Abstract
Damage caused by Orobanchaceae root parasitic weeds is a substantial agricultural problem for global food security. Many studies have been conducted to establish practical methods of control, but efforts are still required for successful management. Seed germination of root parasitic weeds requires host-derived germination stimulants including strigolactones (SLs). Studies on SLs have revealed that a butenolide ring is the essential moiety for SL activity as a germination stimulant. Interestingly, recent studies have revealed that butenolide hormones regulate the biosynthesis of secondary metabolites and mediate communication in actinomycete bacteria. Because of the structural similarity between SLs and the bacterial butenolides, we evaluated the germination stimulatory activity of butenolides isolated from Streptomyces albus J1074 on root parasitic weeds. These butenolides were found to specifically induce seed germination of Orobanche minor. Our findings contribute to understanding the molecular mechanisms of germination stimulant perception and to the development of a method for their biological control.
Keywords: butenolide, broomrape, Orobanche minor, parasitic weed, Streptomyces albus, strigolactone
Introduction
Damage caused by Orobanchaceae root parasitic weeds is a substantial agricultural problem for global food security.1) Many studies have been conducted to establish practical methods for root parasitic weed control,2) but efforts are still required for their successful management.
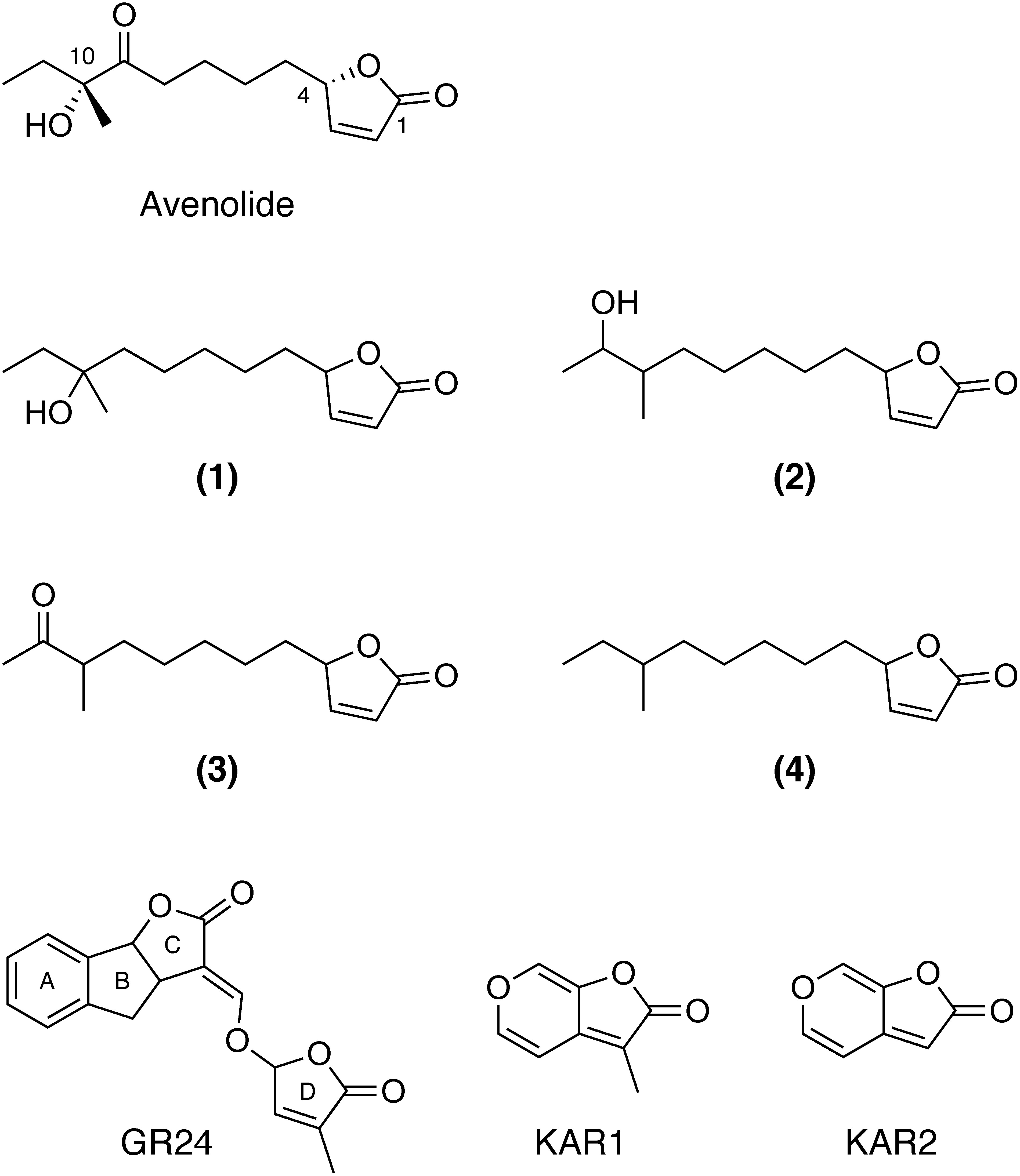
Life cycles of root parasitic weeds depend on their hosts, and seed germination requires host-derived germination stimulants.3–5) The most general germination stimulants are strigolactones (SLs),4–6) which also act as a class of plant hormones7,8) and as signaling molecules in symbiosis with arbuscular mycorrhizal fungi.9,10) If seeds of root parasitic weeds are forced to germinate by exogenous application of germination stimulants, they wither and die soon after because of the lack of a vitalizing host. This control strategy is called “suicidal germination”, and many synthetic germination stimulants have been designed based on SL biochemisty.11) Expanding structural information on natural SLs enables their classification into canonical SLs composed of four ring structures (A–D rings) (Fig. 1) and non-canonical SLs, possessing diverse skeletons with butenolide rings (D rings).5,6) All active SLs possess D rings, indicating that they are a minimum and essential requirement for SL activity.11)
Fig. 1. Structures of butenolides 1–4 isolated from Streptomyces albus J1074; strigolactone, GR24; and karrikins, KAR1 and KAR2. Canonical SLs like GR24 consist of four ring structures, A, B, C, and D rings.
