Abstract
Macrophages residing in various tissue types are unique in terms of their anatomical locations, ontogenies, developmental pathways, gene expression patterns, and immunological functions. Alveolar macrophages (AMs) reside in the alveolar lumen of the lungs and serve as the first line of defense for the respiratory tract. The immunological functions of AMs are implicated in the pathogenesis of various pulmonary diseases such as allergic asthma, chronic obstructive pulmonary disorder (COPD), pulmonary alveolar proteinosis (PAP), viral infection, and bacterial infection. Thus, the molecular mechanisms driving the development and function of AMs have been extensively investigated. In this review article, we discuss the roles of granulocyte-macrophage colony-stimulating factor (GM-CSF) and transforming growth factor (TGF)-β in AM development, and provide an overview of the anti-inflammatory and pro-inflammatory functions of AMs in various contexts. Notably, we examine the relationships between the metabolic status of AMs and their development processes and functions. We hope that this review will provide new information and insight into AM development and function.
Keywords: alveolar macrophage, development, fine-tuning, GM-CSF, immunological functions, immunometabolism, ontogeny
INTRODUCTION
Macrophages are a major group of innate immune cells that reside in various tissues, where they play essential roles as immune system sentinels. Macrophages express various proteins such as pattern recognition receptors and scavenger receptors that enable them to sense immunological stimuli, and to initiate and amplify immunological responses (Canton et al., 2013; Kawai and Akira, 2010). In addition, macrophages participate in homeostatic functions, such as clearance of dead cells and secretion of tissue repair factors (Elliott et al., 2017; Wynn et al., 2016). Therefore, understanding the molecular mechanisms that drive the development and functions of macrophages is crucial to understanding the immune system.
Recent studies suggest that subsets of tissue-resident macrophages differ in terms of their ontological backgrounds, developmental pathways, and immunological functions, among other features (Davies et al., 2013; Gentek et al., 2014; Hoeffel and Ginhoux, 2018). Investigations into the molecular mechanisms behind the development and functions of tissue-resident macrophages have revealed that tissue environment, signaling pathways, and transcription factors drive variations in tissue-resident macrophages among various organs (Lavin et al., 2015). Alveolar macrophages (AMs) are resident macrophages of the airways and lungs, where they serve as the primary immune sentinels of the respiratory tract, express specific surface markers such as Siglec-F and CD11c, and participate in surfactant clearance (Lavin et al., 2015). AM dysfunction is associated with various lung diseases such as chronic obstructive pulmonary disorder (COPD), acute lung injury, pulmonary fibrosis, and pulmonary alveolar proteinosis (PAP) (Antoniu et al., 2020; Li et al., 2019; O'Beirne et al., 2020; Song et al., 2019). Currently, conventional therapeutic strategies for these lung diseases are limited in their effectiveness (Gross and Barnes, 2017; Hindelang et al., 2020). Thus, the application of an AM-targeted strategy could be a promising new therapeutic approach. Therefore, a comprehensive understanding of the developmental pathways and immunological functions of AMs could aid in the development of novel therapeutics for lung diseases.
In this review, we discuss the current models for AM development and immunological functions of AMs, as well as the relationships of metabolic status of AMs and their development and function.
ONTOGENY AND ORIGIN OF AMs
Circulating monocytes minimally contribute to the AM pool
Until recently, macrophages were thought to originate from circulating monocytes. However, recent studies using genetically engineered, parabiotic, or fate-mapping mice have suggested otherwise. Monocytes infiltrate target tissues under pathological conditions such as osteoarthritis, pancreatic cancer, and steatohepatitis by expressing the C-C chemokine receptor type 2 (CCR2) (Han et al., 1998; Krenkel et al., 2018). Thus, researchers expected that CCR2 knockout mice would have a lower AM count, compared with control mice. However, the proportions and numbers of AMs were comparable between CCR2 knockout and control mice, suggesting that circulating monocytes contribute minimally to the AM pool (Hashimoto et al., 2013). Furthermore, circulating monocytes exhibited almost perfect chimerism in parabiotic mice, but AMs did not exhibit chimerism, despite 5 months of parabiosis (Hashimoto et al., 2013). Moreover, the tdTomato signals of Mx1-cre × R26Tomato and S100a4-cre × R26Tomato fate-mapping mice were high in circulating monocytes, but low in AMs, indicating that circulating monocytes rarely serve as precursors of AMs (Hoeffel et al., 2015). Taken together, these findings indicate that circulating monocytes contribute minimally to the AM pool.
Embryonic origin of AMs
Recent evidence suggests that AMs may originate from fetal macrophages during embryonic hematopoiesis, which occurs in two waves. The first wave, termed primitive hematopoiesis, takes place in the blood islands of mouse embryonic yolk sacs (YSs) around embryonic day 7.5 and gives rise to YS monocytes, which migrate into and seed peripheral tissues such as the lungs (Yamane, 2018). The second wave, termed definitive hematopoiesis, occurs in the fetal liver (FL) around embryonic day 12.5 and gives rise to FL monocytes, which also migrate into and seed peripheral tissues (Sugiyama et al., 2011). Upon analysis of c-fms-EGFP mice that enables tracing of embryonic lung macrophages, it has been demonstrated that two distinct subsets of AM precursors such as F4/80+ and Mac2+ cells derived from YS and FL, respectively (Tan and Krasnow, 2016). Although the contribution of each wave to the AM pool remains unclear, studies using runt-related transcription factor 1 (RUNX1) and colony-stimulating factor 1 receptor (CSFR1) fate-mapping mice suggest that AMs originate from FL monocytes (Hoeffel et al., 2015). RUNX1 is expressed by cells of YS monocytic origin. In the fate-mapping study, AMs exhibited low RUNX1 expression levels, whereas brain microglia exhibited high RUNX1 levels. These findings imply that microglia, but not AMs, originate from YS monocytes. Moreover, the AMs of CSFR1 fate-mapping mice, which also enable the tracing of cells originating from YS monocytes, exhibited low CSFR1 expression.
