Abstract
Introduction
Chronic kidney disorder is a main public health concern. Inflammatory processes and oxidative stress are common in end-stage renal disease patients. We aimed to evaluate the effect of the hydroalcoholic extract of watercress (WC) on the inflammatory cytokines and protein carbonyl (PCO) contents in chronic hemodialysis patients.
Methods
This was a double-blind randomized clinical trial performed on 46 hemodialysis patients. The participants were randomly divided into two groups: intervention group (500 mg hydroalcoholic extract of WC every day for 4 weeks) and control group (500 mg of white flour every night for 4 weeks). The blood samples were taken to determine the levels of vitamin E, PCO, and inflammatory cytokines at baseline and the end of treatment.
Results
Forty-five patients completed the study (22 patients in the intervention group and 23 patients in the control group). There was a significant reduction in the PCO level (20.33 ± 4.40 vs. 15.06 ± 6.41, P=0.001) in the intervention group; also, this change was statistically significant relative to the control group. Furthermore, there were significant reductions in hs-CRP (8953.30 ± 5588.06 vs. 7249.86 ± 5091.62, P=0.007) and IL-6 (60.10 (55.99, 73.10) vs. 55.21 (53.39, 60.48), P=0.050) in the intervention group, but these changes were not significant in comparison with the control group.
Conclusion
We conclude that the hydroalcoholic extract of WC reduced the PCO content in hemodialysis patients via inhibition of protein oxidation. Although WC administration had caused a significant reduction in IL-6 and CRP levels, these differences were not statistically significant relative to the control group. Further research is needed to identify the antioxidant and anti-inflammatory effects of WC in hemodialysis patients.
1. Introduction
Chronic kidney disease (CKD) is a main public health concern and an important factor in reducing life expectancy [1]. With the quick rise in the incidence of end-stage renal disease (ESRD), the number of renal replacement therapies (RRTs) is steadily increasing. RRT, which includes dialysis and kidney transplantation, is a treatment to extend the lifespan of ESRD patients [2]. Hemodialysis is the major therapy in the controlling of CKD, which can destroy vitamins, water-soluble peptides, and amino acids [3]. Dialysis is required when the glomerular filtration rate (GFR) in ESRD patients is reduced to 10 to 15 mL/min per 1.73 m2 [4, 5].
Increased oxidative stress in dialysis patients is due to the loss of antioxidant compounds during dialysis, bacterial products during dialysis, and malnutrition in these patients [6]. Over the last two decades, oxidative stress has been a risk factor for mortality in CKD patients [7]. In a normal cell, there is a good balance between oxidants such as reactive oxygen species (ROS) and antioxidants; increasing pro-oxidants or decreasing antioxidants led to oxidative stress that it able to cause serious cellular damage [8]. In the hemodialysis patients, oxidative stress increases [9]; then, oxidative compounds can interact with cellular components such as lipids, proteins, and DNA by various reactions and subsequently causes pathological complications [10].
Dialysis patients are known to have a chronic inflammatory condition [11], and inflammatory processes are common in CKD and ESRD patients [12]. Although inflammation is a frequent feature of dialysis patients, its underlying factors and mechanisms are poorly understood [13]. Inflammation in these patients may occur due to dialysis-related factors (such as exposure to endotoxins and cytokine inducers in dialysate) and nondialysis-related factors (such as genetic factors, infection, and other underlying diseases) [14].
Increased oxidative stress and inflammation in hemodialysis patients lead to cardiovascular disease (CVD) [15]; in other words, inflammation as a risk factor for CVD is a main cause of mortality in hemodialysis patients [16]. Studies have shown that proinflammatory cytokines such as IL-6, IL-1, and TNF-α increase in dialysis patients [16, 17]. IL-6 is a proinflammatory cytokine that induces hepatic C-reactive protein (CRP) production and eventually leads to CVD [13, 17].
Watercress (Nasturtium officinale, WC) is a plant that grows in arid and aquatic situations and is rich in vitamins (such as A, B, C, K, E, and folic acid), ions/elements (such as iron, chromium, calcium, magnesium, phosphorus, iron, potassium, zinc, and sodium), and bioactive substances (for instance, β-carotene, lutein, and quercetin). It is used to treat diabetes, anemia, and eczema, as well as renal and hepatic disorders [18–20]. Previous studies showed that WC reduces the risk of colon, lung, lymphatic, and prostate cancers [21, 22]. In addition, in vivo and in vitro studies have reported that WC has antidiabetic [23, 24], anti-inflammatory [25], antioxidant [26–28], nephroprotective [29], and hepatoprotective effects [30, 31].
Therefore, based on anti-inflammatory and antioxidant properties of WC, the purpose of the present study was to evaluate the effect of the hydroalcoholic extract of WC on the inflammatory cytokines and protein carbonyl contents in chronic hemodialysis patients.
