions (Neudorfer et al., 2007; Schmitt et al., 2011).
Choice of fluorophore
The quality of streptavidin-fluorophore conjugates seems to affect the efficiency of MHC multimer staining. Commercially available streptavidin reagents are generally conjugated to PE and APC using hetero-bifunctional cross-linking reagents. This process may result in the different ratios of fluorophores to streptavidin, and consequently, variable staining intensity of MHC multimers depending on the source of streptavidin dye reagents. If the heterogeneous mixtures of streptavidin-fluorophore conjugates are fractionated with size exclusion chromatography, the higher-order conjugates, which may be brighter than lower-order ones, yields enhanced staining efficiency (Altman and Davis, 2003). Variable attachment points on streptavidin during cross-linking may have an influence on biotin-binding function depending on the location of the points. Therefore, careful selection of streptavidin-fluorophore reagents and titration procedures is important for achieving optimal performance of MHC multimers.
Choice of anti-CD8 antibody
The CD8 and CD4 coreceptors bind to the invariable regions of MHC I and MHC II molecules, respectively, and facilitate antigen recognition by T cells. CD8 stabilizes pMHC-TCR interactions through its binding to the MHC I molecule. Thus, anti-CD8 antibodies with different specificities likely affect the binding of MHC I molecules depending on their binding sites. Some anti-CD8 antibody clones have been shown to enhance pMHC-TCR on-rates and improve MHC I tetramer staining, while some other clones can disrupt staining (Clement et al., 2011; Wooldridge et al., 2006). The mechanism by which anti-CD8 antibodies exert enhancing or inhibitory effects on MHC I multimer binding remains unclear, but it is likely that anti-CD8 engages directly in MHC I multimer binding and/or local rearrangement of CD8 relative to TCR by anti-CD8 antibodies enhance or inhibit pMHC-TCR interactions (Dolton et al., 2015). Therefore, the choice of anti-CD8 antibody clones and the order of staining (e.g., staining with an MHC multimer before staining with an anti-CD8 antibody) is also important in achieving optimal performance of MHC I multimers.
Reducing the pMHC off-rate and TCR internalization
As mentioned above, low avidity between MHC and the TCR can be overcome by using MHC multimerization. In addition, several new techniques are being applied to further improve MHC multimer staining. For example, the pMHC off-rate can be reduced by adding an anti-fluorophore antibody and/or more secondary antibodies after MHC multimer staining, and MHC multimers on the cell surface can be stabilized further to increase the staining intensity (Tungatt et al., 2015). Another technique employs protein kinase inhibitors to reduce the internalization of pMHC-bound TCR, and the MHC tetramer staining intensity was reported to be significantly increased upon treatment with a protein kinase inhibitor, which is particularly effective in lower affinity T cells such as anticancer or autoimmune T cells (Lissina et al., 2009).
APPLICATION
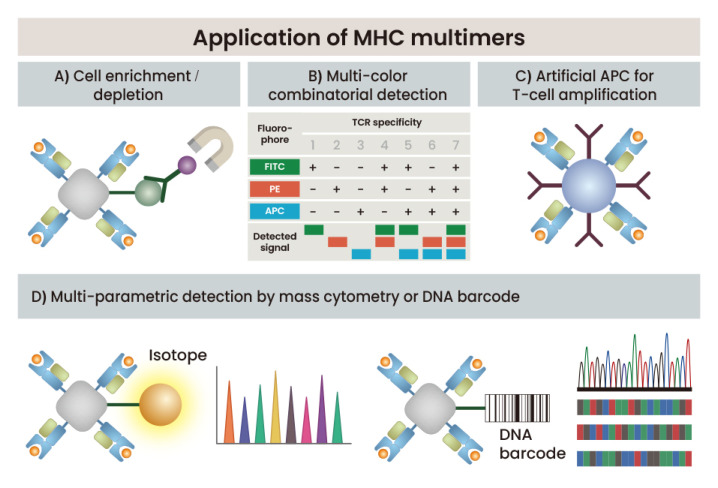
Cell enrichment using MHC multimers and detection of rare antigen-specific T cells
The number of antigen-specific T cells present in vivo is generally very low. As the immune response progresses, the number of antigen-specific T cells increases and become much greater than that of naïve T cells but is still far below 1 in 104 among all T cells. Therefore, in the past, a special system using TCR-transgenic T cells and clonotype antibodies was used for antigen-specific T-cell detection, but the advent of MHC multimer technology has made it generally possible to detect and analyze low-frequency antigen-specific T cells. Recently, a method that improves detection by more than 100 times by combining MHC multimer staining and magnetic enrichment has been reported (Fig. 3A) (Day et al., 2003). Even by detecting rare naïve CD4 T cells through this enrichment method, a new prospective approach to assessing naive T-cell repertoires, which have been very difficult to analyze, has become possible (Moon et al., 2007).