Adoptive transfer experiments, in which YS monocytes, FL monocytes, and bone marrow-derived monocytes were transferred into AM-deficient mice (CSF2 receptor subunit beta-knockout mice), revealed that AM pool colonization was dominated by FL monocytes. This suggests that FL monocytes are more likely to be precursors to AMs, compared with the potential for YS monocytes or bone marrow-derived macrophages to serve as precursors (van de Laar et al., 2016). However, these data should be interpreted with caution. Although these studies suggest that FL monocytes contribute to AM formation, they do not exclude the possibility that other macrophage/monocytic cell types could also serve as precursors for AMs. Indeed, individual adoptive transfer experiments of YS monocytes, FL monocytes, or bone marrow-derived monocytes into AM-deficient mice resulted in similar populations of AMs, suggesting that each precursor has the potential to differentiate into AMs. Furthermore, it has been demonstrated that circulating monocytes replace AMs in γ-herpesvirus-infected or fibrotic lungs. Based on these findings, it is also feasible that circulating monocytes might be one of AM precursors under inflammatory conditions (Machiels et al., 2017; Misharin et al., 2017).
The studies discussed above suggest that AMs originate from FL monocytes and do not require continuous replenishment of circulating monocytes to maintain their pool. The abilities of YS monocytes and bone marrow-derived monocytes to differentiate into AMs indicate the existence of compensatory mechanisms for AM development. YS monocytes or circulating monocytes might serve as compensatory AM precursors when the differentiation of FL monocytes into AMs is limited due to inflammation.
DEVELOPMENT OF AMs
GM-CSF–PPAR-γ axis critically contributes to the development of AMs
Macrophage colony-stimulating factor (M-CSF) is a master cytokine that regulates the overall developmental processes of several monocyte/macrophage lineage cells (Ushach and Zlotnik, 2016). However, M-CSF is minimally involved in the development and cellular responses of AMs (Draijer et al., 2019). Thus, unlike several other monocyte/macrophage lineage subsets, AMs are likely to rely on factors other than M-CSF for their development. Moreover, several studies have demonstrated the critical role of granulocyte-macrophage colony-stimulating factor (GM-CSF) in AM development. The functional link between GM-CSF and AMs was first recognized in the 1990s, on the basis of studies with GM-CSF-deficient and GM-CSF receptor β-chain (GM-CSFR βc)-deficient mice (Nishinakamura et al., 1996; Stanley et al., 1994). Histological analyses of the lungs from the GM-CSF-deficient mice revealed pathological alterations that resembled human PAP, characterized by the accumulation of amorphous eosinophilic materials in alveolar spaces due to impaired surfactant production by AMs. Furthermore, intranasal instillation of exogenous GM-CSF restored AM populations in GM-CSF-deficient mice, indicating the critical role of GM-CSF in AM development (Guilliams et al., 2013). Recent studies suggest that GM-CSF is involved in the early development of AMs, beginning during their embryonic developmental stages. GM-CSF levels are relatively high in mouse lungs from embryonic day 17.5 and increase sharply on the day of mouse birth (Guilliams et al., 2013). This sharp increase in lung GM-CSF levels coincides temporally with AM precursor differentiation into AMs (Guilliams et al., 2013). Furthermore, GM-CSF-deficient mice have comparatively fewer AM precursors at various embryonic stages. GM-CSF increases the expression and functional activity of the transcription factor peroxisome proliferator-activated receptor gamma (PPAR-γ) (Schneider et al., 2014), which regulates AM developmental processes as a master transcription factor. The AMs of PPAR-γ-deficient mice are relatively low in number and exhibit multiple abnormalities; this phenotype is similar to that of GM-CSF-deficient or GM-CSFR βc-deficient mice. Collectively, these findings indicate that the GM-CSF–PPAR-γ axis plays a critical role in the development of AMs.
Fine-tuning of GM-CSFR signaling in AM development and function
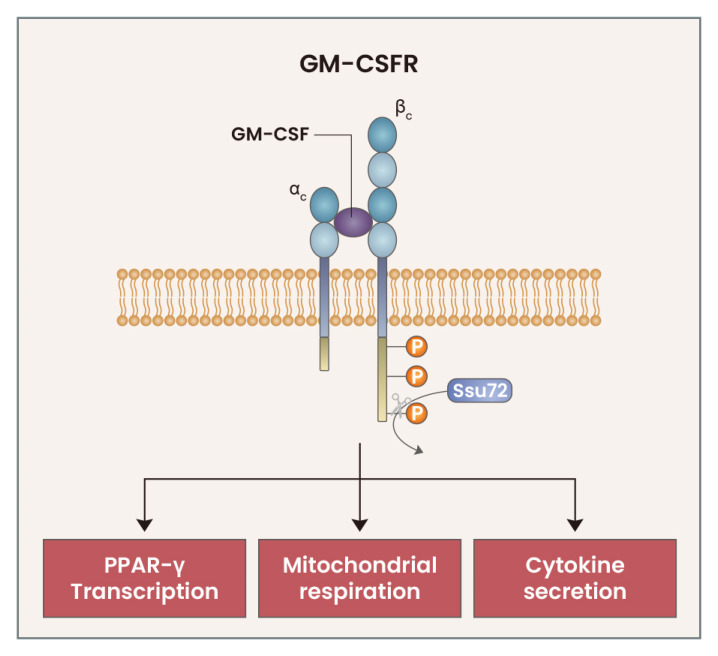
The absence of GM-CSF or GM-CSFR signaling results in AM developmental defects; paradoxically, the overexpression of GM-CSF or GM-CSFR signaling components produces similar defective developmental phenotypes in AMs. Surfactant protein surfactant protein C-granulocyte macrophage (SPC-GM) mice, which have a marked elevation in lung GM-CSF levels compared with wild-type mice, exhibit increased AM accumulation in juvenile stages, but have multiple AM abnormalities following mouse maturity (Huffman Reed et al., 1997). Although the previous study of SPC-GM mice indicated the detrimental effects of increased GM-CSF levels in AM development, we recently found that excessive GM-CSFR signaling also exerts detrimental effects on AM development (Woo et al., 2021). GM-CSFR is composed of two chains: an α-chain, which directly binds to GM-CSF, and a β-chain, which is mainly involved in signal transduction (Hercus et al., 2012). Upon binding of GM-CSF to the GM-CSFR α-chain, GM-CSFR forms a heterodimer complex. Then, signal transduction is initiated by the phosphorylation of GM-CSFR βc, followed by the phosphorylation of downstream proteins such as Janus kinase (JAK)2, signal transducer and activator of transcription (STAT)5, and protein kinase b (PKB) (Hercus et al., 2009). Following GM-CSF treatment, AMs rapidly upregulate expression of the RNA Polymerase II subunit A C-terminal domain phosphatase SSU72 (Ssu72), which directly binds to and de-phosphorylates GM-CSFR βc, thus reducing GM-CSFR signaling strength. GM-CSFR βc and its downstream signaling proteins are excessively phosphorylated in Ssu72-deficient AMs, which leads to AM developmental defects and multiple abnormalities in cell number, cell cycle, proliferation, cell death, and various functions. These abnormalities were fully rescued following treatment with a JAK2 inhibitor, which led to downregulation of the excessive GM-CSF signaling (Woo et al., 2021). These results indicate that the strength of GM-CSF signaling plays a critical role in the development and functions of AMs, and that Ssu72 is an important regulatory protein involved in the fine-tuning of GM-CSFR signaling (Fig. 1).