2. Materials and Methods
2.1. Chemicals
Trichloroacetic acid (TCA), guanidinium chloride (GdnHCl), 2,4-dinitrophenylhydrazine (DNPH), acetic acid, acetonitrile, acetate sodium, and methanol were obtained from Merck (Germany). All other reagents and chemicals utilized were of analytical grade.
2.2. Plant Material and Extraction
Aerial parts of WC were provided in March–May 2019 from the Shehniz area placed in Yasuj, Iran. The WC plant was identified by the botanist (Herbarium No. HYU30230). The aerial parts of the plant were dried in the shade at 25°C and finely ground. Briefly, 100 g powder of the plant was suspended in 70% ethanol (1000 ml) at 25°C for 48 hours. The extract was filtered by a filter paper, transferred to a vacuum distillation apparatus, and concentrated as far as possible. Then, the extract was dried in a 50°C incubator and stored at −20°C.
2.3. Clinical Trial
This double-blind randomized clinical trial was performed on chronic hemodialysis patients in Shahid Beheshti Hospital, Yasuj University of Medical Sciences, between July 2019 and August 2019. The study was permitted by the Research Committee of Yasuj University of Medical Sciences (Ethical code: YUMS.REC.1398.112); then, it was confirmed in the Iranian Clinical Trial System (http://www.irct.ir) with the registration number IRCT20201228049866N1. Before starting the study, all patients completed an informed consent form. Ethical considerations include confidentiality of information, imposing no cost on subjects and assigning code to each subject. Inclusion criteria were ≥18 years, ≥3 months on hemodialysis and three times a week hemodialysis, not taking medications like corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) at least for 4 weeks, and no acute infectious or inflammatory disease. Exclusion criteria were as follows: (1) active hepatic disease, malignancy, AIDS, rheumatic diseases, and other inflammatory diseases; (2) presence of any infection in late two months; (3) rejected renal transplantation; (4) allergy to the hydroalcoholic extract of WC; (5) having active infectious disease during the study; (6) pre-existing cardiac arrhythmias; (7) having of hypotension; (8) immunodeficiency; (9) malnutrition and cachexia (BMI <18.5 kg/m2); (10) albumin ≤3 mg/dL; (11) patient's displeasure to continue the study; and (12) kidney transplantation.
From the 110 hemodialysis patients, 46 patients were selected using simple random sampling. The mean year that the patients have been in the hemodialysis unit was about 2 years. The patients were randomly (with 1 : 1 ratio) divided into two groups: intervention (n = 23) and control (n = 23). During a four weeks' period, the patients in the intervention group took 500 mg hydroalcoholic extract of WC once a day and those in the control group received drug-like (capsule color, packing) containing 500 mg of white flour. Hydroalcoholic extract of WC dose was selected according to previous clinical and pharmacologic studies [3, 21]. To confirm that the supplement was taken, the patient was asked to bring the capsule bottle to the next dialysis session. Patients were recommended not to consume any other nutritional or antioxidant supplements during the trial in order to sustain their daily dietary patterns, diet, and physical activity. The supplementation and placebo were packaged in similar color, form, wrapping, and size. The patients were checked for WC side effects based on the dialysis frequency of at least three times a week. Patients were monitored weekly for drug use for the assurance of clinical research, and they were asked to bring the empty container.
A 5 mL blood sample was taken (when the dialysis catheter was attached to the patient) at baseline and the end of treatment for measurement of NO (nitric oxide) metabolite, protein carbonyl (PCO), vitamin E, and inflammatory markers (such as TNF-α, IL-6, and hs-CRP).
2.4. HPLC Conditions
Vitamin E in a standard and hydroalcoholic extract of WC was analyzed quantitatively by reverse-phase HPLC via a Knauer column (4.6 mm diameter and 250 mm length) with a precolumn (particle size of 5 μm, Eurospher 100-5 C18) at 220 nm. The final concentrations of all experiments were recorded based on the mobile phase at room temperature and were a mixture of acetonitrile (95%) and water (5.0%) at a flow rate of 1.0 mL·min−1, and the injection loop (20 μL). For the quantification of vitamin E, blood samples of chronic hemodialysis patients were centrifuged at 3000 rpm for 15 min and the serum was then separated. The proteins were precipitated by adding 200 μL of acetonitrile to 100 µL serum in 1 mL microtubes. After centrifugation (15 min, 10000 rpm), 20 μL of the supernatant was injected into the HPLC-UV system for subsequent analysis. The concentration of vitamin E in the samples was determined from the calibration curve.
2.5. Determination of Nitric Oxide Metabolite
The nitrite level was measured as an index of NO production based on the Griess reaction [32]. The NO metabolite level was presented as µmol/L utilizing sodium nitrite as standard (0–100 μmol/L).