Karrikins (Fig. 1) are compounds present in smoke derived from burning plant materials and have been found to promote seed germination.12,13) Karrikins are also butenolides, although they do not induce germination of root parasitic weeds.14) Structural similarity between SLs and karrikins has been focused on in terms of their interaction with receptor proteins.15–17) Karrikins are known to be perceived by KARRIKIN-INSENSITIVE2 (KAI2) receptors, the intrinsic ligands of which still remain to be elucidated.15,16) Recently, sesquiterpene lactones possessing butenolide rings in sunflowers (Helianthus annuus) were shown to be bound with HaKAI2s in silico.18)
SL receptors mediating seed germination of root parasitic weeds are members of a subclade of KAI2 paralogues, KAI2d,15,16,19) while another class of KAI2 paralogues, DWARF14 (D14) consists of SL receptors in other plants that mediate SL signaling to regulate plant architecture.8,15,16,20–22) Genomic information reveals that KAI2ds are highly diversified in Orobanchaceae through multiple gene duplications.15,16,19,23) Diversities of SLs in plant root exudates and KAI2ds might be a result of co-evolution during battles between parasites and hosts.5) Characterization of specificity between each KAI2d and its ligand still remains a big challenge.
A butenolide-type autoregulator, avenolide, acts as a hormone to regulate biosynthesis of secondary metabolites in the actinomycete Streptomyces avermitilis.24) A recent study indicated a wide distribution of butenolide hormones in actinomycetes,25) which were shown to act as external signals in actinomycete communication.26) The structural similarity of these Streptomyces hormones with SLs and karrikins motivated us to evaluate their germination activity in root parasitic weeds. Elucidation of a new class of germination stimulants will contribute to the understanding of molecular mechanisms of germination stimulant perception in root parasitic weeds and to the development of a root parasitic weed control method.
Materials and methods
1. Butenolide preparation
Four butenolides (Fig. 1) were isolated from Streptomyces albus J1074 as reported previously.26) The culture broth of S. albus J1074 was extracted with ethyl acetate, and successively purified through silica gel column chromatography and preparative reversed-phase HPLC, yielding pure butenolide compounds 1–4.
2. Plant material and germination treatment
Orobanche minor seeds were collected in Yokohama, Japan, in June 2013 and stored at 4°C. Seeds of Orobanche crenata, Phelipanche aegyptiaca, and Striga hermonthica were supplied by Dr. Walid El-Rodeny (Agricultural Research Center, Egypt), Dr. Yaakov Goldwasser (The Hebrew University of Jerusalem, Israel) and Prof. Abdel Gabar Babiker (National Center for Research, Sudan), respectively. The seed germination assay was conducted as reported previously.27,28) Surface-sterilized seeds were conditioned on two layers of glass filters (Whatman GF/D; GE Healthcare, Chicago, IL, USA) moistened with distilled water in a Petri dish in the dark for 7 days at 23°C for O. minor, for 5 days at 21°C for O. crenata, for 5 days at 23°C for P. aegyptiaca, and for 9 days at 21°C for S. hermonthica. After conditioning, the upper layer of the glass filter with the seeds was transferred to a new Petri dish containing a fresh glass filter (Whatman GF/D). Germination was induced by the application of a solution of each compound, and the seeds were incubated at the same temperature for conditioning. Synthetic strigolactone, rac-GR24 (Chiralix, Nijmegen, Netherlands), at 1 ppm was used as a positive control. The number of germinated seeds was counted under a microscope, and the germination stimulatory activity of each compound was evaluated as the percentage of germinated seeds per total treated seeds.
