Abstract
We synthesized 33 new phenylpiperazine derivatives and assessed their acaricidal activity. These derivatives were synthesized through sequential reactions consisting of the sulfonylation of 2-substituted 4-methylaniline with chlorosulfonic acid, reduction with red phosphorus and iodine, alkylation by alkyl halide, cyclization with bis(2-chloroethyl)amine hydrochloride, and N-substitution reaction of phenylpiperazines with various reagents. All phenylpiperazines synthesized were evaluated for acaricidal activity and their structure–activity relationships discussed, it was found that 4-substituted 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]piperazine derivatives exhibited good acaricidal activity. Among them, 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]-4-(trifluoromethylsulfonyl) piperazine showed the highest level of activity against Tetranychus urticae and provided a high level of activity against Tetranychus kanzawai and Panonychus citri. In addition, studies on the effect at various stages of T. urticae exhibited that this compound showed good activity against both adults and eggs.
Keywords: phenylpiperazine, acaricidal activity, Tetranychus urticae, Tetranychus kanzawai and Panonychus citri
Introduction
About 300,000 to 500,000 species of mites are distributed throughout the world. Among them, spider mites perform a piercing and sucking process to remove cell contents, resulting in reduced photosynthesis and transpiration rates in plants.1,2) Plants slightly infected by spider mites show leaf discoloration and defoliation, bud and fruit shedding, and reduced fruit yield and quality; in serious cases, the entire plant may die.3) As a result, more than 150 crops types, such as cotton, citrus, apples, vegetables, tea, and flowers are damaged, causing significant losses to agriculture and forestry every year.4–11)
On the other hand, 53 species of resistant mites have been reported to exist in the world.9) This resistance is caused by the inappropriate and frequent use of pesticides and a series of characteristics of the mites themselves, such as a short generation cycle, rapid reproduction, a high inbreeding rate, and a high mutation rate, the number of mites with serious resistance to existing acaricides is increases every year.12–14) Therefore, it is always desirable to develop innovative agrochemicals with new modes of action and structures for spider mite control.
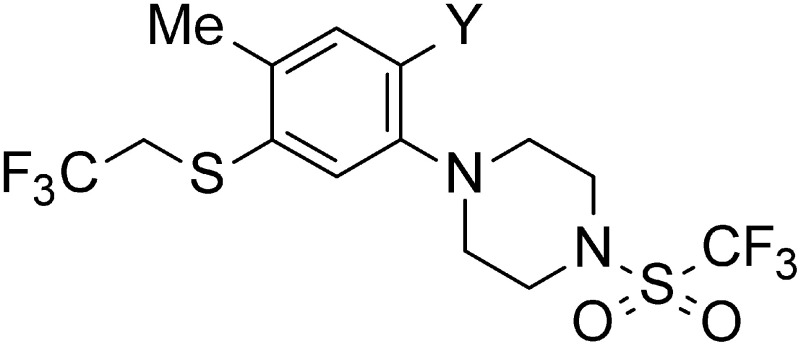
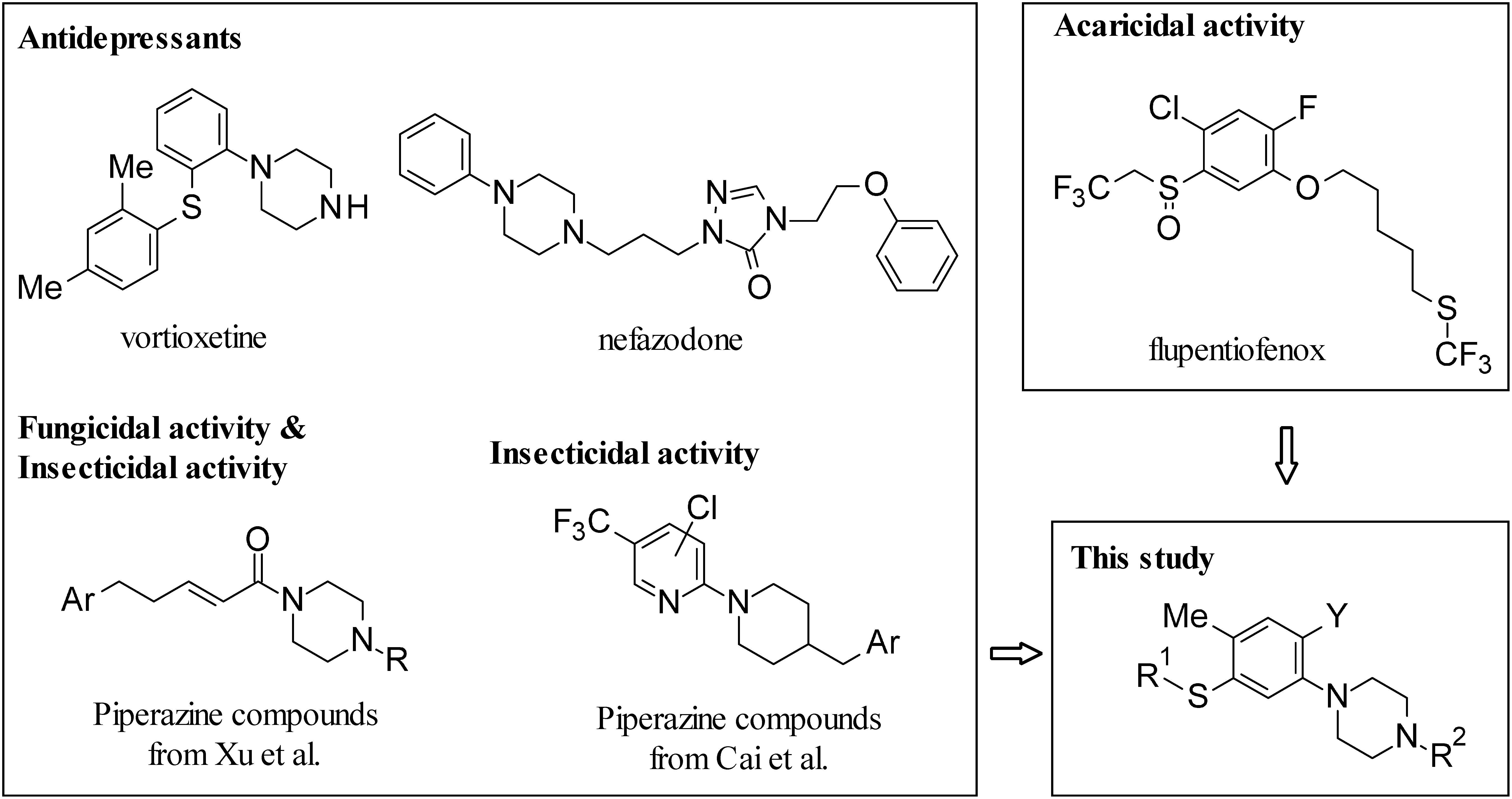
In the process of finding promising new acaricides, we focused on phenylpiperazines such as vortioxetine and negazodone, launched as antidepressants, which are attracting attention as target molecules for drug development. In addition, piperazine derivatives have been studied for applications in insecticides and fungicides.15,16) On the other hand, we also focused on a recently developed acaricide, flupentiofenox, having a 2,2,2-trifluoroethylsulfanyl group in the molecule.17) With these things in mind, we developed a series of new synthetic phenylpiperazine derivatives having a 2,2,2-trifluoroethylsulfanyl group and other related sulfanyl groups (Fig. 1). The present paper deals with the synthesis and acaricidal activity of various phenylpiperazine derivatives against Tetranychus urticae (two-spotted spider mite) and their structure–activity relationships. For the most active compound selected, further studies comparing its efficacy against T. urticae, Tetranychus kanzawai (Kanzawa spider mite) and Panonychus citri (citrus red mite), as well as its efficacy against T. urticae eggs, were conducted.