2.6. Determination of the Protein Carbonyl Level
The carbonyl content of the protein was calculated by a spectrophotometric method [33]. After treatment with DNPH (10 mmol/L) in HCl (2 mol/L) and 50% TCA, the deposit was washed with a mixture of ethanol and ethyl acetate (1 : 1, v/v) and dissolved in GdnHCl (6 mol/L). At the end of the procedure, the PCO level was determined using the molar absorption coefficient of 2.2 × 104 M−1·cm−1 and presented as μmol/mg protein.
2.7. Determination of Inflammatory Markers
In serum samples on 0th and 28th days, the levels of hs-CRP, IL-6, and TNF-α were measured using ELISA kits (Karmania Pars Gene, Kerman, Iran) based on the manufacturer's guidelines. The lower detection limit was 2 pg/mL for TNF-α, 3 pg/mL for IL-6, and 10 ng/mL for hs-CRP. The intra- and inter assay CVs were 10 and 12% for hs-CRP, 3 and 9% for IL-6, and 3 and 8% for TNF-α, respectively. Absorption was measured using an ELISA reader (BioTek, Winooski, Vermont, USA) at 450 nm.
2.8. Statistical Analyses
Results are presented as mean ± SD or median (25th to 75th interquartile range). The normality test was performed to select the appropriate statistical test. Parametric tests were used for normal distribution, and nonparametric tests were used for data without normal distribution. Baseline data were evaluated to determine the possible significant intergroup variations. An independent t-test and paired t-test were used for parametric data; also, the Mann–Whitney U test and Wilcoxon signed-rank test were used for nonparametric data. The significance level was assumed as P < 0.05.
3. Results
The flow diagram of the present study is shown in Figure 1. Out of 46 hemodialysis patients, 23 patients entered in each group (23 patients in the intervention group and 23 patients in the control group). Among the patients of the intervention group, one patient left the study due to kidney transplantation. Finally, 22 patients in the intervention group (12 males and 10 females) and 23 patients in the control group (13 males and 10 females) completed the study. In the current study, the main cause of renal failure was diabetes in 22 (47.82%) patients. One patient showed some gastrointestinal symptoms of pain and diarrhea that were tolerable, and the patient continued the study.
Figure 1.
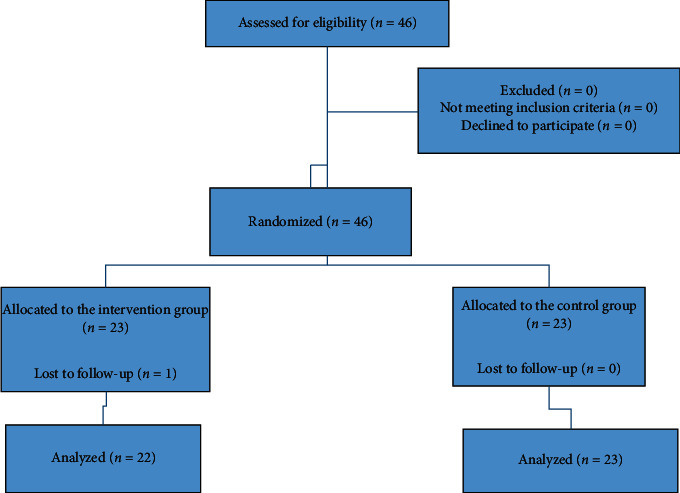
Schematic flow diagram of the study.
3.1. RP-HPLC Results
The RP-HPLC quantitative analyses were carried out by the standard addition method. The typical chromatograms and calibration curve of vitamin E and the hydroalcoholic extract of WC are displayed in Figures 2(a) and 2(b), respectively. The retention time (tR) of vitamin E was 18.1 ± 0.20 min. The linear range of injected concentrations in the standard and hydroalcoholic extract of WC was obtained in the range of 0.0–100 mg·L−1 (correlation coefficient 0.999). Finally, on the basis of obtained data on injected samples of the hydroalcoholic extract of WC, the amount of vitamin E was measured as 52.0 ± 2.0 mg·g−1.
Figure 2.
