Abstract
Introduction
The hydatid cyst (HC) of the right ventricle (RV) is an extremely uncommon and a serious location that can cause sudden death following pulmonary embolism, obstruction of the valvular orifice or anaphylactic shock.
Case presentation
We report a case of a 14 years-old girl with a HC of the RV. Surgical excision of the HC under Cardiopulmonary bypass (CPB) was successful in managing this rare case.
Clinical discussion
Cardiac HC is extremely rare. It represents only 0.5–2% of all hydatid cases. However, RV location is very severe. It has a tendency to rupture intracavitarily and causes sudden death in 30% of cases. Its diagnosis is based on echocardiography, computed tomography scan and magnetic resonance imaging. The surgical treatment under CPB with anthelmintic therapy seems to improve the prognostic outcomes.
Conclusion
Cardiac HC must be always suspected in endemic countries, especially in patients with a family history of HC.
Keywords: Hydatid cyst, Heart, Right ventricle, Case report, SCARE guidelines
Highlights
-
•
Hydatid cyst of the right ventricle is an extremely uncommon location.
-
•
Right ventricle location is very severe that can cause sudden death in 30% of cases.
-
•
The optimal treatment is still based on surgery under cardiopulmonary bypass with anthelmintic therapy.
-
•
Hydatid cyst of the right ventricle should be diagnosed and treated early to prevent sudden death.
1. Introduction
Hydatid disease (HD) or cystic hydatidosis (CH) is a systemic zoonosis caused by the larval stages of cestodes Echinococcus granulosus [1]. This parasitosis can occur in any organ of the body from head to toe via the general or lymphatic circulation [2]. Cardiac involvement in HD is rare (0.5–2%) compared to hepatic (65%) and pulmonary (25%) locations [3]. While right ventricular location is an extremely uncommon entity that can cause sudden death following pulmonary embolism or anaphylactic shock [3]. In this paper, we report a very rare case of hydatid cyst (HC) of the right ventricle (RV) occurring in a young patient according to SCARE guidelines [4].
2. Case report
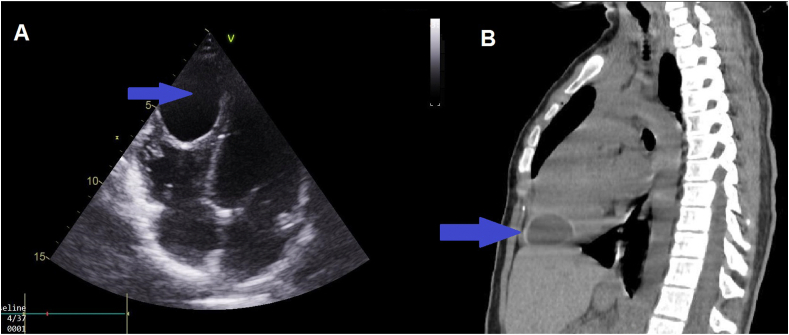
A 14 year old Moroccan girl resident in a sheep farming province, with a family history of hepatic HC in her father and brother was admitted to the emergency room for the management of dyspnea associated with palpitations that started 3 weeks before her consultation. The physical examination was unremarkable apart of tachycardia. Moreover, the electrocardiogram (ECG) found a regular sinus rhythm at 98 cpm. Chest x-ray showed a normal cardiothoracic index. Transthoracic echocardiography (TTE) showed a cystic mass measuring 47 × 33 mm attached to the right ventricular apex protruding into the lumen (Fig. 1A). Heart valves, left and right ventricular function were normal. In addition, the thoracoabdominal CT scan confirmed the cystic nature of the apical mass measuring 47x33 × 39 mm in the right ventricle wall protruding into the lumen (Fig. 1B). It also excluded any other location within the other organs including the liver and lungs. The serologic tests for hydatid disease were positive and the rest of the laboratory investigations were normal. Based on these aforementioned results, the diagnosis of HC of the RV was made and oral albendazole (15 mg/kg) was administered three days before surgery.
Fig. 1.
Imaging of the hydatid cyst. (A): Echocardiography image of the four chamber cardiac view showing cystic mass attached to the right ventricular apex protruding into the lumen. (B): Thoracic CT scan showing the hydatid cyst located in the right ventricular apex.