Fig. 1. Fine-tuning of GM-CSFR signaling in AMs.
Upon binding of GM-CSF to the GM-CSFR, the GM-CSFR βc is phosphorylated, which initiates downstream signaling. GM-CSFR signaling triggers the rapid upregulation of the phosphatase Ssu72, which directly binds to and dephosphorylates the GM-CSFR βc. This fine-tuning mechanism for GM-CSFR signaling is critical for the PPAR-γ, as well as mitochondrial respiration and cytokine secretion, in AMs.
Role of TGF-β in AM development
In addition to GM-CSF, transforming growth factor (TGF)-β plays a critical role in AM development (Yu et al., 2017). FL monocytes continuously produce TGF-β, which drives the differentiation of FL monocytes into AMs via autocrine and paracrine processes. The selective deletion of TGF-β receptor 2 (TGFβR2) in AMs of adult mice using a human estrogen receptor-Cre recombinase system leads to the dysregulation of AM homeostasis and a reduction in PPAR-γ expression; this suggests that AMs require continuous TGF-β signaling to maintain homeostasis, which might be associated with PPAR-γ. However, the exact mechanism by which TGF-β regulates PPAR-γ expression and transcriptional activity in AMs remains unclear. Han et al. (2000) demonstrated that TGF-β treatment increased the phosphorylation of mitogen-activated protein kinase (MAPK) and of PPAR-γ in THP-1 macrophages, while treatment of low dose of TGF-β induced upregulation of PPAR-γ expression and transcriptional activity via homologues of the Drosophila protein, mothers against decapentaplegic (Mad) and the Caenorhabditis elegans protein Sma-3 (SMAD3) signaling in murine lung fibroblasts (Ramirez et al., 2012). These could be the molecular mechanism behind the TGF-β-mediated regulation of PPAR-γ in AMs, but further investigation is needed to confirm this hypothesis.
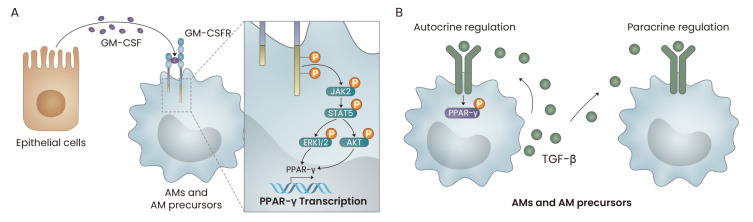
GM-CSF, PPAR-γ and TGF-β-mediated signaling pathways are critical for AM development. Mechanistically, signaling induced by GM-CSF increases the expression level and transcriptional activity of PPAR-γ. In addition, the fine-tuning of the strength of GM-CSF signaling is required for proper AM development. However, the fine-tuning of TGF-β-mediated signaling in AMs has not yet been reported; such investigations could provide valuable insights into the fine-tuning machinery of innate immune cells such as AMs (Fig. 2).
Fig. 2. The roles of GM-CSF and TGF-β in the development of AMs.
(A) GM-CSF, secreted by lung epithelial cells, induces the JAK2/STAT5-mediated transcription of PPAR-γ in AMs. (B) TGF-β is produced by adult AMs, and the cellular precursors of AMs then phosphorylate PPAR-γ in an autocrine and/or paracrine manner.
IMMUNOLOGICAL FUNCTIONS OF AMs
Anti-inflammatory roles of AMs under homeostatic conditions
Under homeostatic conditions, AMs reside in alveolar spaces, where they are the main effectors of immune responses (Rubins, 2003). Following exposure of the respiratory tract to various external stimuli, AMs exhibit anti-inflammatory activity within the alveolar spaces by means of efferocytosis (Ortega-Gomez et al., 2013). Various in vivo and in vitro studies have demonstrated that AM-driven efferocytosis prevents dead cells from inducing pro-inflammatory or immunological responses in alveoli (Grabiec and Hussell, 2016; Kim et al., 2018; Krysko et al., 2006; Mohning et al., 2018). Moreover, efferocytosis promotes the secretion of anti-inflammatory factors such as TGF-β, prostaglandin E2 (PGE2), and platelet-activating factor (PAF) by AMs, further suppressing inflammatory responses (Fadok et al., 1998; Huynh et al., 2002). Notably, macrophages from patients with severe asthma or COPD have poor phagocytic ability, compared with macrophages from healthy controls. This difference may contribute to chronic inflammation in patients with respiratory diseases (Fitzpatrick et al., 2008; Hodge et al., 2003).
AMs also participate in immunosuppression by promoting the generation of regulatory T (Treg) cells (Coleman et al., 2013; Soroosh et al., 2013). Compared with ovalbumin-pulsed dendritic cells, ovalbumin-pulsed AMs more strongly induce the differentiation of OT-II CD4+T cells into functional Foxp3+ Treg cells in co-culture systems (Soroosh et al., 2013). Furthermore, adoptive transfer of antigen-pulsed AMs inhibits lung inflammation by promoting Treg cells, indicating that AMs contribute to the generation of functional Treg cells in vivo. AMs promote Treg cell generation by producing key factors, such as TGF-β and the retinal dehydrogenases 1 & 2 (RALDH1 and RALDH2) (Bazewicz et al., 2019). In laryngeal squamous cell carcinoma, Treg cells promote the differentiation of monocytes into AM-like macrophages, which suggests that there is a positive feedback loop between AM and Treg cell generation (Sun et al., 2017).