Fig. 3. Application of MHC multimers.
A schematic illustration is shown for the application of MHC multimer technology, including cell enrichment or depletion (A), combinatorial detection in a single sample (B), antigen-specific T-cell stimulation and amplification using artificial antigen-presenting complex (C), and multi-parametric detection of rare T cells using mass cytometry with isotope-labeled MHC multimers or DNA barcoded MHC multimers (D).
Multiplex MHC multimer strategies for detection of antigen-specific T cells
In general, researchers have used one color for each specific TCR in T cell detection using MHC multimers. However, if a mixture of the same MHC multimer bound to different fluorochromes is used, each specific T-cell can be distinguished according to the combination of fluorochromes (Fig. 3B) (Newell et al., 2009). Indeed, 25 different specific T-cells have been detected in a single sample through combinatorial staining with quantum dot-conjugated MHC I tetramers (Hadrup et al., 2009).
One of the disadvantages of multicolor-based flow cytometry is the need for compensation of the overlapping spectra of different fluorochromes. CyTOF eliminates the compensation problem of flow cytometry by using unique heavy metal ions to label cells (Fig. 3D) (Bandura et al., 2009; Bendall et al., 2011). This also dramatically increases the sensitivity of detection because the background noise and the interference between the metal ions is low. In addition, CyTOF with heavy metal labels is better suited for combinatorial staining because it is relatively free of spillover between labels. For example, 109 different MHC tetramers and additional 23 cellular markers have been used to characterize a single human blood sample (Newell et al., 2013). This technology might be a useful tool for identifying hard-to-find epitopes from neoantigens in a high-throughput manner (Fehlings et al., 2017).
Another approach for multi-parametric detection of antigen-specific T cells is to use unique DNA barcodes that form considerable diversity of specific tags for the given epitopes (Fig. 3D). In this method, antigen-responsive T cells are detected with a retrospective analysis of amplified sequence reads after staining of DNA barcode-labeled MHC multimers and sorting of binding T cells (Bentzen et al., 2016). The most obvious advantage of DNA barcode-based MHC multimer technology is the possibility of screening for T-cell specificity using large libraries of epitopes with a single sample. The DNA barcode-based approach is also advantageous for detecting low avidity T cells because it provides enough signals with lower levels of MHC multimer interactions than fluorochromes-based approaches.
Therapeutic use of MHC multimers
MHC multimer technology has been also used for therapeutic purposes. For example, MHC I tetramers have been used to enrich cytomegalovirus-specific CD8 T cells in adoptive cell transfer therapy for patients undergoing stem cell transplantation (Cobbold et al., 2005). The use of reversible MHC multimers might further improve the efficiency of clinical application by preserving the functional status of T lymphocytes (Knabel et al., 2002). In other studies, tetramer variants conjugated either to a radioisotope or toxin have been developed, and specific T-cell populations have been shown to be modulated or depleted by direct in vivo administration of these variants (Hess et al., 2007; Yuan et al., 2004).
Specific T-cell monitoring is important for vaccines for cancers and infectious diseases and for immunotherapy that induces T-cell responses. From this point of view, T-cell monitoring using MHC multimers is useful because in many cases, the degree of T-cell response and clinical outcomes are correlated. If MHC multimer technology is used to more closely examine the characteristics and phenotypic markers of antigen-specific T cells, our capacity to identify relevant immune correlates will be increased for the success of the treatment.
With the advent of technologies such as adaptive cell transfer and checkpoint blockade, interest in immunotherapy using T lymphocytes continues to increase. Usually, the process for immunotherapy is patient-specific, and it is essential to secure a large number of antigen-specific T cells that maintain their functionality and activity. However, APCs extracted from a patient's own body are limited in number and are often deteriorated functionally, which is disadvantageous for use in in vitro amplification of autologous T cells. Soluble forms of pMHC molecules can be used as artificial APCs when immobilized on solid supports (Curtsinger et al., 1997; Oelke et al., 2003) and have the potential to overcome the limitations associated with autologous dendritic cell-based stimulation (Fig. 3C). Artificial APC systems using soluble pMHC complexes have several advantages in expanding antigen-specific T cells for adoptive transfer immunotherapy, such as ease of optimization, prepared development under good manufacturing practice conditions, and provision of off-the-shelf reagents for clinical use.