Fig. 1. Piperazine compounds with biological activity and flupentiofenox as an acaricide, and target compounds in this study.
Materials and methods
1. Synthesis of chemicals
1.1. General procedure
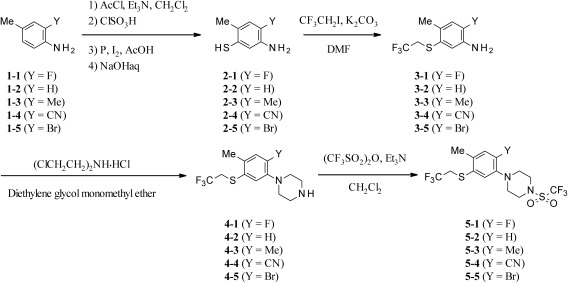
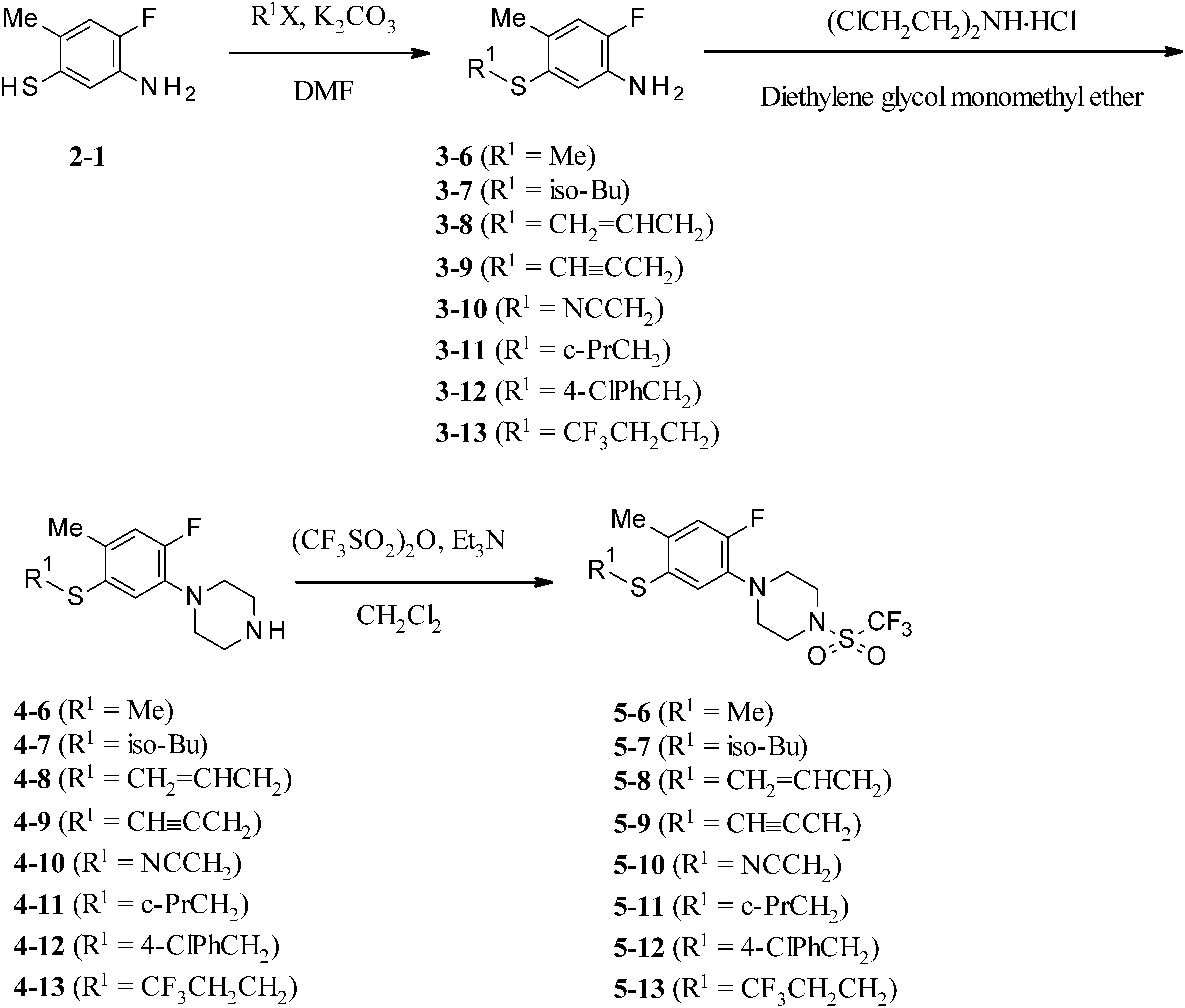
The synthetic route of 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]-4-(trifluoromethylsulfonyl)piperazine (5-1) is depicted in Fig. 2. Commercially available 2-fluoro-4-methylaniline (1-1) was reacted with acetyl chloride to give N-acetyl 2-fluoro-4-methylaniline, which was treated with chlorosulfonic acid, giving 4-fluoro-5-amino-2-methylbenzenesulfonyl chloride. The obtained benzenesulfonyl chloride was converted to 4-fluoro-5-amino-2-methylbenzenethiol (2-1) with a good yield by reducing it with red phosphorus in the presence of a catalytic amount of iodine. Then the benzenethiol derivative (2-1) was reacted with 2,2,2-trifluoroethyl iodide to give the 2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)aniline (3-1). The reaction of 3-1 with bis(2-chloroethyl)amine hydrochloride was carried out in diethylene glycol monomethyl ether and the corresponding phenylpiperazine (4-1) was obtained. Finally, trifluoromethanesulfonation of 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl] piperazine (4-1) with trifluoromethansulfonic anhydride in the presence of triethylamine afforded 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]-4-(trifluoromethylsulfonyl)piperazine (5-1). A low-field chemical shift of the piperazine ring protons in 1H NMR spectra certainly suggested the addition of a trifluoromethylsulfonyl group.