The alveolar microenvironment actively participates in continuous signaling to promote immunosuppressive activity in AMs (Guth et al., 2009). In vitro co-culturing of bronchial epithelial cells and AMs reduces the AM inflammatory response via soluble factors produced by the bronchial epithelial cells and cell-to-cell contact (Mayer et al., 2008). Alveolar epithelial cells promote anti-inflammatory activity in AMs by producing interleukin (IL)-10 and TGF-β-activating integrin αvβ6 (Mayer et al., 2008). Moreover, continuous cluster of differentiation 200 (CD200)-mediated CD200 receptor signaling by type II alveolar epithelial cells suppresses the c-Jun N-terminal kinases (JNK), p38 mitogen-activated protein kinases (p38), and extracellular signal-regulated kinases (ERK) signaling pathways in AMs, which in turn suppresses the expression of pro-inflammatory cytokines (Koning et al., 2010). In addition, several mannose receptor ligands expressed on type II alveolar epithelial cells are recognized by AM mannose receptors, which blocks the recognition of toll-like receptor (TLR) 4 ligands (Steele et al., 2003; Zhang et al., 2005). The activation of tripartite motif-containing protein 2 (TRIM2) expressed by AMs also restricts pro-inflammatory AM activity (Gao et al., 2013); however, evidence of TRIM2 ligand expression by type II alveolar epithelial cells has not been found. Taken together, these results suggest that AMs play anti-inflammatory roles in the alveolar microenvironment via various mechanisms.
Pro-inflammatory roles of AMs under inflammatory conditions
Although AMs play an immunosuppressive role in non-inflammatory alveolar spaces, AMs can switch to perform various pro-inflammatory functions (Duan et al., 2017; Huang et al., 2018; Soni et al., 2016; Tsai et al., 2019; Wilson et al., 2020; Yeligar et al., 2016). The destruction of airway epithelia and the associated loss of immunosuppressive ligands induces switching of immunosuppressive AMs into their pro-inflammatory state (Bissonnette et al., 2020; Fujii et al., 2002; Kaur et al., 2015; Moon et al., 2015). The ligands of several pattern recognition receptors such as TLR 2, 4 and 9, inhibit IL-10 receptor signaling and activate IL-1R-associated kinase, p38, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling in AMs (Fernandez et al., 2004), which triggers the pro-inflammatory state of AMs (Chen et al., 2007). Furthermore, neutrophil extracellular traps induce the pro-inflammatory state of AMs in acute lung injury models, which indicates that AMs can be triggered to perform pro-inflammatory roles through various mechanisms (Song et al., 2019). Following their shift into a pro-inflammatory state, AMs exhibit greater phagocytic activity and increase their secretion of oxygen metabolites, pro-inflammatory cytokines (e.g., IL-1, IL-6, and tumor necrosis factor α), chemokines, lysozyme, antimicrobial peptides, and proteases (Belchamber and Donnelly, 2017; Haslett, 1999; Hodge et al., 2019; Mariencheck et al., 1999; Nagre et al., 2019; Schagat et al., 2001; Soni et al., 2016).
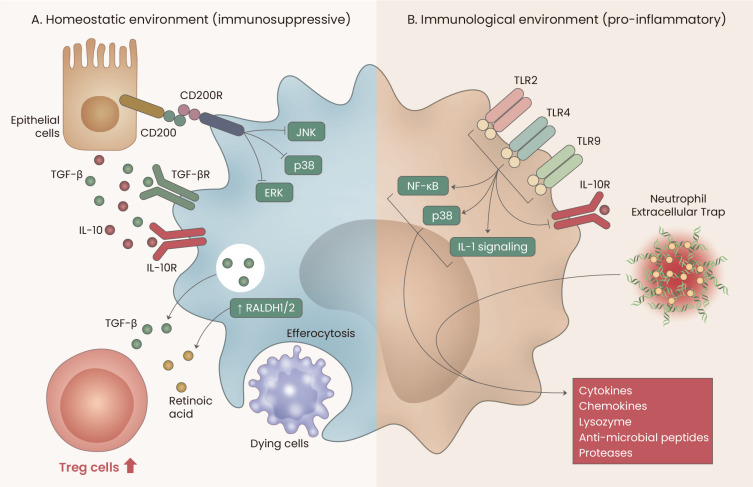
AMs can exert both immunosuppressive and pro-inflammatory functions, depending on the nature of the alveolar microenvironment (Fig. 3). Although non-inflammatory alveolar microenvironments continuously promote immunosuppressive activity in AMs, inflammatory alveolar microenvironments induce AMs to perform pro-inflammatory functions. Thus, developing our understanding of the dual functions of AMs in various pulmonary diseases may help to identify novel and effective pharmaceutical targets.
Fig. 3. Immunological functions of AMs.
(A) Under homeostatic conditions, lung epithelial cells continuously provide immunosuppressive signals to AMs by producing CD200, IL-10, and TGF-β. Subsequently, AMs contribute to regulatory T cell generation by secreting TGF-β and by expressing retinal dehydrogenases 1/2 (RALDH1/2). Moreover, AMs phagocytose dying cells to maintain the alveolar homeostatic environment. (B) Under inflammatory conditions, toll-like receptor (TLR) signals and/or neutrophil extracellular traps (NETs) trigger AMs to functionally switch to pro-inflammatory roles.
METABOLISM IN AMs
Recent studies emphasize the intimate relationship between the metabolic status of immune cells and their development and functions (Pearce and Pearce, 2013). For example, the differentiation of macrophages into classically or alternatively activated macrophages is determined by their use of glycolysis or the tricarboxylic acid cycle, respectively (Viola et al., 2019). However, there have been few reports concerning the relationships of AM metabolic status with their development and function. Recently, mammalian target of rapamycin (mTOR)-deficient mice show a reduction of number and disruptions of fatty acid oxidation and amino acid pathway in AMs, suggesting that lipid and amino acid metabolism might be critical for AM development (Sinclair et al., 2017). mTOR forms two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which perform distinct functions (Saxton and Sabatini, 2017). mTORC1 forms when mTOR binds to regulatory-associated protein of mTOR (RAPTOR), which regulates lipogenesis and sterol homeostasis via sterol regulatory element-binding proteins 1 & 2 (SREBP1/2). mTORC2 forms when mTOR binds to rapamycin-insensitive companion of mTOR (RICTOR), which regulates cell growth, proliferation, and cytoskeletal remodeling (Saxton and Sabatini, 2017). While deletion of RICTOR has no significant effect on AMs, the deletion of RAPTOR leads to a significant reduction in AMs (Deng et al., 2017). Moreover, RAPTOR-deficient AMs show disruption in fatty acid and amino acid metabolism, which is similar to the mTOR-deficient AMs, suggesting that regulation of fatty acid and amino acid metabolism might be attributable to mTOR1 rather than mTOR2 in AMs.