Results
1. Germination stimulatory activity of butenolides toward O. minor seed
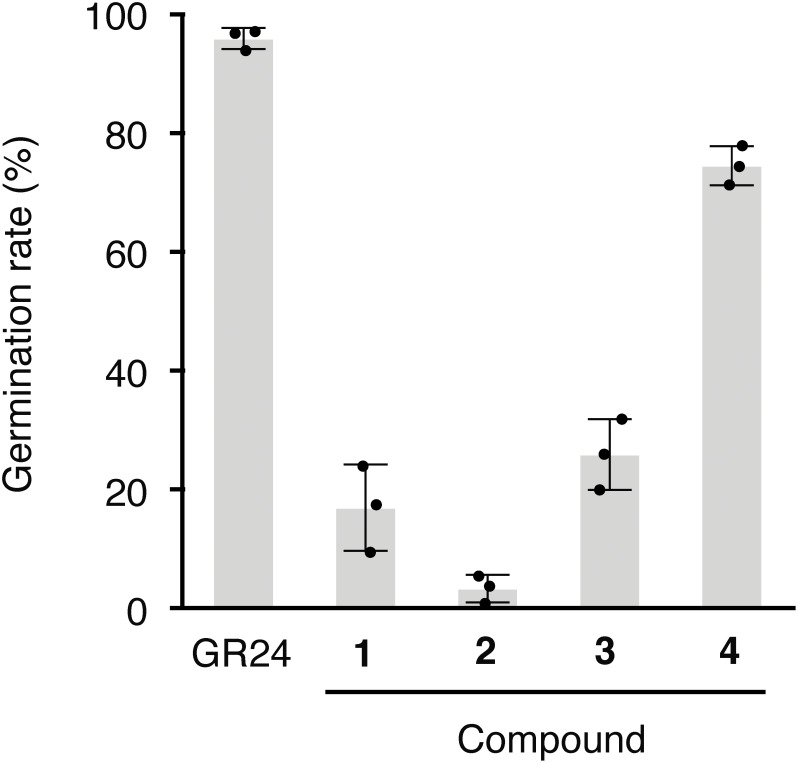
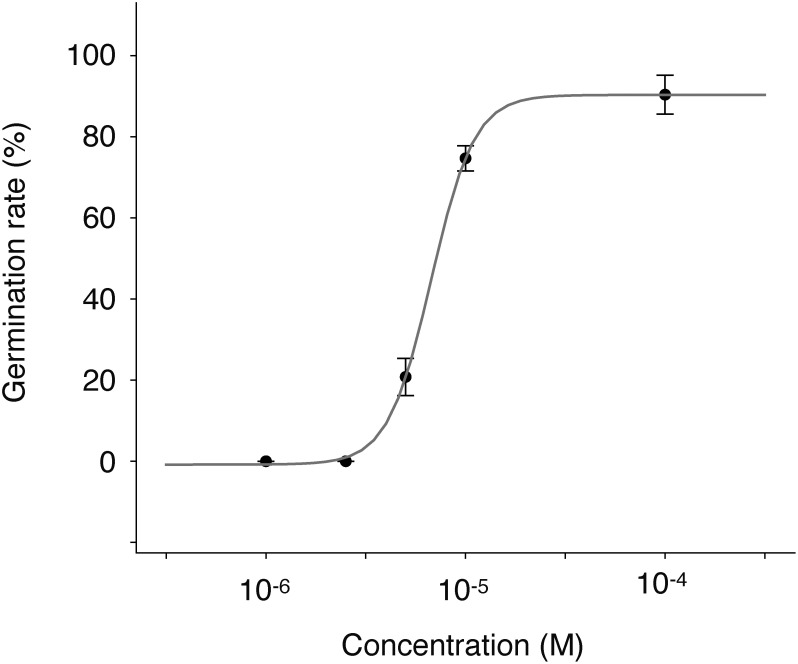
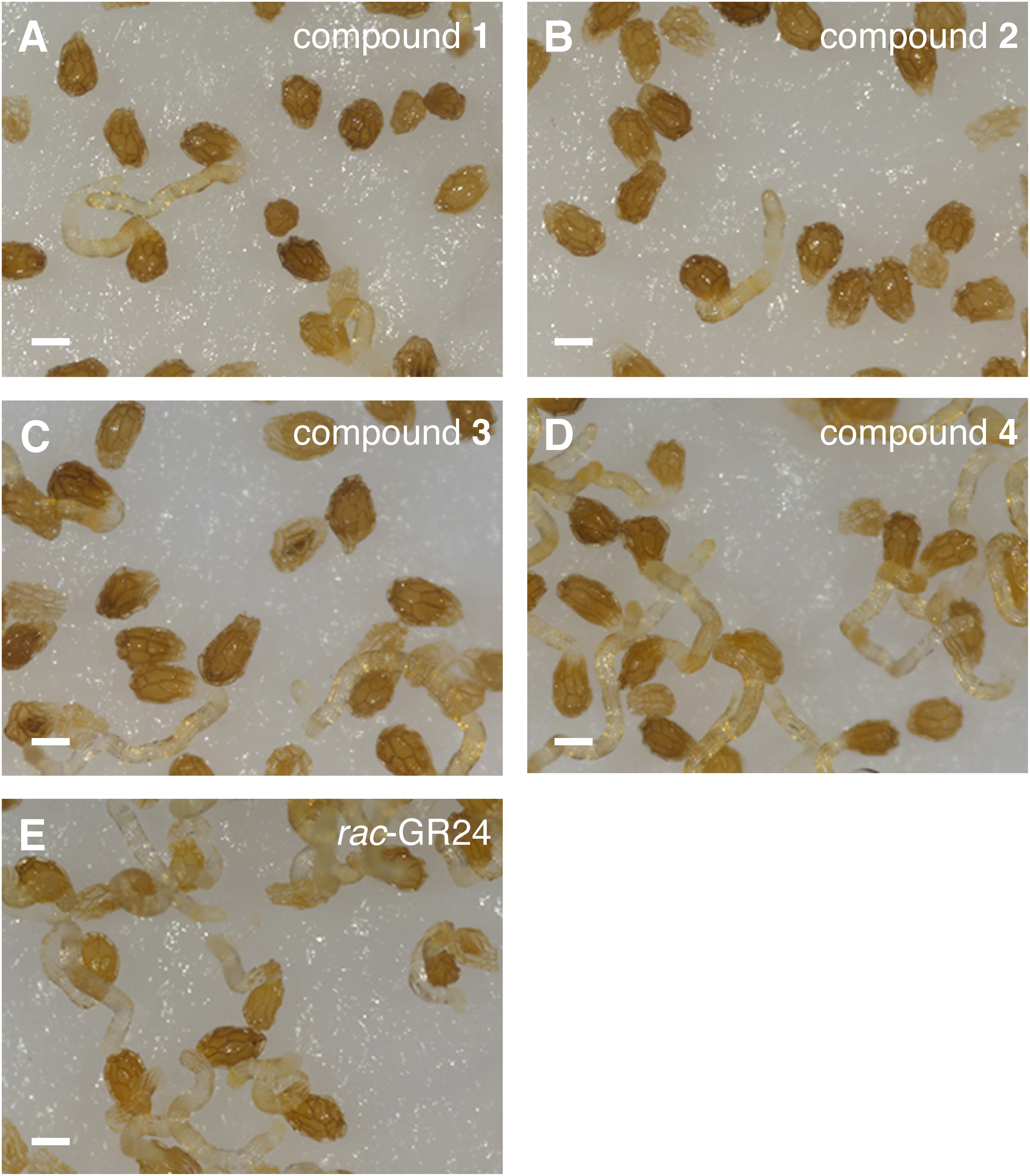
All butenolides isolated from S. albus J1074 induced the germination of O. minor seeds. Compound 4 was the most potent germination stimulant among the tested butenolides (Fig. 2), inducing germination of more than 70% of the treated seeds, while other compounds stimulated less than 30% at 10−5 M. Higher concentrations of compounds 1, 2, and 3 did not improve the efficiency (data not shown). On the other hand, compound 4 induced the germination of O. minor in a dose–dependent manner (Fig. 3). The maximum germination rate (90.4%) was achieved at 10−4 M, and the no activity was confirmed at 10−6 M. EC50 value for germination stimulatory activity of compound 4 was calculated to be 6.3×10−6 M. Although germination stimulatory activity of butenolides other than compound 4 was low, radicles induced by compounds 1–3 exhibited normal growth (Fig. 4).
Fig. 2. Germination stimuratory activity of butenolides on O. minor seeds. Each butenolide was applied at 10−5 M, and germination rates were calculated 7 days after treatment. rac-GR24 at 1 ppm was applied as a positive control. Data are presented as means of three replicates. Error bars indicate standard deviations.
Fig. 3. Dose–dependent germination stimuratory activity of compound 4 on O. minor seeds. Germination rate was calculated 7 days after treatment. Data are presented as means of three replicates. Error bars indicate standard deviations.
Fig. 4. Stimulation of O. minor seed germination by butenolide compounds 1 (A), 2 (B), 3 (C), and 4 (D) at 10−5 M. rac-GR24 (1 ppm) was applied as a positive control (E). Microscopic images were taken 7 days after treatment of each compond. Scale bar, 0.2 mm.