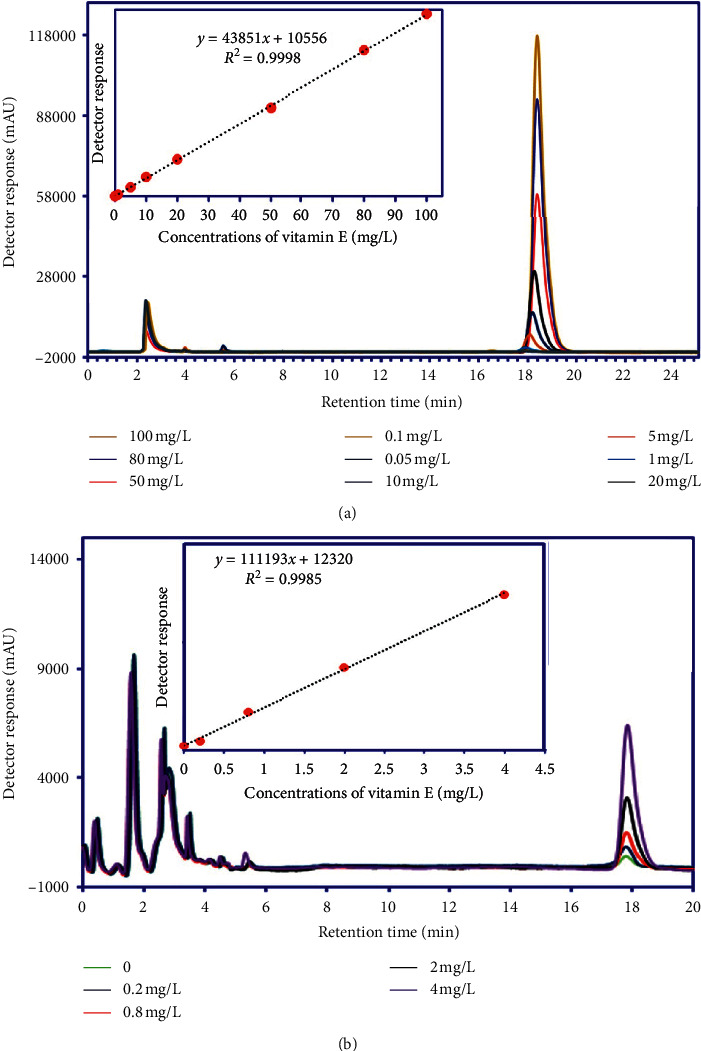
The typical chromatograms and calibration curves obtained from standard vitamin E (a) and vitamin E in the hydroalcoholic extract of WC by the standard addition method (b).
3.2. Baseline Characteristics
Baseline data of the patients are presented in Table 1. The baseline data showed that there was no statistically significant change among the variables between intervention and control groups.
Table 1.
Baseline characteristics of control and intervention groups.
| Variable | Control | Intervention | P value |
|---|---|---|---|
| Age | 63.08 ± 13.9 | 58.86 ± 16.68 | 0.357 |
| BMI (kg/m2) | 23.79 ± 4.17 | 25.59 ± 4.25 | 0.153 |
| hs-CRP | 10508.52 ± 5479.98 | 8953.30 ± 5588.06 | 0.346 |
| PCO | 22.19 ± 4.26 | 20.33 ± 4.40 | 0.153 |
| NO metabolite | 9.12 ± 5.88 | 12.25 ± 7.66 | 0.142 |
| TNF-α (pg/mL) | 15.95 (15.17, 16.66) | 15.96 (15.16, 16.66) | 0.785 |
| IL-6 (pg/mL) | 58.24 (55.19, 61.12) | 60.10 (55.99, 73.10) | 0.075 |
| Vit E | 740.00 ± 104.12 | 703.05 ± 73.91 | 0.186 |
Values are mean ± SD for the data with normal distribution and median (interquartile ranges) for the data not normally distributed. BMI: body mass index; CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; PCO: protein carbonyl; NO metabolite: nitric oxide metabolite; Vit E: vitamin E.
3.3. Effects of the WC Extract on NO Metabolite and PCO Levels
Table 2 shows the comparisons of NO metabolite and PCO variables between groups at the beginning and the end of the study. There was no statistically significant change between groups regarding NO metabolite. There was a significant reduction in the PCO level (20.33 ± 4.40 vs. 15.06 ± 6.41, P=0.001) in the intervention group; also, this change was statistically significant relative to the control group.
Table 2.
Comparison of changes in the oxidant-antioxidant parameters and inflammatory markers during the study period between the intervention and control groups.
| Variable | Baseline | After 4 weeks | P value | Net differences of groups | P value | |
|---|---|---|---|---|---|---|
| PCO | Control | 22.19 ± 4.26 | 26.46 ± 7.72 | 0.022 | 4.26 ± 8.28 | 0.001 |
| Intervention | 20.33 ± 4.40 | 15.06 ± 6.41 | 0.006 | −5.26 ± 8.34 | ||
| NO | Control | 9.12 ± 5.88 | 14.25 ± 9.41 | 0.060 | 5.12 ± 11.97 | 0.525 |
| Intervention | 12.25 ± 7.66 | 15.00 ± 12.10 | 0.312 | 2.75 ± 11.46 | ||
| hs-CRP | Control | 10508.52 ± 5479.98 | 9104.60 ± 4901.67 | 0.142 | −1403.91 ± 4425.17 | 0.785 |
| Intervention | 8953.30 ± 5588.06 | 7249.86 ± 5091.62 | 0.007 | −1703.43 ± 2770.61 | ||
| TNF-α | Control | 15.95 (15.17, 16.66) | 15.87 (14.75, 16.22) | 0.330 | −11.00 (−2.30, 0.59) | 0.276 |
| Intervention | 15.96 (15.16, 16.66) | 15.94 (15.44, 16.53) | 0.615 | 0.37 (−1.31, 1.28) | ||
| IL-6 | Control | 58.24 (55.19, 61.12) | 56.57 (53.05, 62.63) | 0.738 | −2.66 (−7.21, 12.42) | 0.196 |
| Intervention | 60.10 (55.99, 73.10) | 55.21 (53.39, 60.48) | 0.050 | −7.27 (−21.33, 1.96) | ||
| Vit E | Control | 740.00 ± 104.12 | 594.07 ± 86.91 | 0.001 | −145.92 ± 157.84 | 0.218 |
| Intervention | 703.05 ± 73.91 | 607.86 ± 69.22 | 0.001 | −95.18 ± 102.56 | ||
Values are mean ± SD for the data with normal distribution and median (interquartile ranges) for the data not normally distributed. PCO: protein carbonyl; NO metabolite: nitric oxide metabolite; hs-CRP: high-sensitivity C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor-α; Vit E: vitamin E.