Fig. 2. Synthetic routes of 2-substituted phenylpiperazine derivatives (5-1–5).
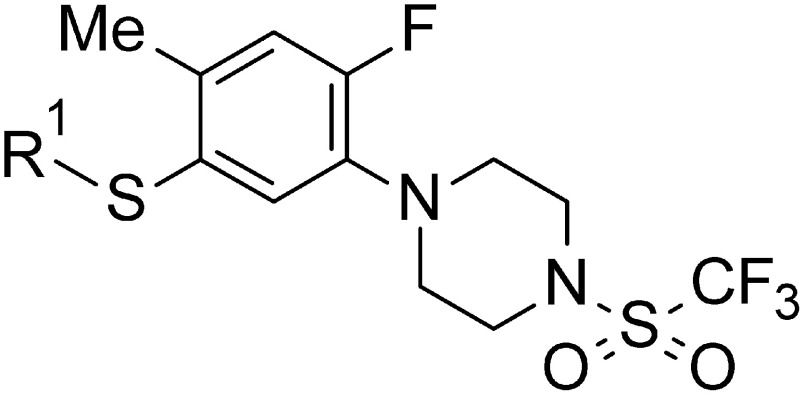
The synthetic route of 1-[5-substituted sulfanyl-2-fluoro-4-methylphenyl]-4-(trifluoromethylsulfonyl) piperazine derivatives (5-6–13) is shown in Fig. 3. The key intermediate 2-1 from Fig. 2 was treated with several alkyl halides and potassium carbonate in N,N-dimethylformamide (DMF) to afford 2-fluoro-4-methyl-5-substituted sulfanyl aniline derivatives (3-6–13). The corresponding phenylpiperazine derivatives (4-6–13) were prepared by cyclizing the aniline derivatives (3-6–13) with bis(2-chloroethyl)amine hydrochloride. Compounds (4-6–13) were reacted with trifluoromethanesulfonic anhydride to give the desired final products (5-6–13) under the same condition as that for 5-1.
Fig. 3. Synthetic routes of 5-substituted sulfanyl phenylpiperazine derivatives (5-6–13).
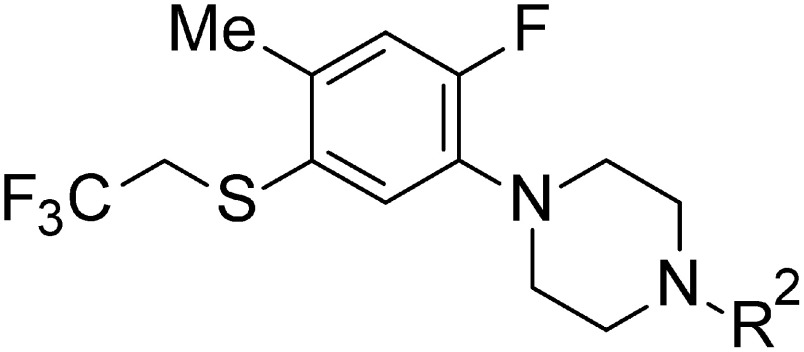
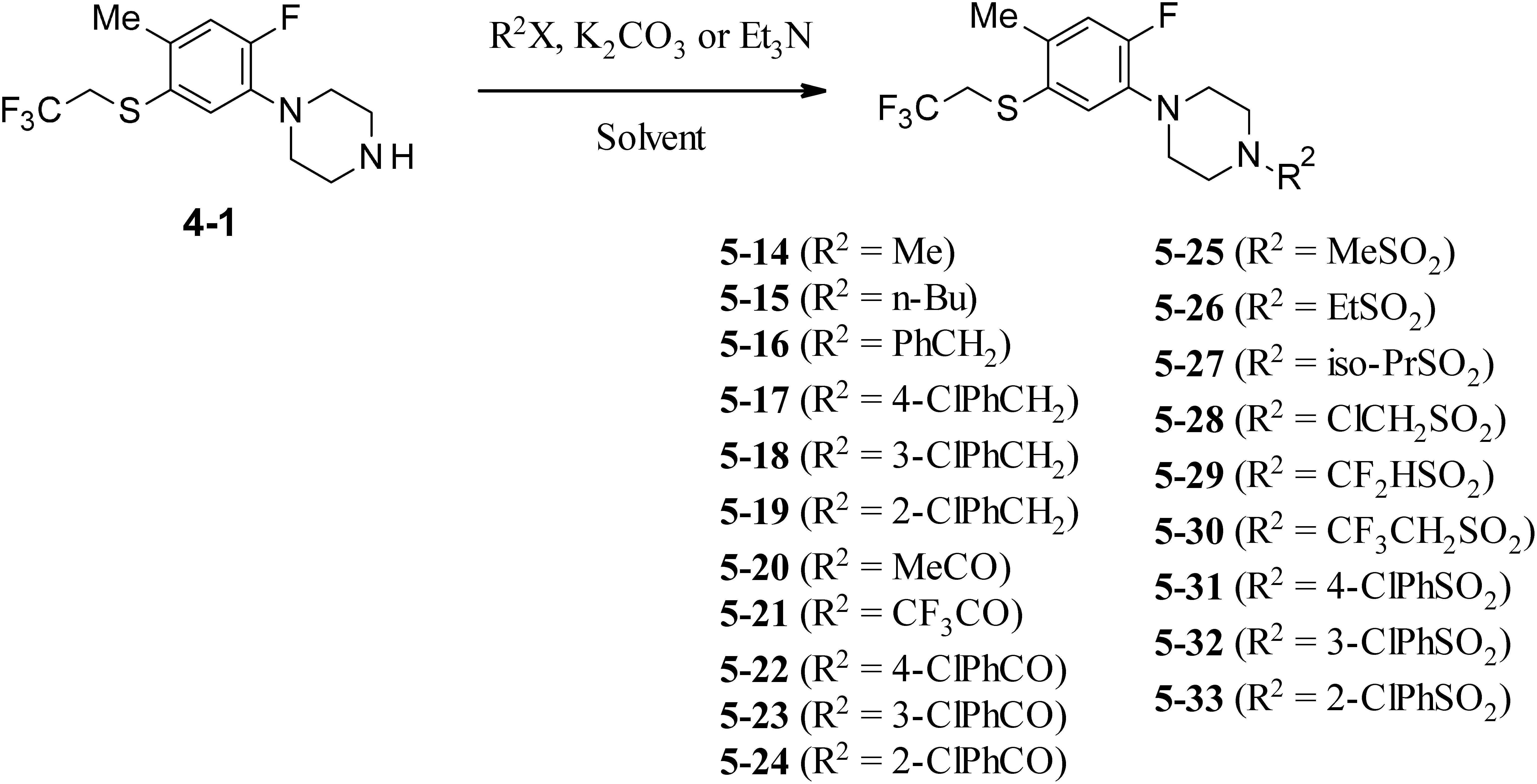
1-[2-Fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]piperazine derivatives substituted on the nitrogen atom of a piperazine ring were prepared as outlined in Fig. 4. The alkyl and benzyl derivatives were synthesized via alkylation and benzylation of a key intermediate 4-1 from Fig. 2. The reaction with several alkyl halides and substituted benzyl halides in the presence of potassium carbonate in DMF gave the corresponding 4-alkyl-1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl] piperazine derivatives (5-14, 15) and substituted 4-benzyl-1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl] piperazine derivatives (5-16–19). Similarly, 4-substituted carbonyl-1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl] piperazines (5-20–24) and 4-substituted sulfonyl-1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]piperazines (5-25–33) were synthesized from the same intermediate 4-1 as shown in Fig. 2. Amidation and sulfonamidation of compound 4-1 with several acid halides and sulfonyl halides in the presence of triethylamine afforded the desired 4-substituted 1-phenylpiperazine derivatives (5-20–33).
Fig. 4. Synthetic routes of N-substituted phenylpiperazine derivatives (5-14–33).
1.2. Typical procedure
The general synthetic methods for the representative compounds are described below. The chemical structures of all compounds were confirmed by 1H NMR spectra, which were recorded on a JEOL JNM-400 (400 MHz) spectrometer using tetramethylsilane (TMS) as an internal standard. All melting points (mps) were measured by a Mettler Toledo MP90 melting point system and are uncorrected.
1.2.1. 5-Amino-4-fluoro-2-methylbenzenethiol (2-1)