2. Germination stimulatory activity of compound 4 toward root parasitic weeds
Since compound 4 exhibited dose–dependent germination stimulatory activity on O. minor seeds, its activity was also evaluated for the Orobanchaceae root parasitic weeds, O. crenata, P. aegyptiaca, and S. hermonthica. However, compound 4 did not induce the germination of O. crenata or S. hermonthica in the concentration range of 10−7–10−3 M (data not shown). Gemination of P. aegyptiaca was induced occasionally, but the germination rate was very low and the result was not reproducible. From these experiments, we concluded that the germination stimulatory activity of compound 4 is specific to O. minor, at least among the tested root parasitic weeds.
Discussion
SLs are key germination stimulants for root parasitic weeds, and the structural diversity of canonical and non-canonical SLs have been identified.5,6) The diversity of SLs is proposed to be one of the molecular bases of host specificity in root parasitic weeds.5,15,16,29) Host-specific induction of germination of a range of root parasitic weeds has been shown using root exudates from various host and non-host plants.30) A synthetic SL, rac-GR24 is used widely as a germination stimulant for root parasitic plants.31) However, rac-GR24 does not induce germination of Striga gesnerioides.32) Precise evaluation of germination induction using optical isomers of GR24 derivatives revealed that S. gesnerioides perceives specific optical isomers as germination stimulants and some isomers as SL antagonists, while O. minor and S. hermonthica perceive all isomers as germination stimulants.33) Those results clearly indicate species-dependent structural requirements of SLs as germination stimulants. Species-specific germination stimulants other than SLs have also been reported. For example, isothiocyanates released as glucosinolate-breakdown products from Brassica napus roots strongly and specifically induce germination of Phelipanche ramosa.14,34) Since glucosinolates are secondary metabolites characteristic to Brassicaceae, response to isothiocyanates might have evolved in P. ramosa under host-driven selection pressure.34)
As expected considering the diversity of germination strategies in Orobanchaceae, transcriptomic and genomic information reveals highly diversified SL receptors.15,16,19,23) Most plants perceive SLs by α/β hydrolase D14s,8,15,16,20–22) while SL receptors in Orobanchaceae are encoded by paralogues, KAI2ds, in a divergent subclade of KAI2.15,16,19,23) Since KAI2 is paralogous to D14, KAI2ds in Orobanchaceae and D14s in other flowering plants may have undergone convergent evolution to perceive SLs.15,16,23) Four to six copies of KAI2d were confirmed in Orobanche and Phelipanche spp.,15) and more than ten KAI2d copies exist in Striga genome.23) Here we found that butenolides from S. albus J1074 specifically induced O. minor gemination. It is highly possible that these butenolides were perceived by one or more KAI2ds in O. minor, resulting in the induction of seed germination.
Structure–activity relationship studies of SL and its mimics as germination stimulants of root parasitic weeds have been widely conducted, because synthetic germination stimulants can be utilized in suicidal gemination for root parasitic weed control.11,35) Numerous studies concluded that a terminal butenolide (D-ring, Fig. 1) is essential for the germination stimulatory activity.11) A methyl substituent on the D-ring is important for high activity but not essential.36) It is possible that the introduction of a methyl substituent on the butenolide rings of compounds 1–4 will improve their germination stimulatory activities. Naturally, enol-ether bridges between C and D rings are hydrolyzed by D14 upon their reception to reduce active SLs.22) This structure can be changed to others, such as carbamate, in germination stimulants for root parasitic weeds.36,37) Hydrolysis-resistant analogs of potent carbamate-type germination stimulants still possess germination stimulatory activity on S. hermonthica, indicating that hydrolysis of the bridge between the C and D rings is not essential for the activity.36) Germination stimulatory activities of compounds 1–4, substances with octyl residues directly attached to the butenolide rings, indicated that hydrolytic release of the butenolide ring is not necessary for stimulation to occur.
These butenolide hormones trigger avermectin production in S. avermitilis, and the C-10 hydroxy group is important for the activity, suggesting the importance of the C-10 hydroxy group in the interaction with the receptor, AvaR1 (Fig. 1).24,26) The minimum effective concentration of compound 1 for the induction of avermectin production is 6×10−9 M, whereas that of compound 4 is 3×10−6 M.26) On the contrary, this study demonstrated that compound 4 is the most potent germination stimulants toward O. minor, implying that hydrophobic interaction may be a major factor in the binding to receptor(s). Elucidation of the receptor(s) will reveal the precise binding-mechanism and structural requirements for their germination stimulatory activity.