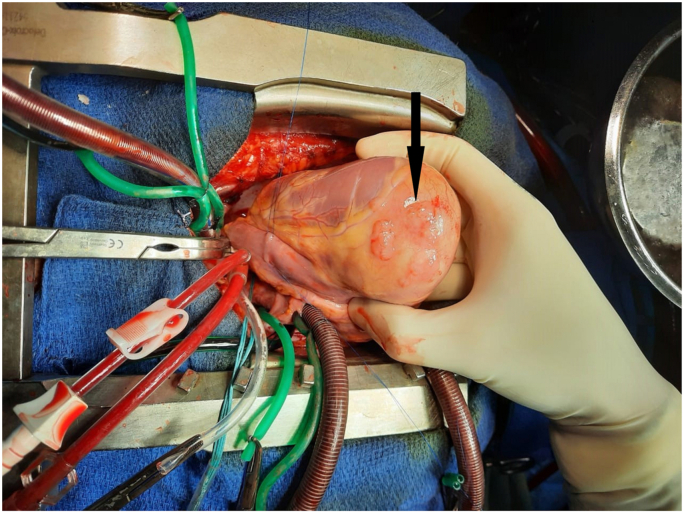
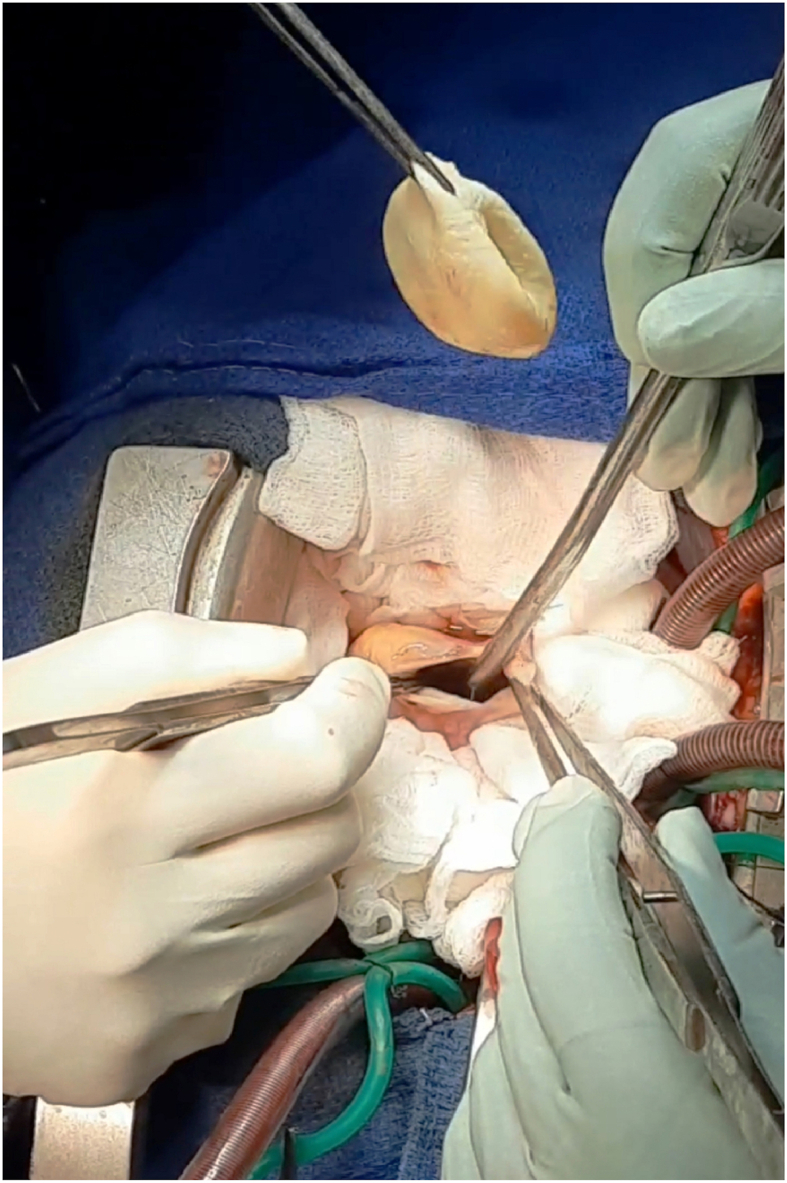
The surgical treatment was performed by a senior cardiac surgeon aided by a junior resident using a median sternotomy with a longitudinal pericardiotomy. A careful inspection revealed a HC on the muscle of the apex of the RV (Fig. 2). A cardiopulmonary bypass (CPB) was performed between aortic and bicaval cannulation and then, a superior and inferior vena cava were snared. The ascending aorta and the pulmonary trunk were cross-clamped. In order to minimize the risk of contamination, the surrounding areas of the cyst were covered with a gauze compress soaked by 20% of hypertonic saline. The cyst was neatly aspirated after injection of the hypertonic saline (20%) and then the germinative membrane was totally removed (Fig. 3). The RV was opened and the exploration did not show any communication between the residual cavity and the right ventricle. After abundant washing with hypertonic saline, the defect and the right ventriculotomy were closed with continuous 5/0 polypropylene suture which was reinforced with autologous pericardial patch. No postoperative events were noticed in our patient. She was extubated 4 h after surgery and discharged eight days later. The histopathological analyses confirmed the diagnosis of a HD and oral albendazole was continued for six months postoperatively using the preoperative dose. Importantly the 6 months follow-up was unremarkable.