CONCLUSION
Since its introduction, MHC multimer technology has been very useful in many areas of immunological research. Over the past few years, its scope, availability, and usage have continuously increased, and further improvements will continue to be made in the future. Remarkably, the development of high-throughput production techniques of MHC multimers and the combination of multi-parameter analysis are making MHC multimer technology more and more applicable to suitable fields. Growing epitope databases will also allow researchers to efficiently apply MHC multimer technology more widely. Therefore, users and potential users of MHC multimers will be able to greatly contribute to the advancement of immunology by studying how the advantages of these reagents can be used in their own research and to the development of new vaccines and therapeutics.
ACKNOWLEDGMENTS
This study was supported by a grant of Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant No. HV20C0049 and HV20C0156).
Footnotes
CONFLICT OF INTEREST
The author has no potential conflicts of interest to disclose.
REFERENCES
- Altman J.D., Davis M.M. MHC-peptide tetramers to visualize antigen-specific T cells. Curr. Protoc. Immunol. 2003;53:17.3.1–17.3.33. doi: 10.1002/cpim.14. [DOI] [PubMed] [Google Scholar]
- Altman J.D., Moss P.A., Goulder P.J., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Bandura D.R., Baranov V.I., Ornatsky O.I., Antonov A., Kinach R., Lou X., Pavlov S., Vorobiev S., Dick J.E., Tanner S.D. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 2009;81:6813–6822. doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- Bendall S.C., Simonds E.F., Qiu P., Amir el A.D., Krutzik P.O., Finck R., Bruggner R.V., Melamed R., Trejo A., Ornatsky O.I., et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzen A.K., Marquard A.M., Lyngaa R., Saini S.K., Ramskov S., Donia M., Such L., Furness A.J., McGranahan N., Rosenthal R., et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat. Biotechnol. 2016;34:1037–1045. doi: 10.1038/nbt.3662. [DOI] [PubMed] [Google Scholar]
- Cebecauer M., Guillaume P., Mark S., Michielin O., Boucheron N., Bezard M., Meyer B.H., Segura J.M., Vogel H., Luescher I.F. CD8+ cytotoxic T lymphocyte activation by soluble major histocompatibility complex-peptide dimers. J. Biol. Chem. 2005;280:23820–23828. doi: 10.1074/jbc.M500654200. [DOI] [PubMed] [Google Scholar]
- Clement M., Ladell K., Ekeruche-Makinde J., Miles J.J., Edwards E.S., Dolton G., Williams T., Schauenburg A.J., Cole D.K., Lauder S.N., et al. Anti-CD8 antibodies can trigger CD8+ T cell effector function in the absence of TCR engagement and improve peptide-MHCI tetramer staining. J. Immunol. 2011;187:654–663. doi: 10.4049/jimmunol.1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold M., Khan N., Pourgheysari B., Tauro S., McDonald D., Osman H., Assenmacher M., Billingham L., Steward C., Crawley C., et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J. Exp. Med. 2005;202:379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M.P., Fahrer A.M., Baumgarth N., Hampl J., Gutgemann I., Teyton L., Chien Y. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- Curtsinger J., Deeths M.J., Pease P., Mescher M.F. Artificial cell surface constructs for studying receptor-ligand contributions to lymphocyte activation. J. Immunol. Methods. 1997;209:47–57. doi: 10.1016/s0022-1759(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Davis M.M., Altman J.D., Newell E.W. Interrogating the repertoire: broadening the scope of peptide-MHC multimer analysis. Nat. Rev. Immunol. 2011;11:551–558. doi: 10.1038/nri3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C.L., Seth N.P., Lucas M., Appel H., Gauthier L., Lauer G.M., Robbins G.K., Szczepiorkowski Z.M., Casson D.R., Chung R.T., et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Invest. 2003;112:831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolton G., Tungatt K., Lloyd A., Bianchi V., Theaker S.M., Trimby A., Holland C.J., Donia M., Godkin A.J., Cole D.K., et al. More tricks with tetramers: a practical guide to staining T cells with peptide-MHC multimers. Immunology. 2015;146:11–22. doi: 10.1111/imm.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlings M., Simoni Y., Penny H.L., Becht E., Loh C.Y., Gubin M.M., Ward J.P., Wong S.C., Schreiber R.D., Newell E.W. Checkpoint blockade immunotherapy reshapes the high-dimensional phenotypic heterogeneity of murine intratumoural neoantigen-specific CD8(+) T cells. Nat. Commun. 2017;8:562. doi: 10.1038/s41467-017-00627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosig T.M., Yap J., Seremet T., Lyngaa R., Svane I.M., Thor Straten P., Heemskerk M.H., Grotenbreg G.M., Hadrup S.R. Design and validation of conditional ligands for HLA-B*08:01, HLA-B*15:01, HLA-B*35:01, and HLA-B*44:05. Cytometry A. 2015;87:967–975. doi: 10.1002/cyto.a.22689. [DOI] [PubMed] [Google Scholar]