3.4. Effects of the WC Extract on Inflammatory Markers
Differences between intervention and control groups in inflammatory markers at the end of the study are indicated in Table 2. There was a significant reduction in hs-CRP (8953.30 ± 5588.06 vs. 7249.86 ± 5091.62, P=0.007) and IL-6 (60.10 (55.99, 73.10) vs. 55.21 (53.39, 60.48), P=0.050) in the intervention group, but these change was not significant in comparison with the control group. No statistically significant change was observed between intervention and control groups in hs-CRP (P=0.785), IL-6 (P=0.196), and TNF-α (P=0.276) levels.
3.5. Effects of the WC Extract on the Vit E Level
Table 2 shows the Vit E level of the participants at the baseline and after 28 days of taking the WC extract or without taking the WC extract (control). No significant change in the Vit E level was determined between WC extracts and control groups.
4. Discussion
Oxidative stress is defined as an imbalance between oxidant and antioxidant molecules. Oxidant products include ROS (such as superoxide and hydroxyl radical) and reactive nitrogen species (such as nitric oxide (NO)), while antioxidant molecules include Vit C and E, glutathione, and antioxidant enzymes [9]. Oxidative stress in CKD patients is mediated by several factors such as chronic inflammation, low-molecular-weight uremic toxins, elevated homocysteine level, nutritional inadequacy, and anemia [34, 35].
Oxidative stress is occurring during renal failure and hemodialysis, and it may lead to protein oxidation. The formation of PCO in proteins happens by direct oxidation via ROS, with the final production of oxidized amino acids [35]. To the best of our knowledge, the current study is the first work carried out to investigate the effects of the WC extract on serum levels of PCO and inflammatory markers in hemodialysis patients.
Agreeing with the current results, some studies have indicated that the PCO content increased significantly in hemodialysis and chronic renal failure groups relative to the controls [35–39]. Our finding indicated that the serum PCO contents (a well-confirmed marker of protein oxidation) significantly increased in the control group during the study; this result showed that there is a low-molecular-weight dialyzable substance in the serum of patients on chronic hemodialysis [39] that may lead to protein oxidation.
The main finding of the current study is that the consumption of WC extracts (a dose of 500 mg) for 28 days significantly decreased PCO levels in hemodialysis patients in the intervention group. Furthermore, daily consumption of WC extracts was associated with a marked reduction in serum levels of PCO in the intervention group compared with control subjects. The use of the WC extract in gentamicin- (GM-) induced and vancomycin- (VCM-) induced nephrotoxicity has been studied in animal models [29, 40]. Shahani et al. concluded that treatment with the WC extract (100 and 200 mg/kg) significantly reduced the ROS formation and serum levels of blood urea nitrogen and creatinine and modulated the pathological changes in kidney tissue [40]. Karami et al. revealed that administration of the WC extract (500 mg/kg) significantly decreased the levels of uric acid, creatinine, and malondialdehyde in the blood and kidney in VCM-induced nephrotoxicity [29]. Furthermore, Bahramikia et al. showed that adding Fe2+/ascorbic acid to the liver homogenate markedly augmented ROS and PCO production, while the WC extract (0.1 mL) showed preventing activity against ROS and PCO formation [41]. Also, a previous study demonstrated that the WC extract significantly diminished the PCO level in the liver tissue of bile duct-ligated rats [42]. The strong antioxidant activity of WC has been attributed to various mechanisms such as direct trapping of ROS, removal of peroxides, inhibition of lipid peroxidation, binding to transition metal ion, and inhibition of chain beginning and reductive capacity [43–45].
The use of WC extracts as an anti-inflammatory factor has been studied in various animal models [25, 40, 46]. Shahani et al. indicated that the WC extract (100 and 200 mg/kg) markedly reduced the levels of TNF-α and NO in GM-induced nephrotoxicity [40]. In another study, the WC extract and WC gel decreased the inflammatory cells infiltration and proinflammatory cytokines (such as macrophage inflammatory protein 2 and IL-1β) levels in acute inflammation induced by croton oil [46]. The current study showed significant decreases in serum CRP and IL-6 levels in the intervention group after 4 weeks. Of note, these changes were not statistically significant between intervention and control groups. The insignificant change of inflammation markers between intervention and control groups in the present study may be the result of small sample size, duration of WC extract consumption, and variation in the WC extract type.