Both GM-CSF and PPAR-γ-deficient AMs demonstrate massive accumulation of cholesterol ester-rich lipid-droplets, enhancement of cholesterol proportion in surfactants and upregulation of adenosine triphosphate (ATP)-binding cassette sub-family G member 1 (ABCG1) expression, a PPAR-γ-regulated ATP binding cassette lipid transporter, which leads to development of PAP (Sallese et al., 2017; Thomassen et al., 2007). PPAR-γ-deficient AMs also exhibit enhanced cholesterol esterification accompanied by reduced expression levels of lipid metabolism-related genes such as Fabp1, Fabp4, Cd36, Olr1, and Cidec, which play critical roles in lipid uptake, transport, storage, and processing (Schneider et al., 2014). Treatment of recombinant GM-CSF, PPAR-γ agonist or cholesterol synthesis inhibitor (statin) restores cholesterol clearance in macrophages and reduces severity of PAP in GM-CSF-deficient mice (McCarthy et al., 2018; Sallese et al., 2017; Thomassen et al., 2007). Furthermore, AMs taken from patients with PAP often exhibit a foamy and lipid-filled cytoplasm and have reduced expression levels of PPAR-γ and ABCG1 (Malur et al., 2012), indicating that the lipid metabolism of AMs contributes to modulation of their function, particularly in PAP. Moreover, AMs derived from asthma patients showed increased uptake of leukotriene C4 (LTC4) (a precursor to leukotriene E4 [LTE4]) to generate lipid metabolites such as leukotriene B4 (LTB4) and 5-hydroxyeicosatetraenoic acid (5-HETE) which may be correlated with enhanced migratory ability of AMs in asthmatic condition (Chavis et al., 1991; Damon et al., 1989). In contrast, AMs derived from severe asthma patients show low levels of PGE2, 15-HETE (Huynh et al., 2005), lipoxin A4 (LTA4), and LTB4 (Bhavsar et al., 2010), which is accompanied with defective efferocytosis. These findings suggest a functional link between lipid metabolite generation and functions in AMs. Consistently, lipid metabolism of AMs is implicated in bacterial clearance. Upon infection with Mycobacterium tuberculosis (Mtb), AMs upregulate their oxidative phosphorylation and fatty acid metabolic pathways, which paradoxically creates a favorable environment for the growth and survival of Mtb (Huang et al., 2018). Thus, the depletion of AMs improves Mtb clearance during Mtb infection. However, it is unclear whether oxidative phosphorylation or fatty acid metabolism in AMs regulates Mtb clearance in the lungs.
There is evidence to suggest a link between glycolytic capacity and overall function in AMs. AMs deficient in the E3 ligase Von Hipple-Lindau protein have increased glycolytic capacities and exhibit impaired induction of group 2 innate lymphoid cells through reduced osteopontin expression, as demonstrated in the mouse model for COPD. This finding suggests that glycolysis inhibits AM function (Zhang et al., 2018). Notably, the microenvironments of alveoli maintain remarkably low glucose concentrations, which enables the maintenance of AM function (Woods et al., 2020). Moreover, following glycolysis inhibition by lipopolysaccharide or oxamate, no substantial differences in glycolytic capacity or production of pro-inflammatory cytokines in AMs were found. Similar phenomena have been observed in vivo in the AMs of an influenza infection model. Thus, these studies suggest that glycolysis may be minimally involved in the regulation of AM function. Although PPAR-γ- and mTORC1-mediated fatty acid metabolism is necessary for the proper development of AMs, overall AM function is dependent on oxidative phosphorylation, rather than glycolysis (Table 1). Although studies thus far have demonstrated close links of AM metabolic status with their functions and development, further investigations examining contributions of major metabolic pathways (e.g., glycolysis, oxidative phosphorylation, and amino acid metabolism) and major metabolites in the development and functions of AMs are still necessary. The use of genetically modified mice and AMs derived from patients with pulmonary diseases in future experiments could develop our understanding of the relationships of metabolism with AM development and function.
Table 1.
Relationships of AM metabolic status with AM development and function
| Metabolism | Molecule | Roles | Reference |
|---|---|---|---|
| Fatty acids metabolism | PPAR-Y mTORCI RAPTOR |
Lipid metabolism-related gene expressions Lipid uptake, transport, storage, and processing Amino acids pathways → Development of AMS |
Deng et al., 2017 Schneider et al., 2014 Sinclair et al., 2017 |
| Oxidative phosphorylation | Up-regulated upon Mtb infection → Makes AMs preferred sites for Mtb growth and survival |
Huang et al., 2018 | |
| Glycolysis | von Hippel-Lindau | Upregulation of glycolytic capacity → Decreased functional capacities of Ams |
Zhang et al., 2005 |
CONCLUDING REMARKS
AMs are major immune cells that reside in and continuously patrol the alveoli, where they function to maintain homeostasis. Human lungs are continuously exposed to external stimuli and respiratory infections. An enhanced understanding of AM biology will lead to the development of suitable pharmaceutical targets for various lung diseases. Although the key soluble factors involved in AM development and function are relatively well-established, studies examining the relationships of AM metabolic status with their functions and development are lacking. We hope that this review will provide new insights that can aid in the establishment of new lung disease treatments.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF) grants funded by Ministry of Science and ICT (MSIT) - grant No. 2020R1A2C2008312 and 2020R1A4A1017515.