Biological control of weeds is becoming increasingly important in terms of sustainable agriculture, especially in developing countries. Screening of herbivorous insects and pathogenic fungi specific to root parasitic weeds has been conducted, and some Fusarium species were found to reduce the emergence of root parasitic weeds.38) Striga germination is induced by ethylene, and ethylene application succeeded in eradicating Striga asiatica in the United States.39,40) Ethylene-producing bacterium Pseudomonas syringae induced germination of Striga spp. efficiently, and is expected to be utilized as a biological inducer of suicidal germination.41,42) Since ethylene is a volatile plant hormone, the effect of applied P. synringae on crops and surrounding ecosystems should be carefully assessed. In this study, we demonstrated that bacterial butenolide hormones specifically induce O. minor germination. Thao et al. reported that the concentration of active hormones towards actinomycetes is 1,000 units/mL in the culture broth, corresponding to ca. 8.0×10−6 M in total.25) However the concentration of each butenolide hormone in the culture broth remains to be determined. Here, we determined that EC50 of compound 4 is 6.3×10−6 M, which might be comparable to the concentration in the culture broth. Further evaluation of germination stimulatory activity of the culture broth will help us to consider the feasibility of its utilization as a germination stimulant for root parasitic weed control. Bioinformatics revealed the diversity of secondary metabolism in Streptomyces even at a strain level.43) There is a possibility that other metabolites in actinomycetes also exhibit germination stimulatory activity toward root parasitic weeds. Previously, we found that nojirimycin, an antibiotic produced by Streptomyces ficellus, inhibits the germination of root parasitic weeds.28,44,45) Based on these findings, the application of Streptomyces spp. as a novel approach to biological control of root parasitic weeds is expected.
Acknowledgments
This research is supported in part by JST/JICA SATREPS (JPMJSA1607 to A.O. and Y.S.), the JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (JP20H02924 to A.O. and D.O.) and the Fund for the Promotion of Joint International Research (Fostering Joint International Research (B) (JP20KK0131 to A.O. and Y.S.). The authors would like to thank Dr. Walid El-Rodeny, Dr. Yaakov Goldwasser, and Prof. Abdel Gabar Babiker for providing the seeds of root parasitic weeds, and Shiori Motoyama for technical assistance.
References
- 1).1) C. Parker: The Parasitic weeds of the Orobanchaceae. In “Parasitic Orobanchaceae,” ed. by D. M. Joel, J. Gressel and L. J. Musselman, Springer-Verlag, Berlin, pp. 313–344, 2013.
- 2).M. Fernández-Aparicio, P. Delavault and M. P. Timko: Management of infection by parasitic weeds: A review. Plants 9, 1184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).3) K. Yoneyama, C. Ruyter-Spira and H. Bouwmeester: Induction of germination. In “Parasitic Orobanchaceae,” ed. by D. M. Joel, J. Gressel and L. J. Musselman, Springer-Verlag, Berlin, pp. 313–344, 2013.
- 4).G. Brun, L. Braem, S. Thoiron, K. Gevaert, S. Goormachtig and P. Delavault: Seed germination in parasitic plants: what insights can we expect from strigolactone research? J. Exp. Bot. 69, 2265–2280 (2018). [DOI] [PubMed] [Google Scholar]
- 5).H. Bouwmeester, C. Li, B. Thiombiano, M. Rahimi and L. Dong: Adaptation of the parasitic plant lifecyle: Germination is controlled by essential host signaling molecules. Plant Physiol. 2020, kiaa066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).K. Yoneyama: Recent progress in the chemistry and biochemistry of strigolactones. J. Pestic. Sci. 45, 45–53 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).S. Al-Babili and H. J. Bouwmeester: Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 66, 161–186 (2015). [DOI] [PubMed] [Google Scholar]
- 8).M. Bürger and J. Chory: The many models of strigolactone signaling. Trends Plant Sci. 25, 395–405 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).K. Akiyama, K. Matsuzaki and H. Hayashi: Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827 (2005). [DOI] [PubMed] [Google Scholar]
- 10).L. Lanfranco, V. Fiorilli, F. Venice and P. Bonfante: Strigolactones cross the kingdoms: plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. J. Exp. Bot. 69, 2175–2188 (2018). [DOI] [PubMed] [Google Scholar]