To a dichloromethane (200 mL) solution of 2-fluoro-4-methlyaniline (25.3 g, 202 mmol) and triethylamine (30.7 g, 304 mmol) was added acetyl chloride (17.4 g, 222 mmol) at 0°C and the mixture was stirred at room temperature for 4 hr. The resulting mixture was poured into water and extracted with chloroform. The organic layer was washed with brine, dried over anhydrous sodium sulfate, and evaporated. A solid deposit was washed with hexane to give 2-fluoro-4-methly-acetanilide (28.0 g, 83%) as a white solid. The obtained solid was added to chlorosulfonic acid (58.0 g, 498 mmol) at 0°C, and the mixture was stirred at 80°C for 2 hr. The resulting mixture was poured into ice water and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and evaporated. A solid deposit was washed with diisopropyl ether to give 5-acetoamino-4-fluoro-2-methylbenzenesulfonyl chloride (19.3 g, 43%) as a white solid. To an acetic acid (80 mL) solution of the obtained solid were added red phosphorus (7.87 g, 254 mmol) and iodine (921 mg, 3.63 mmol) at room temperature, and the mixture was stirred under reflux for 2 hr. The resulting mixture was poured into ice water and extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and evaporated. A solid deposit was washed with hexane to give 5-acetylthio-2-fluoro-4-methly-acetanilide (16.3 g, 93%) as a white solid. The obtained solid was added to a 10% aqueous solution of sodium hydroxide (400 mL), and the mixture was stirred under reflux for 3 hr. A diluted hydrochloric acid was added to the mixture to adjust it to pH 7. The resulting mixture was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and evaporated. The residue was purified via silica gel column chromatography (ethyl acetate / hexane=1 / 3) to give 2-1 (8.50 g, 80%) as a white solid. mp: 55–58°C; 1H NMR δH (CDCl3, TMS): 6.80 (1H, d, J H,F=12.0 Hz), 6.75 (1H, d, J H,F=8.4 Hz), 3.57 (2H, br.s), 3.14 (1H, br.s), 2.22 (3H, s).
1.2.2. 2-Fluoro-4-methyl-5-(2,2,2-trifluoroethylthio)aniline (3-1)
To a N,N-dimethylformamide (5 mL) solution of 5-amino-4-fluoro-2-methylbenzenethiol (950 mg, 6.04 mmol) were added potassium carbonate (1.00 g, 7.24 mmol) and 2,2,2-trifluoro-1-iodoethane (1.27 g, 6.05 mmol) at room temperature and the mixture was stirred at room temperature for 4 hr. The resulting mixture was poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate and evaporated. The residue was purified via silica gel column chromatography (ethyl acetate / hexane=1 / 3) to give 3-1 (1.16 g, 80%) as a yellow oil. 1H NMR δH (CDCl3, TMS): 6.98 (1H, d, J H,F=9.0 Hz), 6.86 (1H, d, J H,F=10.4 Hz), 3.63 (2H, br.s), 3.28 (2H, q, J=9.9 Hz), 2.36 (3H, s).