Footnotes
AUTHOR CONTRIBUTIONS
Y.D.W. wrote the manuscript, D.J. designed the figures & the table, and D.H.C. secured funding, provided feedback, and supervised the overall process.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Antoniu S.A., Rajnoveanu R., Grigore M., Antohe I. Pharmacotherapy options in pulmonary alveolar proteinosis. Expert Opin. Pharmacother. 2020;21:1359–1366. doi: 10.1080/14656566.2020.1757650. [DOI] [PubMed] [Google Scholar]
- Bazewicz C.G., Dinavahi S.S., Schell T.D., Robertson G.P. Aldehyde dehydrogenase in regulatory T-cell development, immunity and cancer. Immunology. 2019;156:47–55. doi: 10.1111/imm.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belchamber K.B.R., Donnelly L.E. Macrophage dysfunction in respiratory disease. Results Probl. Cell Differ. 2017;62:299–313. doi: 10.1007/978-3-319-54090-0_12. [DOI] [PubMed] [Google Scholar]
- Bhavsar P.K., Levy B.D., Hew M.J., Pfeffer M.A., Kazani S., Israel E., Chung K.F. Corticosteroid suppression of lipoxin A4 and leukotriene B4 from alveolar macrophages in severe asthma. Respir. Res. 2010;11:71. doi: 10.1186/1465-9921-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette E.Y., Lauzon-Joset J.F., Debley J.S., Ziegler S.F. Cross-talk between alveolar macrophages and lung epithelial cells is essential to maintain lung homeostasis. Front. Immunol. 2020;11:583042. doi: 10.3389/fimmu.2020.583042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton J., Neculai D., Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- Chavis C., Godard P., Michel F.B., Crastes, de Paulet A., Damon M. Sulfidopeptide leukotrienes contribute to human alveolar macrophage activation in asthma. Prostaglandins Leukot. Essent. Fatty Acids. 1991;42:95–100. doi: 10.1016/0952-3278(91)90074-f. [DOI] [PubMed] [Google Scholar]
- Chen H., Cowan M.J., Hasday J.D., Vogel S.N., Medvedev A.E. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J. Immunol. 2007;179:6097–6106. doi: 10.4049/jimmunol.179.9.6097. [DOI] [PubMed] [Google Scholar]
- Coleman M.M., Ruane D., Moran B., Dunne P.J., Keane J., Mills K.H. Alveolar macrophages contribute to respiratory tolerance by inducing FoxP3 expression in naive T cells. Am. J. Respir. Cell Mol. Biol. 2013;48:773–780. doi: 10.1165/rcmb.2012-0263OC. [DOI] [PubMed] [Google Scholar]
- Damon M., Chavis C., Daures J.P., Crastes, de Paulet A., Michel F.B., Godard P. Increased generation of the arachidonic metabolites LTB4 and 5-HETE by human alveolar macrophages in patients with asthma: effect in vitro of nedocromil sodium. Eur. Respir. J. 1989;2:202–209. [PubMed] [Google Scholar]
- Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Yang J., Lin X., Shin J., Gao J., Zhong X.P. Essential role of mTORC1 in self-renewal of murine alveolar macrophages. J. Immunol. 2017;198:492–504. doi: 10.4049/jimmunol.1501845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draijer C., Penke L.R.K., Peters-Golden M. Distinctive effects of GM-CSF and M-CSF on proliferation and polarization of two major pulmonary macrophage populations. J. Immunol. 2019;202:2700–2709. doi: 10.4049/jimmunol.1801387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M., Hibbs M.L., Chen W. The contributions of lung macrophage and monocyte heterogeneity to influenza pathogenesis. Immunol. Cell Biol. 2017;95:225–235. doi: 10.1038/icb.2016.97. [DOI] [PubMed] [Google Scholar]
- Elliott M.R., Koster K.M., Murphy P.S. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol. 2017;198:1387–1394. doi: 10.4049/jimmunol.1601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez S., Jose P., Avdiushko M.G., Kaplan A.M., Cohen D.A. Inhibition of IL-10 receptor function in alveolar macrophages by Toll-like receptor agonists. J. Immunol. 2004;172:2613–2620. doi: 10.4049/jimmunol.172.4.2613. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick A.M., Holguin F., Teague W.G., Brown L.A. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J. Allergy Clin. Immunol. 2008;121:1372–1378.e1. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Hayashi S., Hogg J.C., Mukae H., Suwa T., Goto Y., Vincent R., van Eeden S.F. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am. J. Respir. Cell Mol. Biol. 2002;27:34–41. doi: 10.1165/ajrcmb.27.1.4787. [DOI] [PubMed] [Google Scholar]
- Gao X., Dong Y., Liu Z., Niu B. Silencing of triggering receptor expressed on myeloid cells-2 enhances the inflammatory responses of alveolar macrophages to lipopolysaccharide. Mol. Med. Rep. 2013;7:921–926. doi: 10.3892/mmr.2013.1268. [DOI] [PubMed] [Google Scholar]
- Gentek R., Molawi K., Sieweke M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014;262:56–73. doi: 10.1111/imr.12224. [DOI] [PubMed] [Google Scholar]
- Grabiec A.M., Hussell T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin. Immunopathol. 2016;38:409–423. doi: 10.1007/s00281-016-0555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross N.J., Barnes P.J. New therapies for asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2017;195:159–166. doi: 10.1164/rccm.201610-2074PP. [DOI] [PubMed] [Google Scholar]
- Guilliams M., De Kleer I., Henri S., Post S., Vanhoutte L., De Prijck S., Deswarte K., Malissen B., Hammad H., Lambrecht B.N. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth A.M., Janssen W.J., Bosio C.M., Crouch E.C., Henson P.M., Dow S.W. Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L936–L946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Hajjar D.P., Tauras J.M., Feng J., Gotto A.M., Jr., Nicholson A.C., Jr. Transforming growth factor-beta1 (TGF-beta1) and TGF-beta2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-gamma. J. Biol. Chem. 2000;275:1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- Han K.H., Tangirala R.K., Green S.R., Quehenberger O. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler. Thromb. Vasc. Biol. 1998;18:1983–1991. doi: 10.1161/01.atv.18.12.1983. [DOI] [PubMed] [Google Scholar]
- Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M., Becker C.D., See P., Price J., Lucas D., et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 1999;160(5 Pt 2):S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- Hercus T.R., Broughton S.E., Ekert P.G., Ramshaw H.S., Perugini M., Grimbaldeston M., Woodcock J.M., Thomas D., Pitson S., Hughes T., et al. The GM-CSF receptor family: mechanism of activation and implications for disease. Growth Factors. 2012;30:63–75. doi: 10.3109/08977194.2011.649919. [DOI] [PubMed] [Google Scholar]