Forgarty et al. observed that the lipid-soluble antioxidants such as α-tocopherol, γ-tocopherol, and xanthophyll increased after WC consumption [47]. Gill et al. showed that the consumption of 85 g of raw WC for 8 weeks significantly increased serum antioxidants (β-carotene and lutein) compared with the control group [21]. Also, they indicated that WC can increase lipid and aqueous soluble antioxidants in healthy participants as follows: α-tocopherol (26%), β-carotene (33%), and ascorbic acid (35%) [21]. However, our findings showed no significant change in the Vit E level between WC extract and control groups.
5. Conclusion
In summary, our results indicated that the hydroalcoholic extract of WC reduced the PCO content in hemodialysis patients via inhibition of protein oxidation. Although WC administration had caused significant reductions in IL-6 and CRP levels, these differences were not statistically significant relative to the control group. Further research is needed to identify the antioxidant and anti-inflammatory effects of WC in hemodialysis patients and to improve therapeutic approaches to decrease oxidative stress.
Acknowledgments
This study was financially maintained by the Deputy of Research of the Yasuj University of Medical Sciences (Grant no. 960587), Yasuj, Iran.
Contributor Information
Rozina Abbasi Larki, Email: larki1353@gmail.com.
Amir Hossein Doustimotlagh, Email: amirhosseindoustimotlagh@gmail.com.
Data Availability
The data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Lamarche C., Iliuta I.-A., Kitzler T. Infectious disease risk in dialysis patients: a transdisciplinary approach. Canadian Journal of Kidney Health and Disease. 2019;6 doi: 10.1177/2054358119839080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li D.-Y., Yin W.-J., Zhou L.-Y., et al. Utility of cystatin C-based equations in patients undergoing dialysis. Clinica Chimica Acta. 2018;485:282–287. doi: 10.1016/j.cca.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Sedaghattalab M., Razazan M., Sadeghi H., et al. Effects of nasturtium officinale extract on antioxidant and biochemical parameters in hemodialysis patients: a randomized double-blind clinical trial. Evidence-Based Complementary and Alternative Medicine. 2021;2021:8. doi: 10.1155/2021/1632957.1632957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chua A. N., Warady B. A. Care of the pediatric patient on chronic dialysis. Advances in Chronic Kidney Disease. 2017;24(6):388–397. doi: 10.1053/j.ackd.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Velloso M. S. S., Otoni A., de Paula Sabino A., et al. Peritoneal dialysis and inflammation. Clinica Chimica Acta. 2014;430:109–114. doi: 10.1016/j.cca.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Coombes J. S., Fassett R. G. Antioxidant therapy in hemodialysis patients: a systematic review. Kidney International. 2012;81(3):233–246. doi: 10.1038/ki.2011.341. [DOI] [PubMed] [Google Scholar]
- 7.Roumeliotis S., Eleftheriadis T., Liakopoulos V. Seminars in Dialysis. Hoboken, NJ, USA: Wiley Online Library; 2019. Is oxidative stress an issue in peritoneal dialysis? [DOI] [PubMed] [Google Scholar]
- 8.Bom A. P. A., Rangel L. P., Costa D. C. F., et al. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils implications for cancer. Journal of Biological Chemistry. 2012;287(33):28152–28162. doi: 10.1074/jbc.M112.340638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liakopoulos V., Roumeliotis S., Gorny X., Dounousi E., Mertens P. R. Oxidative stress in hemodialysis patients: a review of the literature. Oxidative Medicine and Cellular Longevity. 2017;2017:22. doi: 10.1155/2017/3081856.3081856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geetha A., Lakshmi Priya M. D, Jeyachristy S. A, Surendran R. Level of oxidative stress in the red blood cells of patients with liver cirrhosis. The Indian Journal of Medical Research. 2007;126(3):204–10. [PubMed] [Google Scholar]
- 11.Haverkamp G. L., Loosman W. L., Schouten R. W., et al. Differences in the association of inflammation and tryptophan with depressive symptoms between white and non-white chronic dialysis patients. General Hospital Psychiatry. 2018;50:76–82. doi: 10.1016/j.genhosppsych.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K., Ikizler T. A., Block G., Avram M. M., Kopple J. D. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. American Journal of Kidney Diseases. 2003;42(5):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Snaedal S., Qureshi A. R., Lund S. H., et al. Dialysis modality and nutritional status are associated with variability of inflammatory markers. Nephrology Dialysis Transplantation. 2016;31(8):1320–1327. doi: 10.1093/ndt/gfw104. [DOI] [PubMed] [Google Scholar]