- Garboczi D.N., Hung D.T., Wiley D.C. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc. Natl. Acad. Sci. U. S. A. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten T.F., Slansky J.E., Kubota R., Soldan S.S., Jaffee E.M., Leist T.P., Pardoll D.M., Jacobson S., Schneck J.P. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19- specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc. Natl. Acad. Sci. U. S. A. 1998;95:7568–7573. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup S.R., Bakker A.H., Shu C.J., Andersen R.S., van Veluw J., Hombrink P., Castermans E., Thor Straten P., Blank C., Haanen J.B., et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat. Methods. 2009;6:520–526. doi: 10.1038/nmeth.1345. [DOI] [PubMed] [Google Scholar]
- Hess P.R., Barnes C., Woolard M.D., Johnson M.D., Cullen J.M., Collins E.J., Frelinger J.A. Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood. 2007;109:3300–3307. doi: 10.1182/blood-2006-06-028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J.D., Eslamizar L., Filanoski B.J., Malekzadeh N., Haugland R.P., Beechem J.M., Haugland R.P. Easily reversible desthiobiotin binding to streptavidin, avidin, and other biotin-binding proteins: uses for protein labeling, detection, and isolation. Anal. Biochem. 2002;308:343–357. doi: 10.1016/s0003-2697(02)00201-4. [DOI] [PubMed] [Google Scholar]
- Huppa J.B., Axmann M., Mortelmaier M.A., Lillemeier B.F., Newell E.W., Brameshuber M., Klein L.O., Schutz G.J., Davis M.M. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabel M., Franz T.J., Schiemann M., Wulf A., Villmow B., Schmidt B., Bernhard H., Wagner H., Busch D.H. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat. Med. 2002;8:631–637. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- Kozono H., White J., Clements J., Marrack P., Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- Leisner C., Loeth N., Lamberth K., Justesen S., Sylvester-Hvid C., Schmidt E.G., Claesson M., Buus S., Stryhn A. One-pot, mix-and-read peptide-MHC tetramers. PLoS One. 2008;3:e1678. doi: 10.1371/journal.pone.0001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissina A., Ladell K., Skowera A., Clement M., Edwards E., Seggewiss R., van den Berg H.A., Gostick E., Gallagher K., Jones E., et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J. Immunol. Methods. 2009;340:11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luimstra J.J., Garstka M.A., Roex M.C.J., Redeker A., Janssen G.M.C., van Veelen P.A., Arens R., Falkenburg J.H.F., Neefjes J., Ovaa H. A flexible MHC class I multimer loading system for large-scale detection of antigen-specific T cells. J. Exp. Med. 2018;215:1493–1504. doi: 10.1084/jem.20180156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile R., Wang B., Schooler W., Meyer A., Collins E.J., Frelinger J.A. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J. Immunol. 2001;167:3708–3714. doi: 10.4049/jimmunol.167.7.3708. [DOI] [PubMed] [Google Scholar]
- Moon J.J., Chu H.H., Pepper M., McSorley S.J., Jameson S.C., Kedl R.M., Jenkins M.K. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorfer J., Schmidt B., Huster K.M., Anderl F., Schiemann M., Holzapfel G., Schmidt T., Germeroth L., Wagner H., Peschel C., et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J. Immunol. Methods. 2007;320:119–131. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Newell E.W., Klein L.O., Yu W., Davis M.M. Simultaneous detection of many T-cell specificities using combinatorial tetramer staining. Nat. Methods. 2009;6:497–499. doi: 10.1038/nmeth.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell E.W., Sigal N., Nair N., Kidd B.A., Greenberg H.B., Davis M.M. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat. Biotechnol. 2013;31:623–629. doi: 10.1038/nbt.