- 11).B. Zwanenburg, A. S. Mwakaboko and C. Kannan: Suicidal germination for parasitic weed control. Pest Manag. Sci. 72, 2016–2025 (2016). [DOI] [PubMed] [Google Scholar]
- 12).G. R. Flematti, E. L. Ghisalberti, K. W. Dixon and R. D. Trengove: A compound from smoke that promotes seed germination. Science 305, 977 (2004). [DOI] [PubMed] [Google Scholar]
- 13).S. D. S. Chiwocha, K. W. Dixon, G. R. Flematti, E. L. Ghisalberti, D. J. Merritt, D. C. Nelson, J.-A. M. Riseborough, S. M. Smith and J. C. Stevens: Karrikins: A new family of plant growth regulators in smoke. Plant Sci. 177, 252–256 (2009). [Google Scholar]
- 14).G. Brun, S. Thoiron, L. Braem, J.-B. Pouvreau, G. Montiel, M.-M. Lechet, P. Simier, K. Gevaert, S. Goormachtig and P. Delavault: CYP707As are effectors of karrikin and strigolactone signalling pathways in Arabidopsis thaliana and parasitic plants. Plant Cell Environ. 42, 2612–2626 (2019). [DOI] [PubMed] [Google Scholar]
- 15).C. E. Conn, R. Bythell-Douglas, D. Neumann, S. Yoshida, B. Whittington, J. H. Westwood, K. Shirasu, C. S. Bond, K. A. Dyer and D. C. Nelson: Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 349, 540–543 (2015). [DOI] [PubMed] [Google Scholar]
- 16).C. E. Conn and D. C. Nelson: Evidence that KARRIKIN-INSENSITIVE2 (KAI2) receptors may perceive an unknown signal that is not karrikin or strigolactone. Front. Plant Sci 6, 1219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).N. Morffy, L. Faure and D. C. Nelson: Smoke and hormone mirrors: action and evolution of karrikin and strigolactone signaling. Trends Genet. 32, 176–188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).M. Rahimi and H. Bouwmeester: Are sesquiterpene lactones the elusive KARRIKIN-INSENSITIVE2 ligand? Planta 253, 54 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Y. Tsuchiya, M. Yoshimura, Y. Sato, K. Kuwata, S. Toh, D. Holbrook-Smith, H. Zhang, P. McCourt, K. Itami, T. Kinoshita and S. Hagihara: Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 349, 864–868 (2015). [DOI] [PubMed] [Google Scholar]
- 20).C. Hamiaux, R. S. M. Drummond, B. J. Janssen, S. E. Ledger, J. M. Cooney, R. D. Newcomb and K. C. Snowden: DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 22, 2032–2036 (2012). [DOI] [PubMed] [Google Scholar]
- 21).H. Nakamura, Y.-L. Xue, T. Miyakawa, F. Hou, H.-M. Qin, K. Fukui, X. Shi, E. Ito, S. Ito, S.-H. Park, Y. Miyauchi, A. Asano, N. Totsuka, T. Ueda, M. Tanokura and T. Asami: Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 4, 2613 (2013). [DOI] [PubMed] [Google Scholar]
- 22).Y. Seto, R. Yasui, H. Kameoka, M. Tamiru, M. Cao, R. Terauchi, A. Sakurada, R. Hirano, T. Kisugi, A. Hanada, M. Umehara, E. Seo, K. Akiyama, J. Burke, N. Takeda-Kamiya, W. Li, Y. Hirano, T. Hakoshima, K. Mashiguchi, J. P. Noel, J. Kyozuka and S. Yamaguchi: Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 10, 191 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).S. Yoshida, S. Kim, E. K. Wafula, J. Tanskanen, Y.-M. Kim, L. Honaas, Z. Yang, T. Spallek, C. E. Conn, Y. Ichihashi, K. Cheong, S. Cui, J. P. Der, H. Gundlach, Y. Jiao, C. Hori, J. K. Ishida, H. Kasahara, T. Kiba, M.-S. Kim, N. Koo, A. Laohavisit, Y.-H. Lee, S. Lumba, P. McCourt, J. C. Mortimer, J. M. Mutuku, T. Nomura, Y. Sasaki-Sekimoto, Y. Seto, Y. Wang, T. Wakatake, H. Sakakibara, T. Demura, S. Yamaguchi, K. Yoneyama, R. Manabe, D. C. Nelson, A. H. Schulman, M. P. Timko, C. W. dePamphilis, D. Choi and K. Shirasu: Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr. Biol. 29, 3041–3052 (2019). [DOI] [PubMed] [Google Scholar]
- 24).S. Kitani, K. T. Miyamoto, S. Takamatsu, E. Herawati, H. Iguchi, K. Nishitomi, M. Uchida, T. Nagamitsu, S. Omura, H. Ikeda and T. Nihira: Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc. Natl. Acad. Sci. U.S.A. 108, 16410–16415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).N. B. Thao, S. Kitani, H. Nitta, T. Tomioka and T. Nihira: Discovering potential Streptomyces hormone producers by using disruptants of essential biosynthetic genes as indicator strains. J. Antibiot. (Tokyo) 70, 1004–1008 (2017). [DOI] [PubMed] [Google Scholar]
- 26).T. B. Nguyen, S. Kitani, S. Shimma and T. Nihira: Butenolides from Streptomyces albus J1074 act as external signals to stimulate avermectin production in Streptomyces avermitilis. Appl. Environ. Microbiol. 84, e02791–e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Y. Sugimoto, A. M. Ali, S. Yabuta, H. Kinoshita, S. Inanaga and A. Itai: Germination strategy of Striga hermonthica involves regulation of ethylene biosynthesis. Physiol. Plant. 119, 137–145 (2003). [Google Scholar]