- 14.Wang A. Y.-M. Seminars in Nephrology. Amsterdam, Netherlands: Elsevier; 2011. Consequences of chronic inflammation in peritoneal dialysis. [DOI] [PubMed] [Google Scholar]
- 15.Himmelfarb J., Phinney S., Ikizler T. A., Kane J., McMonagle E., Miller G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. Journal of Renal Nutrition. 2007;17(5):296–304. doi: 10.1053/j.jrn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Korevaar J. C., van Manen J. G., Dekker F. W., et al. Effect of an increase in C-reactive protein level during a hemodialysis session on mortality. Journal of the American Society of Nephrology. 2004;15(11):2916–2922. doi: 10.1097/01.asn.0000143744.72664.66. [DOI] [PubMed] [Google Scholar]
- 17.Cobo G., Lindholm B., Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrology Dialysis Transplantation. 2018;33(3):iii35–iii40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisten K., Gounden D., Moodley R., Jonnalagadda S. B. Elemental distribution and uptake by watercress (Nasturtium aquaticum) as a function of water quality. Journal of Environmental Science and Health, Part B. 2015;50(6):439–447. doi: 10.1080/03601234.2015.1011971. [DOI] [PubMed] [Google Scholar]
- 19.Casanova N. A., Carballo M. A. Antigenotoxic activity of watercress extract in an in vitro mammalian system using comet assay. Phytotherapy Research. 2011;25(12):1743–1746. doi: 10.1002/ptr.3474. [DOI] [PubMed] [Google Scholar]
- 20.Syamsianah A., Anggraini H. Control of lipid profile on diabetes mellitus animal models with watercress and black rice bran. Proceedings of the Prosiding Seminar Nasional & Internasional; 2016; Jawa Tengah, Indonesia. [Google Scholar]
- 21.Gill C. I., Haldar S., Boyd L. A., et al. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. The American Journal of Clinical Nutrition. 2007;85(2):504–510. doi: 10.1093/ajcn/85.2.504. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann T., Kuhnert A., Schubert A., et al. Modulation of detoxification enzymes by watercress: in vitro and in vivo investigations in human peripheral blood cells. European Journal of Nutrition. 2009;48(8):483–491. doi: 10.1007/s00394-009-0039-5. [DOI] [PubMed] [Google Scholar]
- 23.Mousa-Al-Reza Hadjzadeh Z. R., Moradi R., Ghorbani A. Effects of hydroalcoholic extract of watercress (Nasturtium officinale) leaves on serum glucose and lipid levels in diabetic rats. Indian Journal of Physiology and Pharmacology. 2015;59:223–230. [PubMed] [Google Scholar]
- 24.Fenton-Navarro B., Urquiza-Martinez M., Fiscal-Castro B., et al. Evaluation of the hypoglycemic and oxidative stress effect of watercress (Nasturtium officinale) on hyperglycemic rats. Planta Medica. 2016;82(1):p. P181. doi: 10.1055/s-0036-1596338. [DOI] [Google Scholar]
- 25.Sadeghi H., Mostafazadeh M., Sadeghi H., et al. In vivoanti-inflammatory properties of aerial parts ofNasturtium officinale. Pharmaceutical Biology. 2014;52(2):169–174. doi: 10.3109/13880209.2013.821138. [DOI] [PubMed] [Google Scholar]
- 26.Spínola V., Pinto J., Castilho P. C. In vitro studies on the effect of watercress juice on digestive enzymes relevant to type 2 diabetes and obesity and antioxidant activity. Journal of Food Biochemistry. 2017;41(1):p. e12335. doi: 10.1111/jfbc.12335. [DOI] [Google Scholar]
- 27.Boligon A. A., Janovik V., Boligon A. A., et al. HPLC analysis of polyphenolic compounds and antioxidant activity inNasturtium officinale. International Journal of Food Properties. 2013;16(1):61–69. doi: 10.1080/10942912.2010.528111. [DOI] [Google Scholar]
- 28.Payne A. C., Mazzer A., Clarkson G. J. J., Taylor G. Antioxidant assays—consistent findings from FRAP and ORAC reveal a negative impact of organic cultivation on antioxidant potential in spinach but not watercress or rocket leaves. Food Science & Nutrition. 2013;1(6):439–444. doi: 10.1002/fsn3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karami M., Mostafazade M., Sadeghi H., et al. Nephroprotective effect of Nasturtium officinale (watercress) ethanol extract and Vitamin E on vancomycin-induced nephrotoxicity in rats. Jundishapur Journal of Natural Pharmaceutical Products. 2018;13(1) doi: 10.5812/jjnpp.67178. [DOI] [Google Scholar]
- 30.Karami M. Protective effects of Nasturtium officinale against gamma-irradiation-induced hepatotoxicity in C57 mice. Research Journal of Pharmacognosy. 2015;2(2):19–25. [Google Scholar]