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemiec P.K., Read L.R., Sharif S. Synthesis of chicken major histocompatibility complex class II oligomers using a baculovirus expression system. Protein Expr. Purif. 2006;46:390–400. doi: 10.1016/j.pep.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Oelke M., Maus M.V., Didiano D., June C.H., Mackensen A., Schneck J.P. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat. Med. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- O’Herrin S.M., Slansky J.E., Tang Q., Markiewicz M.A., Gajewski T.F., Pardoll D.M., Schneck J.P., Bluestone J.A. Antigen-specific blockade of T cells in vivo using dimeric MHC peptide. J. Immunol. 2001;167:2555–2560. doi: 10.4049/jimmunol.167.5.2555. [DOI] [PubMed] [Google Scholar]
- Rahimpour A., Koay H.F., Enders A., Clanchy R., Eckle S.B., Meehan B., Chen Z., Whittle B., Liu L., Fairlie D.P., et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reantragoon R., Corbett A.J., Sakala I.G., Gherardin N.A., Furness J.B., Chen Z., Eckle S.B., Uldrich A.P., Birkinshaw R.W., Patel O., et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenko B., Toebes M., Hadrup S.R., van Esch W.J., Molenaar A.M., Schumacher T.N., Ovaa H. Generation of peptide-MHC class I complexes through UV-mediated ligand exchange. Nat. Protoc. 2006;1:1120–1132. doi: 10.1038/nprot.2006.121. [DOI] [PubMed] [Google Scholar]
- Saini S.K., Tamhane T., Anjanappa R., Saikia A., Ramskov S., Donia M., Svane I.M., Jakobsen S.N., Garcia-Alai M., Zacharias M., et al. Empty peptide-receptive MHC class I molecules for efficient detection of antigen-specific T cells. Sci. Immunol. 2019;4:eaau9039. doi: 10.1126/sciimmunol.aau9039. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Guillaume P., Irving M., Baumgaertner P., Speiser D., Luescher I.F. Reversible major histocompatibility complex I-peptide multimers containing Ni(2+)-nitrilotriacetic acid peptides and histidine tags improve analysis and sorting of CD8(+) T cells. J. Biol. Chem. 2011;286:41723–41735. doi: 10.1074/jbc.M111.283127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A., Tonn T., Busch D.H., Grigoleit G.U., Einsele H., Odendahl M., Germeroth L., Ringhoffer M., Ringhoffer S., Wiesneth M., et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51:591–599. doi: 10.1111/j.1537-2995.2010.02940.x. [DOI] [PubMed] [Google Scholar]
- Schneck J.P., Slansky J.E., O'Herrin S.M., Greten T.F. Monitoring antigen-specific T cells using MHC-Ig dimers. Curr. Protoc. Immunol. 2001;35:17.2.1–17.2.17. doi: 10.1002/0471142735.im1702s35. [DOI] [PubMed] [Google Scholar]
- Scholler J., Singh M., Bergmeier L., Brunstedt K., Wang Y., Whittall T., Rahman D., Pido-Lopez J., Lehner T. A recombinant human HLA-class I antigen linked to dextran elicits innate and adaptive immune responses. J. Immunol. Methods. 2010;360:1–9. doi: 10.1016/j.jim.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Sidobre S., Kronenberg M. CD1 tetramers: a powerful tool for the analysis of glycolipid-reactive T cells. J. Immunol. Methods. 2002;268:107–121. doi: 10.1016/s0022-1759(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Toebes M., Coccoris M., Bins A., Rodenko B., Gomez R., Nieuwkoop N.J., van de Kasteele W., Rimmelzwaan G.F., Haanen J.B., Ovaa H., et al. Design and use of conditional MHC class I ligands. Nat. Med. 2006;12:246–251. doi: 10.1038/nm1360. [DOI] [PubMed] [Google Scholar]
- Tungatt K., Bianchi V., Crowther M.D., Powell W.E., Schauenburg A.J., Trimby A., Donia M., Miles J.J., Holland C.J., Cole D.K., et al. Antibody stabilization of peptide-MHC multimers reveals functional T cells bearing extremely low-affinity TCRs. J. Immunol. 2015;194:463–474. doi: 10.4049/jimmunol.1401785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge L., Scriba T.J., Milicic A., Laugel B., Gostick E., Price D.A., Phillips R.E., Sewell A.K. Anti-coreceptor antibodies profoundly affect staining with peptide-MHC class I and class II tetramers. Eur. J. Immunol. 2006;36:1847–1855. doi: 10.1002/eji.200635886. [DOI] [PubMed] [Google Scholar]
- Wu J., Groh V., Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J. Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- Yuan R.R., Wong P., McDevitt M.R., Doubrovina E., Leiner I., Bornmann W., O'Reilly R., Pamer E.G., Scheinberg D.A. Targeted deletion of T-cell clones using alpha-emitting suicide MHC tetramers. Blood. 2004;104:2397–2402. doi: 10.1182/blood-2004-01-0324. [DOI] [PubMed] [Google Scholar]