1.2.3. 1-[2-Fluoro-4-methyl-5-(2,2,2-trifluoroethylthio)phenyl]piperazine (4-1)
To a diethylene glycol monomethyl ether (5 mL) solution of 2-fluoro-4-methyl-5-(2,2,2-trifluoroethylthio)aniline (3.58 g, 15.0 mmol) was added bis(2-chloroethyl)amine hydrochloride (2.70 g, 15.0 mmol), and the mixture was stirred at 150°C for 10 hr. The resulting mixture was poured into an aqueous solution of saturated sodium bicarbonate and extracted with chloroform. The organic layer was dried over anhydrous sodium sulfate and evaporated. The residue was purified via silica gel column chromatography (methanol / chloroform=1 / 5) to give 4-1 (4.00 g, 86%) as a brown oil. 1H NMR δH (CDCl3, TMS): 7.13 (1H, d, J H,F=9.0 Hz), 6.91 (1H, d, J H,F=13.2 Hz), 3.28 (2H, q, J=9.0 Hz), 3.11–3.01 (8H, m), 2.41 (3H, s), 1.70 (1H, br.s).
1.2.4. 1-[2-Fluoro-4-methyl-5-(2,2,2-trifluoroethylthio)phenyl]-4-(trifluoromethylsulfonyl)piperazine (5-1)
To a dichloromethane (3 mL) solution of 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylthio)phenyl]piperazine (150 mg, 0.486 mmol) were added triethylamine (98.0 mg, 0.970 mmol) and trifluoromethanesulfonic anhydride (206 mg, 0.730 mmol) at 0°C, and the mixture was stirred at 0°C for 0.5 hr. The resulting mixture was poured into an aqueous solution of saturated sodium bicarbonate and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate and evaporated. The residue was purified via silica gel column chromatography (ethyl acetate / hexane=1 / 5) to give 5-1 (122 mg, 57%) as a white solid. mp: 84–86°C; 1H NMR δH (CDCl3, TMS): 7.13 (1H, d, J H,F=8.8 Hz), 6.95 (1H, d, J H,F=12.8 Hz), 3.69–3.64 (4H, m), 3.28 (2H, q, J=9.6 Hz), 3.16–3.11 (4H, m), 2.43 (3H, s). ESI-MS: m/z 441.20 [M+H]+.
1.2.5. 1-(4-Chlorobenzyl)-4-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylthio)phenyl])piperazine (5-17)
To a N,N-dimethylformamide (1 mL) solution of 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylthio)phenyl]piperazine (100 mg, 0.324 mmol) were added potassium carbonate (67.0 mg, 0.485 mmol) and 4-chlorobenzyl chloride (57.0 mg, 0.354 mmol) at room temperature, and the mixture was stirred at room temperature for 3 hr. The resulting mixture was poured into water and extracted with ethyl acetate. The organic layer was washed with brine, dried over anhydrous sodium sulfate, and evaporated. The residue was purified via silica gel column chromatography (ethyl acetate / hexane=1 / 3) to give 5-17 (110 mg, 78%) as a yellow oil. 1H NMR δH (CDCl3, TMS): 7.30–7.27 (4H, m), 7.12 (1H, d, J H,F=8.8 Hz), 6.90 (1H, d, J H,F=13.2 Hz), 3.53 (2H, s), 3.27 (2H, q, J=9.6 Hz), 3.07 (4H, t, J=4.8 Hz), 2.60 (4H, t, J=4.8 Hz), 2.42 (3H, s). ESI-MS: m/z 433.15 [M+H]+.
2. Acaricidal assays
2.1. Tetranychus urticae
The acaricidal activity of phenylpiperazine derivatives was evaluated using the foliar spray method with T. urticae, which was multiplied from a sensitive strain. The test solution was prepared as 100 g a.i./L of emulsifiable concentrate (EC) formulation of the phenylpiperazine derivatives and diluted to several concentrations (100, 10, 3, 1 ppm) with water containing a wetting agent (High-ten-Power®, 0.2 mL/L). The day before treatment with the test solution, ten adult female spider mites were released on the leaf of a kidney bean plant (cv.Himetebo, growth stage: first foliage leaf developing). The plant and spider mites were sprayed with an equivalent of 300 L per hectare of the test solution. Control plots were treated with water containing the agent. Each treatment consisted of two replicates. The plants were held in an air-controlled room (16 : 8L : D, 25°C). Eight days after treatment, the surviving mites were counted.
2.2. Tetranychus kanzawai
The acaricidal activity of phenylpiperazine derivatives was evaluated using the foliar spray method with T. kanzawai, which was multiplied from a sensitive strain. The test solution was prepared as 100 g a.i./L of EC formulation of the phenylpiperazine derivatives and diluted to several concentrations (10, 3, 1 ppm) with water containing a wetting agent (High-ten-Power®, 0.2 mL/L). The day before treatment with the test solution, ten adult female spider mites were released on the leaf of a kidney bean plant (cv.Himetebo, growth stage: first foliage leaf developing). The plant and spider mites were sprayed with an equivalent of 300 L per hectare of the test solution. Control plots were treated with water containing the agent. Each treatment consisted of two replicates. The plants were held in an air-controlled room (16 : 8L : D, 25°C). Eight days after treatment, the surviving mites were counted.
2.3. Adults of Panonychus citri
The acaricidal activity of phenylpiperazine derivatives was evaluated using the leaf-disk method with P. citri, which was multiplied from a sensitive strain. The test solution was prepared as 100 g a.i./L of EC formulation of the phenylpiperazine derivatives and diluted to several concentrations (10, 3, 1 ppm) with water containing a wetting agent (High-ten-Power®, 0.2 mL/L). The day before treatment with the test solution, five adult female spider mites were released onto each of five citrus leaf disks (1 cm diameter) on an agar medium (4 g a.i./L) in a Petri dish (9 cm). Five leaf disks of citrus were lay on the agar medium in a petri dish. The leaf disks and spider mites were sprayed with an equivalent of 300 L per hectare of the test solution. Control plots were treated with water containing the agent. The spider mites on leaf disks were held in an air-controlled room (16 : 8L : D, 25°C). Three days after treatment, the surviving mites were counted.