- 31.Azarmehr N., Afshar P., Moradi M., et al. Hepatoprotective and antioxidant activity of watercress extract on acetaminophen-induced hepatotoxicity in rats. Heliyon. 2019;5(7) doi: 10.1016/j.heliyon.2019.e02072.e02072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadeghi H., Mansourian M., Panahi E., et al. Antioxidant and protective effect of Stachys pilifera Benth against nephrotoxicity induced by cisplatin in rats. Journal of Food Biochemistry. 2020;44(5) doi: 10.1111/jfbc.13190.e13190 [DOI] [PubMed] [Google Scholar]
- 33.Mansourian M., Mirzaei A., Azarmehr N., et al. Hepatoprotective and antioxidant activity of hydroalcoholic extract of Stachys pilifera. Benth on acetaminophen-induced liver toxicity in male rats. Heliyon. 2019;5(12) doi: 10.1016/j.heliyon.2019.e03029.e03029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlović D., Savić-Radojević A., Plješa-Ercegovac M., et al. Biomarkers of oxidative damage and antioxidant enzyme activities in pre-dialysis Balkan endemic nephropathy patients. International Urology and Nephrology. 2016;48(2):257–263. doi: 10.1007/s11255-015-1192-9. [DOI] [PubMed] [Google Scholar]
- 35.Albarello K., dos Santos G. A., Bochi G. V., et al. Ischemia modified albumin and carbonyl protein as potential biomarkers of protein oxidation in hemodialysis. Clinical Biochemistry. 2012;45(6):450–454. doi: 10.1016/j.clinbiochem.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 36.Dursun E., Dursun B., Suleymanlar G., Ozben T. Effect of haemodialysis on the oxidative stress and antioxidants in diabetes mellitus. Acta Diabetologica. 2005;42(3):123–128. doi: 10.1007/s00592-005-0191-1. [DOI] [PubMed] [Google Scholar]
- 37.Dursun E., Dursun B., Süleymanlar G., Ozben T. Carbonyl stress in chronic renal failure: the effect of haemodialysis. Annals of Clinical Biochemistry. 2005;42(1):64–66. doi: 10.1258/0004563053026808. [DOI] [PubMed] [Google Scholar]
- 38.Pavone B., Sirolli V., Giardinelli A., et al. Plasma protein carbonylation in chronic uremia. Journal of Nephrology. 2011;24(4):453–464. doi: 10.5301/jn.2011.8342. [DOI] [PubMed] [Google Scholar]
- 39.Himmelfarb J., McMonagle E., McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney International. 2000;58(6):2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- 40.Shahani S., Behzadfar F., Jahani D., Ghasemi M., Shaki F. Antioxidant and anti-inflammatory effects of Nasturtium officinale involved in attenuation of gentamicin-induced nephrotoxicity. Toxicology Mechanisms and Methods. 2017;27(2):107–114. doi: 10.1080/15376516.2016.1258748. [DOI] [PubMed] [Google Scholar]
- 41.Bahramikia S., Ardestani A., Yazdanparast R. Protective effects of four Iranian medicinal plants against free radical-mediated protein oxidation. Food Chemistry. 2009;115(1):37–42. doi: 10.1016/j.foodchem.2008.11.054. [DOI] [Google Scholar]
- 42.Sadeghi H., Jahanbazi F., Sadeghi H., et al. Metformin attenuates oxidative stress and liver damage after bile duct ligation in rats. Research in Pharmaceutical Sciences. 2019;14(2):p. 122. doi: 10.4103/1735-5362.253359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozen T. Investigation of antioxidant properties of Nasturtium officinale (watercress) leaf extracts. Acta Poloniae Pharmaceutica. 2008;66(2):187–193. [PubMed] [Google Scholar]
- 44.Diplock A. T. Will the “good fairies” please prove to us that vitamin E lessens human degenerative disease? Free Radical Research. 1997;27(5):511–532. doi: 10.3109/10715769709065791. [DOI] [PubMed] [Google Scholar]
- 45.Bahramikia S., Yazdanparast R. Antioxidant efficacy of Nasturtium officinale extracts using various in vitro assay systems. Journal of Acupuncture and Meridian Studies. 2010;3(4):283–290. doi: 10.1016/s2005-2901(10)60049-0. [DOI] [PubMed] [Google Scholar]
- 46.Camponogara C., Silva C. R., Brusco I., et al. Nasturtium officinale R. Br. effectively reduces the skin inflammation induced by croton oil via glucocorticoid receptor-dependent and NF-κB pathways without causing toxicological effects in mice. Journal of Ethnopharmacology. 2019;229:190–204. doi: 10.1016/j.jep.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Fogarty M. C., Hughes C. M., Burke G., Brown J. C., Davison G. W. Acute and chronic watercress supplementation attenuates exercise-induced peripheral mononuclear cell DNA damage and lipid peroxidation. British Journal of Nutrition. 2013;109(2):293–301. doi: 10.1017/s0007114512000992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available within the article.