- 28).T. Wakabayashi, B. Joseph, S. Yasumoto, T. Akashi, T. Aoki, K. Harada, S. Muranaka, T. Bamba, E. Fukusaki, Y. Takeuchi, K. Yoneyama, T. Muranaka, Y. Sugimoto and A. Okazawa: Planteose as a storage carbohydrate required for early stage of germination of Orobanche minor and its metabolism as a possible target for selective control. J. Exp. Bot. 66, 3085–3097 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).A. Khosla and D. C. Nelson: Strigolactones, super hormones in the fight against Striga. Curr. Opin. Plant Biol. 33, 57–63 (2016). [DOI] [PubMed] [Google Scholar]
- 30).M. Fernández-Aparicio, K. Yoneyama and D. Rubiales: The role of strigolactones in host specificity of Orobanche and Phelipanche seed germination. Seed Sci. Res. 21, 55–61 (2011). [Google Scholar]
- 31).A. W. Johnson, G. Gowda, A. Hassanali, J. Knox, S. Monaco, Z. Razavi and G. Rosebery: The preparation of synthetic analogues of strigol. J. Chem. Soc. Perkin Trans. 1, 1734–1743 (1981). [Google Scholar]
- 32).I. Igbinnosa and S. N. C. Okonkwo: Stimulation of germination of seeds of cowpea witchweed (Striga gesnerioides) by sodium hypochlorite and some growth regulators. Weed Sci. 40, 25–28 (1992). [Google Scholar]
- 33).K. Ueno, M. Fujiwara, S. Nomura, M. Mizutani, M. Sasaki, H. Takikawa and Y. Sugimoto: Structural requirements of strigolactones for germination induction of Striga gesnerioides seeds. J. Agric. Food Chem. 59, 9226–9231 (2011). [DOI] [PubMed] [Google Scholar]
- 34).B. Auger, J.-B. Pouvreau, K. Pouponneau, K. Yoneyama, G. Montiel, B. L. Bizec, K. Yoneyama, P. Delavault, R. Delourme and P. Simier: Germination stimulants of Phelipanche ramosa in the rhizosphere of Brassica napus are derived from the glucosinolate pathway. Mol. Plant Microbe Interact. 25, 993–1004 (2012). [DOI] [PubMed] [Google Scholar]
- 35).H. Samejima, A. G. Babiker, H. Takikawa, M. Sasaki and Y. Sugimoto: Practicality of the suicidal germination approach for controlling Striga hermonthica. Pest Manag. Sci. 72, 2035–2042 (2016). [DOI] [PubMed] [Google Scholar]
- 36).D. Uraguchi, K. Kuwata, Y. Hijikata, R. Yamaguchi, H. Imaizumi, S. Am, C. Rakers, N. Mori, K. Akiyama, S. Irle, P. McCourt, T. Kinoshita, T. Ooi and Y. Tsuchiya: A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica. Science 362, 1301–1305 (2018). [DOI] [PubMed] [Google Scholar]
- 37).37) M. Sasaki, Y. Sugimoto, H. Takikawa, H. Miyake and N. Matsuo: (Kobe Univ. and Sumitomo Chemical Co., Ltd.): Germination-stimulant carbamate derivatives and process for preparation thereof. US Pat. US8822383B2 (2011).
- 38).J. Sauerborn, D. Müller-Stöver and J. Hershenhorn: The role of biological control in managing parasitic weeds. Crop Prot. 26, 246–254 (2007). [Google Scholar]
- 39).G. H. Egley and J. E. Dale: Ethylene, 2-chloroethylphosphonic acid, and witchweed germination. Weed Sci. 18, 586–589 (1970). [Google Scholar]
- 40).40) R. E. Eplee and R. S. Norris: Chemical control of Striga. In “Parasitic Weeds in Agriculture,” ed. by L. J. Mussleman, CRC Press, Boca Raton, pp. 173–182, 1987.
- 41).D. K. Berner, N. W. Schaad and B. Völksch: Use of ethylene-producing bacteria for stimulation of Striga spp. seed germination. Biol. Control 15, 274–282 (1999). [Google Scholar]
- 42).R. Masteling, L. Lombard, W. de Boer, J. M. Raaijmakers and F. Dini-Andreote: Harnessing the microbiome to control plant parasitic weeds. Curr. Opin. Microbiol. 49, 26–33 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).R. F. Seipke: Strain-level diversity of secondary metabolism in Streptomyces albus. PLoS One 10, e0116457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).K. Harada, Y. Kurono, S. Nagasawa, T. Oda, Y. Nasu, T. Wakabayashi, Y. Sugimoto, H. Matsuura, S. Muranaka, K. Hirata and A. Okazawa: Enhanced production of nojirimycin via Streptomyces ficellus cultivation using marine broth and inhibitory activity of the culture for seeds of parasitic weeds. J. Pestic. Sci. 42, 166–171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).A. Okazawa, T. Wakabayashi, T. Muranaka, Y. Sugimoto and D. Ohta: The effect of nojirimycin on the transcriptome of germinating Orobanche minor seeds. J. Pestic. Sci. 45, 230–237 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]