Fig. 2.
Perioperative view showing cardiac hydatid cyst on the muscle of the apex of the right ventricle wall.
Fig. 3.
Perioperative view showing removal of the germinative membrane through the right ventricle.
3. Discussion
HD is a widespread zoonosis in cattle breeding areas [3].This zoonosis remains a main public health problem in Morocco [5], as well as the rest of endemic countries, such as South America, Mediterranean and Middle East, Africa, New Zealand and Australia [2,6]. The infection of humans can occur directly via close contact with contaminated dogs or indirectly after ingesting contaminated water or food containing tapeworm eggs [1,6]. After ingestion, the oncosphere crosses the duodenal mucosa, easily penetrates the venous or lymphatic system, and can affect any part of the body mainly liver (65%) and lungs (25%) [[2], [3], [4], [5], [6], [7]]. However, cardiac involvement is infrequent, even in endemic countries. It represents only 0.5–2% of all hydatid cases [3].This rareness is due to the continuous contraction of the heart which prevents the attachment of parasite eggs into the cardiac wall [8]. After traversing the heart through the bloodstream, the Echinococcus granulosus penetrates the coronary circulation and lodges into the myocardium [9]. Thereafter, it grows slowly (1 cm per year) and becomes a mature hydatid cyst after 1–5 years [3].CH can occur in any part of the heart. While, right ventricle (RV) CH is even rarer than left (LV) ventricle CH (10% versus 60%). It can be explained by the fact that the muscle of LV is larger and very rich in vascular supply [3].Clinical manifestations of cardiac HC are inconstant and non specific, depending on the number, the size, the evolutionary stage, the location of the cyst and its proximity with valvular orifices and conductive tissue [10]. However, RV location is very severe. It has a tendency to rupture intracavitarily and causes obstruction of the valvular orifice, anaphylactic shock and pulmonary embolism with postembolic cor pulmonale. In this case, the risk of sudden death is estimated at 30% [7,10]. Serological tests are important and have the ability to confirm the diagnosis of HC when the results are positive [5]. In this case, the serology of HC was positive. Chest x-ray may show the presence of multiple small rounded opacities in the two lung fields related to the embologenic dissemination after rupture of a cardiac HC [11]. TTE appears to be the most useful test for a positive diagnosis of cardiac HC. It allows visualizing the cyst with an anechoic aspect. However, the presence of daughter vesicles, multivesicular appearance or membrane detachment is strongly suggestive of the hydatid origin of the cyst [5,10]. In summary, the identification of cardiac cyst lesion (TTE and CT) associated with positive serology makes the diagnosis. The CT scan confirms the diagnosis, specifies the relationship between the HC and the cardiac structures and performs the extension workup in search of other locations. However, the CT may lack sensitivity for small cysts and cause false negatives due to heart movement [10,12]. Magnetic resonance imaging (MRI) is also useful especially in case of doubt about the diagnosis or if there is a discrepancy between TTE and CT scan [12].