- Hercus T.R., Thomas D., Guthridge M.A., Ekert P.G., King-Scott J., Parker M.W., Lopez A.F. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindelang M., Kirsch F., Leidl R. Effectiveness of non-pharmacological COPD management on health-related quality of life - a systematic review. Expert Rev. Pharmacoecon. Outcomes Res. 2020;20:79–91. doi: 10.1080/14737167.2020.1734455. [DOI] [PubMed] [Google Scholar]
- Hodge M.X., Reece S.W., Madenspacher J.H., Gowdy K.M. In vivo assessment of alveolar macrophage efferocytosis following ozone exposure. J. Vis. Exp. 2019;(152):e60109. doi: 10.3791/60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S., Hodge G., Scicchitano R., Reynolds P.N., Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003;81:289–296. doi: 10.1046/j.1440-1711.2003.t01-1-01170.x. [DOI] [PubMed] [Google Scholar]
- Hoeffel G., Chen J., Lavin Y., Low D., Almeida F.F., See P., Beaudin A.E., Lum J., Low I., Forsberg E.C., et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G., Ginhoux F. Fetal monocytes and the origins of tissue-resident macrophages. Cell. Immunol. 2018;330:5–15. doi: 10.1016/j.cellimm.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Huang L., Nazarova E.V., Tan S., Liu Y., Russell D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018;215:1135–1152. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Xiu H., Zhang S., Zhang G. The role of macrophages in the pathogenesis of ALI/ARDS. Mediators Inflamm. 2018;2018:1264913. doi: 10.1155/2018/1264913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman Reed J.A., Rice W.R., Zsengellér Z.K., Wert S.E., Dranoff G., Whitsett J.A. GM-CSF enhances lung growth and causes alveolar type II epithelial cell hyperplasia in transgenic mice. Am. J. Physiol. 1997;273:L715–L725. doi: 10.1152/ajplung.1997.273.4.L715. [DOI] [PubMed] [Google Scholar]
- Huynh M.L., Fadok V.A., Henson P.M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J. Clin. Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh M.L., Malcolm K.C., Kotaru C., Tilstra J.A., Westcott J.Y., Fadok V.A., Wenzel S.E. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am. J. Respir. Crit. Care Med. 2005;172:972–979. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- Kaur M., Bell T., Salek-Ardakani S., Hussell T. Macrophage adaptation in airway inflammatory resolution. Eur. Respir. Rev. 2015;24:510–515. doi: 10.1183/16000617.0030-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kim K.K., Dotson M.R., Agarwal M., Yang J., Bradley P.B., Subbotina N., Osterholzer J.J., Sisson T.H. Efferocytosis of apoptotic alveolar epithelial cells is sufficient to initiate lung fibrosis. Cell Death Dis. 2018;9:1056. doi: 10.1038/s41419-018-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning N., van Eijk M., Pouwels W., Brouwer M.S., Voehringer D., Huitinga I., Hoek R.M., Raes G., Hamann J. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J. Innate Immun. 2010;2:195–200. doi: 10.1159/000252803. [DOI] [PubMed] [Google Scholar]
- Krenkel O., Puengel T., Govaere O., Abdallah A.T., Mossanen J.C., Kohlhepp M., Liepelt A., Lefebvre E., Luedde T., Hellerbrand C., et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- Krysko D.V., D'Herde K., Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis. 2006;11:1709–1726. doi: 10.1007/s10495-006-9527-8. [DOI] [PubMed] [Google Scholar]
- Lavin Y., Mortha A., Rahman A., Merad M. Regulation of macrophage development and function in peripheral tissues. Nat. Rev. Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Jin F., Du J., He Q., Yang B., Luo P. Macrophage-secreted TSLP and MMP9 promote bleomycin-induced pulmonary fibrosis. Toxicol. Appl. Pharmacol. 2019;366:10–16. doi: 10.1016/j.taap.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Machiels B., Dourcy M., Xiao X., Javaux J., Mesnil C., Sabatel C., Desmecht D., Lallemand F., Martinive P., Hammad H., et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 2017;18:1310–1320. doi: 10.1038/ni.3857. [DOI] [PubMed] [Google Scholar]
- Malur A., Kavuru M.S., Marshall I., Barna B.P., Huizar I., Karnekar R., Thomassen M.J. Rituximab therapy in pulmonary alveolar proteinosis improves alveolar macrophage lipid homeostasis. Respir. Res. 2012;13:46. doi: 10.1186/1465-9921-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariencheck W.I., Savov J., Dong Q., Tino M.J., Wright J.R. Surfactant protein A enhances alveolar macrophage phagocytosis of a live, mucoid strain of P. aeruginosa. Am. J. Physiol. 1999;277:L777–L786. doi: 10.1152/ajplung.1999.277.4.L777. [DOI] [PubMed] [Google Scholar]
- Mayer A.K., Bartz H., Fey F., Schmidt L.M., Dalpke A.H. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur. J. Immunol. 2008;38:1689–1699. doi: 10.1002/eji.200737936. [DOI] [PubMed] [Google Scholar]
- McCarthy C., Lee E., Bridges J.P., Sallese A., Suzuki T., Woods J.C., Bartholmai B.J., Wang T., Chalk C., Carey B.C., et al. Statin as a novel pharmacotherapy of pulmonary alveolar proteinosis. Nat. Commun. 2018;9:3127. doi: 10.1038/s41467-018-05491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misharin A.V., Morales-Nebreda L., Reyfman P.A., Cuda C.M., Walter J.M., McQuattie-Pimentel A.C., Chen C.I., Anekalla K.R., Joshi N., Williams K., et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohning M.P., Thomas S.M., Barthel L., Mould K.J., McCubbrey A.L., Frasch S.C., Bratton D.L., Henson P.M., Janssen W.J. Phagocytosis of microparticles by alveolar macrophages during acute lung injury requires MerTK. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314:L69–L82. doi: 10.1152/ajplung.00058.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H.G., Cao Y., Yang J., Lee J.H., Choi H.S., Jin Y. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 2015;6:e2016. doi: 10.1038/cddis.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagre N., Cong X., Pearson A.C., Zhao X. Alveolar macrophage phagocytosis and bacteria clearance in mice. J. Vis. Exp. 2019;(145):e59088. doi: 10.3791/59088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinakamura R., Wiler R., Dirksen U., Morikawa Y., Arai K., Miyajima A., Burdach S., Murray R. The pulmonary alveolar proteinosis in granulocyte macrophage colony-stimulating factor/interleukins 3/5 beta c receptor-deficient mice is reversed by bone marrow transplantation. J. Exp. Med. 1996;183:2657–2662. doi: 10.1084/jem.183.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Beirne S.L., Kikkers S.A., Oromendia C., Salit J., Rostmai M.R., Ballman K.V., Kaner R.J., Crystal R.G., Cloonan S.M. Alveolar macrophage immunometabolism and lung function impairment in smoking and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2020;201:735–739. doi: 10.1164/rccm.201908-1683LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Gomez A., Perretti M., Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol. Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez A., Ballard E.N., Roman J. TGFbeta1 controls PPARgamma expression, transcriptional potential, and activity, in part, through Smad3 signaling in murine lung fibroblasts. PPAR Res. 2012;2012:375876. doi: 10.1155/2012/375876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins J.B. Alveolar macrophages: wielding the double-edged sword of inflammation. Am. J. Respir. Crit. Care Med. 2003;167:103–104. doi: 10.1164/rccm.2210007. [DOI] [PubMed] [Google Scholar]