2.4. Adults of T. urticae
The acaricidal activity of phenylpiperazine derivatives was evaluated using the leaf-disk method with T. urticae, which was multiplied from a sensitive strain. The test solution was prepared as 100 g a.i./L of EC formulation of the phenylpiperazine derivatives and diluted to several concentrations (10, 3, 1 ppm) with water containing a wetting agent (High-ten-Power®, 0.2 mL/L). The day before treatment with the test solution, ten adult female spider mites were released onto each leaf disk (3 cm diameter) of a kidney bean plant on an agar medium (4 g a.i./L) in a Petri dish (9 cm). Three kidney bean leaf disks were laid on the agar medium in a Petri dish. The leaf disks and spider mites were sprayed with an equivalent of 300 L per hectare of the test solution. Control plots were treated with water containing the agent. The spider mites on leaf disks were held in an air-controlled room (16 : 8L : D, 25°C). Three days after treatment, the surviving mites were counted.
2.5. Eggs of T. urticae
The acaricidal activity of phenylpiperazine derivatives was evaluated using the leaf-disk method with eggs of T. urticae, which was multiplied from a sensitive strain. The test solution was prepared as 100 g a.i./L of EC formulation of the phenylpiperazine derivatives and diluted to several concentrations (10, 3, 1 ppm) with water containing a wetting agent (High-ten-Power®, 0.2 mL/L). The day before treatment with the test solution, five adult females of T. urticae were released onto each leaf disk (3 cm diameter) of a kidney bean plant on an agar medium (4 g a.i./L) and allowed to oviposit for 24 hr. Three kidney bean leaf disks were laid on the agar medium in a petri dish. After removal of the adults, the leaf disks with eggs were sprayed with an equivalent of 300 L per hectare of the test solution. Control plots were treated with water containing the agent. The spider mites on leaf disks were held in an air-controlled room (16 : 8L : D, 25°C). Seven days after treatment, the surviving mites and unhatched eggs were counted.
Results and discussion
1. Synthesis
The desired compound 5-1 was synthesized through successive reactions consisting of the formation of 4-fluoro-5-amino-2-methylbenzenethiol (2-1), alkylation of the thiophenol of 2-1, cyclization of the aniline (3-1), and trifluoromethanesulfonation of the piperazine 4-1 as shown in Fig. 2. We found that after the acetylation of aniline (1) to consider the rules of electrophilic aromatic substitution, treatment of the acetyl-protected compound with chlorosulfonic acid led to an almost perfect regioselective reaction. For the subsequent reduction of sulfonyl chloride, we chose to use red phosphorus and iodine, but we could also obtain the desired 2-1 by other common reduction methods. From the J H–F coupling of 1H NMR data on the benzene ring, it is suggested that proton atoms remain in the ortho and meta positions from the fluorine atom, and the sulfonyl group was determined to be located at the para position from the fluorine atom. Next, we attempted to convert the obtained 2-1 into alkylated products. As a result, S-selective alkylation of aminobenzenethiol 2-1 was achieved with a stoichiometric amount of 2,2,2-trifluoroethyl iodide, leading to the compound 3-1 without a N-alkylated product. For the formation of the piperazine ring, we followed the method of Paudel et al. using bis(2-chloroethyl)amine hydrochloride at a high temperature because of its high yield and easy operation18) described in several reports.19–22) In the cyclization reaction of 3-1 with bis(2-chloroethyl)amine hydrochloride leading to the compound 4-1. Finally, we converted the piperazine 4-1 with Tf2O into the compound 5-1. Phenylpiperazine derivatives 5-2–5, having other substituents at the 2-position, were similarly synthesized using the same methods as for 5-1. The selectivity of all sulfonation and alkylation was also good.
Following the synthesis methods for 2-substituted phenylpiperazine derivatives (5-1–5), 5-substituted sulfanyl phenylpiperazine derivatives (5-6–13) were also synthesized, providing 5-substituted sulfanyl aniline derivatives (3-6–13) prepared through various substitution reactions of benzenethiol (2-1) with several alkyl halides (Fig. 3). In the cyclization reactions of 3-6–13, the corresponding phenylpiperazine derivatives (4-6–13) yielded as much as 4-1. The subsequent sulfonation with Tf2O was successfully advanced to give 5-6–13.
As mentioned later, 4-substituted 1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]piperazine was more acaricidally active against T. urticae than were 2-substitued phenylpiperazine derivatives (5-2–5) and 5-substituted sulfanyl phenylpiperazine derivatives (5-6–13); therefore, many kinds of phenylpiperazines (5-14–33) with various substituents on the piperazine-nitrogen atom were synthesized. There are two types of reaction conditions, inorganic base and organic base, shown in Fig. 4. In the alkylation and benzylation reactions, 5-14–19 were synthesized in the presence of K2CO3. On the other hand, amidation and sulfonamidation reactions in the presence of triethylamine afforded 5-20–33.
2. Acaricidal activity
Acaricidal activity against T. urticae was evaluated for 33 types of phenylpiperazine, and the results are summarized in Tables 1–3. Further, acaricidal activity against T. urticae, T. kanzawai, and P. citri was evaluated for 5-1, and the results are summarized in Table 4. Finally, Table 5 shows the acaricidal activity of 5-1 against adults and eggs of T. urticae. Acaricidal activity was graded as follows: 10: mortality 100%; 9: 99–90%; 8: 89–80%; 7: 79–70%; 6: 69–60%; 5: 59–50%; 4: 49–40%; 3: 39–30%; 2: 29–20%; 1: 19–10%; 0: 9–0%.
Table 1. The influence of the substituents of the 2-position on the benzene ring on acaricidal activity against T. urticae.
Table 4. Acaricidal activity of compound 5-1 against T. urticae, T. kanzawai and, P. citri.
| No. | Concentration (ppm) | Acaricidal activity | ||
|---|---|---|---|---|
| T. urticae | T. kanzawai | P. citri | ||
| 5-1 | 10 | 10 | 10 | 10 |
| 3 | 10 | 10 | 10 | |
| 1 | 9 | 7 | 6 | |
| fenpyroximate | 10 | 10 | 10 | 7 |
| 3 | 10 | 10 | 4 | |
| 1 | 9 | 9 | 2 | |
Table 5. Acaricidal activity of the compound 5-1 against adults and eggs of T. urticae.
| No. | Concentration (ppm) | Acaricidal activity (T. urticae) | |
|---|---|---|---|
| Adults | Eggs | ||
| 5-1 | 10 | 10 | 10 |
| 3 | 10 | 10 | |
| 1 | 7 | 6 | |
| fenpyroximate | 10 | 10 | 10 |
| 3 | 9 | 8 | |
| 1 | 6 | 5 | |
2.1. The influence of Y on acaricidal activity against T. urticae
Table 1 summarizes the acaricidal activity of the phenylpiperazine derivatives (5-1–5) substituted with a halogen atom, a methyl group or a cyano group at the 2-position of the benzene ring. In every substituent, phenylpiperazine derivatives (5-1–5) tended to exhibit excellent acaricidal activity against T. urticae at the rate of 100 ppm. Compared with the unsubstituted compound 5-2, the introduction of a fluorine atom at the 2-position of the benzene ring increased activity, and it fully controlled T. urticae even at 3 ppm; however, no considerable influence was recognized in the activity relationships between 5-2 and its methyl derivative (5-3). Furthermore, a marked increase in the activity of 5-4 obviously indicated that the cyano group is also effective for eliciting good efficacy against T. urticae. These findings suggest that the electron-withdrawing group might be of importance for the high activity. On the other hand, the bromo-substituted compound (5-5) was moderately active because of having a bulky halogen atom or a weaker C–Br bond energy than the C–F bond.