- Sallese A., Suzuki T., McCarthy C., Bridges J., Filuta A., Arumugam P., Shima K., Ma Y., Wessendarp M., Black D., et al. Targeting cholesterol homeostasis in lung diseases. Sci. Rep. 2017;7:10211. doi: 10.1038/s41598-017-10879-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Schagat T.L., Wofford J.A., Wright J.R. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J. Immunol. 2001;166:2727–2733. doi: 10.4049/jimmunol.166.4.2727. [DOI] [PubMed] [Google Scholar]
- Schneider C., Nobs S.P., Kurrer M., Rehrauer H., Thiele C., Kopf M. Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- Sinclair C., Bommakanti G., Gardinassi L., Loebbermann J., Johnson M.J., Hakimpour P., Hagan T., Benitez L., Todor A., Machiah D., et al. mTOR regulates metabolic adaptation of APCs in the lung and controls the outcome of allergic inflammation. Science. 2017;357:1014–1021. doi: 10.1126/science.aaj2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Li H., Li Y., Dai M., Zhang L., Liu S., Tan H., Deng P., Liu J., Mao Z., et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization. Exp. Cell Res. 2019;382:111486. doi: 10.1016/j.yexcr.2019.06.031. [DOI] [PubMed] [Google Scholar]
- Soni S., Wilson M.R., O'Dea K.P., Yoshida M., Katbeh U., Woods S.J., Takata M. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax. 2016;71:1020–1029. doi: 10.1136/thoraxjnl-2015-208032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroosh P., Doherty T.A., Duan W., Mehta A.K., Choi H., Adams Y.F., Mikulski Z., Khorram N., Rosenthal P., Broide D.H., et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 2013;210:775–788. doi: 10.1084/jem.20121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E., Lieschke G.J., Grail D., Metcalf D., Hodgson G., Gall J.A., Maher D.W., Cebon J., Sinickas V., Dunn A.R. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C., Marrero L., Swain S., Harmsen A.G., Zheng M., Brown G.D., Gordon S., Shellito J.E., Kolls J.K. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J. Exp. Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D., Inoue-Yokoo T., Fraser S.T., Kulkeaw K., Mizuochi C., Horio Y. Embryonic regulation of the mouse hematopoietic niche. ScientificWorldJournal. 2011;11:1770–1780. doi: 10.1100/2011/598097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Wei F.Q., Li W.J., Wei J.W., Zhong H., Wen Y.H., Lei W.B., Chen L., Li H., Lin H.Q., et al. A positive-feedback loop between tumour infiltrating activated Treg cells and type 2-skewed macrophages is essential for progression of laryngeal squamous cell carcinoma. Br. J. Cancer. 2017;117:1631–1643. doi: 10.1038/bjc.2017.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.Y., Krasnow M.A. Developmental origin of lung macrophage diversity. Development. 2016;143:1318–1327. doi: 10.1242/dev.129122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M.J., Barna B.P., Malur A.G., Bonfield T.L., Farver C.F., Malur A., Dalrymple H., Kavuru M.S., Febbraio M. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J. Lipid Res. 2007;48:2762–2768. doi: 10.1194/jlr.P700022-JLR200. [DOI] [PubMed] [Google Scholar]
- Tsai C.F., Chen J.H., Yeh W.L. Pulmonary fibroblasts-secreted CXCL10 polarizes alveolar macrophages under pro-inflammatory stimuli. Toxicol. Appl. Pharmacol. 2019;380:114698. doi: 10.1016/j.taap.2019.114698. [DOI] [PubMed] [Google Scholar]
- Ushach I., Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 2016;100:481–489. doi: 10.1189/jlb.3RU0316-144R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Laar L., Saelens W., De Prijck S., Martens L., Scott C.L., Van Isterdael G., Hoffmann E., Beyaert R., Saeys Y., Lambrecht B.N., et al. Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue-resident macrophages. Immunity. 2016;44:755–768. doi: 10.1016/j.immuni.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Viola A., Munari F., Sánchez-Rodríguez R., Scolaro T., Castegna A. The metabolic signature of macrophage responses. Front. Immunol. 2019;10:1462. doi: 10.3389/fimmu.2019.01462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.E., McCandless E.E., Olszewski M.A., Robinson N.E. Alveolar macrophage phenotypes in severe equine asthma. Vet. J. 2020;256:105436. doi: 10.1016/j.tvjl.2020.105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Y.D., Koh J., Ko J.S., Kim S., Jung K.C., Jeon Y.K., Kim H.Y., Lee H., Lee C.W., Chung D.H. Ssu72 regulates alveolar macrophage development and allergic airway inflammation by fine-tuning of GM-CSF receptor signaling. J. Allergy Clin. Immunol. 2021;147:1242–1260. doi: 10.1016/j.jaci.2020.07.038. [DOI] [PubMed] [Google Scholar]
- Woods P.S., Kimmig L.M., Meliton A.Y., Sun K.A., Tian Y., O'Leary E.M., Gökalp G.A., Hamanaka R.B., Mutlu G.M. Tissue-resident alveolar macrophages do not rely on glycolysis for LPS-induced inflammation. Am. J. Respir. Cell Mol. Biol. 2020;62:243–255. doi: 10.1165/rcmb.2019-0244OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T. Mouse yolk sac hematopoiesis. Front. Cell Dev. Biol. 2018;6:80. doi: 10.3389/fcell.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeligar S.M., Chen M.M., Kovacs E.J., Sisson J.H., Burnham E.L., Brown L.A. Alcohol and lung injury and immunity. Alcohol. 2016;55:51–59. doi: 10.1016/j.alcohol.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Buttgereit A., Lelios I., Utz S.G., Cansever D., Becher B., Greter M. The cytokine TGF-beta promotes the development and homeostasis of alveolar macrophages. Immunity. 2017;47:903–912.e4. doi: 10.1016/j.immuni.2017.10.007. [DOI] [PubMed] [Google Scholar]
- Zhang J., Tachado S.D., Patel N., Zhu J., Imrich A., Manfruelli P., Cushion M., Kinane T.B., Koziel H. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J. Leukoc. Biol. 2005;78:665–674. doi: 10.1189/jlb.1204699. [DOI] [PubMed] [Google Scholar]
- Zhang W., Li Q., Li D., Li J., Aki D., Liu Y.C. The E3 ligase VHL controls alveolar macrophage function via metabolic-epigenetic regulation. J. Exp. Med. 2018;215:3180–3193. doi: 10.1084/jem.20181211. [DOI] [PMC free article] [PubMed] [Google Scholar]