2.2. The influence of R1 on acaricidal activity against T. urticae
Table 2 shows the influence of the substituents of the sulfur atom on acaricidal activity. 5-Alkyl-substituted sulfanylphenylpiperazine derivatives (5-6–7) showed poor activity in comparison with 5-1, and the alkenyl group (5-8), alkynyl group (5-9) and cyanomethyl group (5-10) exhibited almost the same activity levels as 5-6–7; however, 5-cyclopropylmethylsulfanylphenylpiperazine (5-11) showed exceptional activity. On the other hand, the 4-chlorobenzyl type (5-12) was inactive because of having a bulky substituent. In addition, the introduction of a 3,3,3-trifluoropropyl group as a fluoroalkyl group similar to the 2,2,2-trifluoroethyl group, which is the best substituent for acaricidal activity, decreased activity. We thus assume that the space on the sulfur atom may be important for acaricidal activity.
Table 2. The influence of the substituents of the sulfur atom on acaricidal activity against T. urticae.
2.3. The influence of R2 on acaricidal activity against T. urticae
The efficacy of various substituents for acaricidal activity is compared in Table 3, while keeping the aryl group on the piperazine ring at 1-position a 2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl group which elicited potent activity. Table 3 shows the acaricidal activity of phenylpiperazines, whose nitrogen atoms were substituted with an alkyl group (5-14–19), acyl group (5-20–24), or sulfonyl group (5-25–33). A variety of R2 substituents on the piperazine ring made no significant difference in activity at 100 ppm, and even the simplest substituent, the methyl group (5-14), perfectly controlled T. urticae with the 10 ppm application. Elongation of the alkyl chain, like the butyl group (5-15), led to a decrease in activity, while the bulkier benzyl group (5-16) exhibited good acaricidal activity. Among the substituted benzyl group (5-17–19), the para position was the most active, followed by the meta and the ortho position. On the other hand, all 4-acyl-1-[2-fluoro-4-methyl-5-(2,2,2-trifluoroethylsulfanyl)phenyl]piperazine derivatives (5-20–24) showed good acaricidal activity against T. urticae even in the 3 ppm application; in particular, the acetyl derivative (5-20) fully controlled T. urticae at 3 ppm. Among the substituted benzoyl group (5-22–24), the para-chloro-substituted derivative (5-22) was the most active, with the same tendency as the substituted benzyl group, but there was almost no difference in activity between the meta and ortho positions. Regarding phenylpiperazines having various kinds of alkylsulfonyl groups (5-25–27), ethyl and isopropylsulfonylpiperazine derivatives (5-26, 27) markedly decreased the activity, although the methylsulfonylpiperazine derivative (5-25) exhibited more than 80% mortality against T. urticae even at a low concentration, i.e., 1 ppm. Furthermore, a marked increase in the activity of 5-1, 5-28, 5-29, and 5-30 obviously indicated that the trifluoromethyl, chloromethyl, difluoromethyl, and 2,2,2-trifluoroethyl groups also elicit good efficacy. We thus assumed that the electron-withdrawing group would be effective for a higher level of acaricidal activity. Among the substituted phenylsulfonyl group (5-31–33), the para-chlorophenylsulfonyl derivative (5-31) was more active than the meta-substituted derivative (5-32); on the other hand, the ortho- substituted derivative (5-33) was almost inactive even at 10 ppm. Thus, the substituents on the nitrogen atom of the piperazine ring were well tolerated and showed good activity in many compounds.
Table 3. The influence of the substituents of the nitrogen atom of the piperazine ring on acaricidal activity against T. urticae.
3. Further acaricidal activity of compound 5-1 against T. urticae, T. kanzawai and P. citri
Since compound 5-1 exhibited the highest activity against T. urticae, a comparative study on the acaricidal activity of 5-1 against T. urticae, T. kanzawai, and P. citri was conducted. As shown in Table 4, 5-1 exhibited a similar tendency of activity among the three species (T. urticae, T. kanzawai and P. citri) of mite tested. On the other hand, the commercially available acaricide fenpyroximate showed the same activity levels against T. urticae and T. kanzawai when compared with 5-1 but showed poor activity against P. citri. These results suggest that the phenylpiperazine 5-1 can control mites without interspecies differences.
4. Further acaricidal activity of compound 5-1 against adults and eggs of T. urticae
Next, we evaluated acaricidal activity against T. urticae at various life stages, such as adults and eggs. As shown in Table 5, against eggs, both 5-1 and fenpyroximate exhibited almost the same activity levels as against adults; however, 5-1 showed a higher level of activity against both stages in comparison with fenpyroximate.
Conclusion
Regarding the substituent effect of the 2-position on the benzene ring (Y), a fluorine atom (5-1) and a cyano group (5-4) were found to be especially active against T. urticae. Regarding the substituent effect of R1, the 2,2,2-trifluoroethyl group (5-1) was the most effective substituent with regard to activity. Regarding the substituent effect on the nitrogen atom of the piperazine ring (R2), many compounds exhibited potent activity against T. urticae. In particular, the trifluoromethylsulfonyl group (5-1), acetyl group (5-20), 4-chlorobenzoyl group (5-22), chloromethylsulfonyl group (5-28), and 4-chlorophenylsulfonyl group (5-31) were very active.
Furthermore, our findings indicate that compound 5-1 perfectly controlled T. urticae, T. kanzawai, and P. citri even at 3 ppm and had no interspecies differences. In addition, 5-1 exhibited high activity against both adults and eggs of T. urticae. Though data are not shown for the experiments with mitochondria (Spodoptera litura (Sf9) insect cells), respiration in vitro was not inhibited by 5-1. The mode of action of the present phenylpiperazines is under investigation.
References
- 1).F. V. Sances, J. A. Wyman, I. P. Ting, R. A. Van Steenwyk and E. R. Oatman: Spider mite interactions with photosynthesis, transpiration and productivity of strawberry. Environ. Entomol. 10, 442–448 (1981). [Google Scholar]
- 2).J. L. Bi, Z. M. Niu, L. Yu and N. C. Toscano: Resistance status of the carmine spider mite, Tetranychus cinnabarinus and the two-spotted spider mite, Tetranychus urticae to selected acaricides on strawberries. Insect Sci. 23, 88–93 (2016). [DOI] [PubMed] [Google Scholar]
- 3).J. X. Luo, W. Ding, Y. Q. Zhang, Z. G. Yang and Y. Li: Synthesis and acaricidal activity of curcumin isoxazole and pyrazole derivatives. Chin. J. Pestic. Sci. 15, 372–380 (2013). [Google Scholar]
- 4).Q. S. Chen, S. Zhao, J. Zou, L. Shi and L. He: Monitoring of acaricide resistance in Tetranychus Cinnabarinus. Yingyong Kunchong Xuebao 4, 364–369 (2012). [Google Scholar]
- 5).L. N. Ma, J. H. Hu and H. D. Lei: Research progress of botanical acaricides. Zhongguo Nongxue Tongbao 24, 377–380 (2008). [Google Scholar]
- 6).N. Z. Hua: A review of new high efficiency and low toxicity acaricides. World Pestic. 3, 25–34 (2016). [Google Scholar]
- 7).T. Van Leeuwen, J. Vontas, A. Tsagkarakou, W. Dermauw and L. Tirry: Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 40, 563–572 (2010). [DOI] [PubMed] [Google Scholar]
- 8).Y. L. Ouyang, G. H. Montez, L. Liu and E. E. Grafton-Cardwell: Spirodiclofen and spirotetramat bioassays for monitoring resistance in citrus red mite, Panonychus citri (Acari: Tetranychidae). Pest Manag. Sci. 68, 781–787 (2012). [DOI] [PubMed] [Google Scholar]
- 9).P. Liang, J. Z. Shen and W. L. You: Research on advances of acaricide mechanism. Pestic. Sci. Adm. 20, 16–20 (1999). [Google Scholar]
- 10).A. D. Mark: Acaricide mode of action. Pest Manag. Sci. 61, 103–110 (2005). [DOI] [PubMed] [Google Scholar]
- 11).T. Van Leeuwen, L. Tirry, A. Yamamoto, R. Nauen and D. Wannes: The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic. Biochem. Physiol. 121, 12–21 (2015). [DOI] [PubMed] [Google Scholar]
- 12).M. Khalighi, W. Dermauw, N. Wybouw, S. Bajda, M. Osakabe, L. Tirrya and T. Van Leeuwena: Molecular analysis of cyenopyrafen resistance in the two-spotted spider mite Tetranychus urticae. Pest Manag. Sci. 72, 103–112 (2016). [DOI] [PubMed] [Google Scholar]
- 13).T. Kramer and R. Nauen: Monitoring of spirodiclofen susceptibility in field populations of European red mites, Panonychus ulmi (Koch) (Acari: Tetranychidae), and the cross-resistance pattern of a laboratory-selected strain. Pest Manag. Sci. 67, 1285–1293 (2011). [DOI] [PubMed] [Google Scholar]
- 14).K. Y. Feng, Y. W. Yang, X. Wen, S. Y. Ou, P. Zhang, Q. Yu, Y. C. Zhang, G. M. Shen, Z. F. Xu, J. H. Li and L. He: Stability of cyflumetofen resistance in Tetranychus cinnabarinus and its correlation with glutathione-S-transferase gene expression. Pest Manag. Sci. 75, 2802–2809 (2019). [DOI] [PubMed] [Google Scholar]
- 15).G. Xu, X. Yang, B. Jiang, P. Lei, X. Liu, Q. Wang, X. Zhang and Y. Ling: Synthesis and bioactivities of novel piperazine-containing 1,5-diphenyl-2-penten-1-one analogues from natural product lead. Bioorg. Med. Chem. Lett. 26, 1849–1853 (2016). [DOI] [PubMed] [Google Scholar]
- 16).M. Cai, Z. Li, F. Fan, Q. Huang, X. Shao and G. Song: Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]piperazine (PAPP). J. Agric. Food Chem. 58, 2624–2629 (2010). [DOI] [PubMed] [Google Scholar]
- 17).K. Domon, K. Toriyabe, Y. Ogawa, J. Bessho, K. Kawamoto, A. Watanabe, M. Komatsu, T. Matsuda, S. Ito (Kumiai Chemical Industry Co., Ltd.): PCT Int. Appl. WO2013/157229 (2013).
- 18).S. Paudel, S. Acharya, K. Kim and S. H. Cheon: Design, synthesis, and biological evaluation of arylpiperazine–benzylpiperidines with dual serotonin and norepinephrine reuptake inhibitory activities. Bioorg. Med. Chem. 24, 2137–2145 (2016). [DOI] [PubMed] [Google Scholar]
- 19).H. G. Jaisinghani, B. R. Chowdhury and B. M. Khadilkar: Microwave assisted rapid synthesis of 1-arylpiperazines. Synth. Commun. 28, 1175–1178 (1998). [Google Scholar]
- 20).K. Fujii, K. Tomino and H. Watanabe: Studies of the syntheses of piperazines. I. Syntheses of N-monosubstituted piperazines. Yakugaku Zasshi 74, 1049–1052 (1954), in Japanese. [Google Scholar]
- 21).S. Paudel, S. Acharya, G. Yoon, K. Kim and S. H. Cheon: Exploration of substituted arylpiperazine–tetrazoles as promising dual norepinephrine and dopamine reuptake inhibitors. Bioorg. Med. Chem. 24, 5546–5555 (2016). [DOI] [PubMed] [Google Scholar]
- 22).E. Mishani, C. S. Dence, T. J. McCarthy and M. J. Welch: Formation of phenylpiperazines by a novel alumina supported bis-alkylation. Tetrahedron Lett. 37, 319–322 (1996). [Google Scholar]