Despite therapeutic advances, surgery is the gold standard for the treatment of cardiac HCs [3,5,[7], [8], [9], [10], [11], [12], [13]]. The median sternotomy is the preferred route in the surgical treatment of cardiac HC because it enables a complete exploration and an easy access to all cardiac structures and pericardial cavity [3,5,[7], [8], [9], [10],[12], [13], [14]]. However, left anterolateral thoracotomy has only been described in cases of HC of the LV associated with a HC of the left lung [14]. The excision of the RV HC can be performed with a beating-heart approach or under CPB with an empty beating heart in cases where the HC is superficial [14]. These techniques are challenging as they can be associated with dissemination of the parasite into the pericardium and the pulmonary circulation. Therefore, this may cause anaphylactic shock and pulmonary embolism. However, the resection of the RV HC under CPB with cardioplegic cardiac arrest is the most adopted attitude by the authors because it is a save strategy and it also allows an easy excision of the cyst with a low risk of rupture and provides a complete exploration of the cardiac cavity [3,7,[12], [13], [14]]. The cross clamping of the pulmonary trunk before the resection of the HC can stop the migration of the contents of the HC into the pulmonary circulation [3,7,[12], [13], [14]]. It is recommended to cover the surrounding tissues with a gauze compress soaked by 20% of hypertonic saline before the resection of the cyst in order to minimize the risk of contamination [3,5,[7], [8], [9], [10], [11], [12], [13], [14]]. Surgery of CHC is based on three different techniques including total cyst resection, intact endocyst enucleation and puncture–aspiration of the HC with cystectomy [14]. However, the puncture–aspiration with cystectomy and the intact endocyst enucleation are the mainly suggested techniques in cases of myocardial hydatid cyst especially when they are protruding intracavitarily or when they have a close contact with the conductive tissue or the valvular orifices [14]. These two approaches allow a resection of the cyst while ensuring the preservation of the surrounding tissues [14]. In order to reduce even more the risk of recurrence and dissemination, several authors have recommended the use of albendazole (15mg/kg) therapy before and after surgery [3,5,[7], [8], [9], [10], [11], [12], [13], [14]]. We believe that this combinatorial approach significantly reduces the risk of recurrence and spread. According to our experience and based on the published literature, it is recommended to consider HC resection with a CPB with cardioplegic cardiac arrest and pulmonary artery clamping. This procedure prevents arrhythmia during heart manipulation and also allows an accurate HC resection. In our setting, we prefer puncture–aspiration with cystectomy as the use of hypertonic saline (20%) inside the cyst has anti-protoscolex properties. Moreover, the aspiration of the cyst contents is associated with a reduced risk of dissemination during cystectomy. Finally, our patient and her family were satisfied with our medical and surgical management.
4. Conclusion
Cardiac HC must be always kept in mind in patients with a family history of HC even in endemic countries. Furthermore, the cardiac HC especially in the RV should be diagnosed and treated early to prevent sudden death.
Consent
Written informed consent was obtained from the parents of the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
N/a.
Sources of funding
None.
Author contribution
Professor Selma Lyazidi: writing of the manuscript. Dr Ayoub Abetti: review, editing, visualisation, conceptualization and investigation. Dr Amal Abdellaoui provided the imaging data of the patient. Professors Ahmed El Adaoui, Rachida Habbal and Youssef Ettaoumi supervised the writing of the manuscript.
Research registration
N/a.
Guarantor
Prof. Selma Lyazidi and Prof. Youssef Ettaoumi
Provenance and peer review
Not commissioned, externally peer-reviewed
Declaration of competing interest
The authors declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102427.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Blanton R. Echinococcosis. In: Behrman R.E., KliegmanRM, Jenson H.B., editors. Nelson Textbook of Pediatrics. seventeenth ed. WB Saunders Company; Philadelphia: 2004. pp. 1173–1174. [Google Scholar]
- 2.Polat P., Kantarci M., Alper F., Suma S., Koruyucu M.B., Okur A. Hydatid disease from head to toe. Radiographics. 2003 Mar-Apr;23(2):475–494. doi: 10.1148/rg.232025704. quiz 536-7. [DOI] [PubMed] [Google Scholar]
- 3.L’aarje A., Lyazidi S., Kitane Y., Alami A., Habbal R. Cardiac hydatid cyst of the right ventricle: severe localization. J Cardiol Cases. 2017 Aug 2;16(4):138–140. doi: 10.1016/j.jccase.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., Scare Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Abetti A., Lyazidi S., Qechchar Z., Habbal R., Ettaoumi Y. Hepato-pericardial fistula revealed by a massive pericardial effusion: a case report of an exceptional complication of the hydatid liver cyst. Int J Surg Case Rep. 2020;73:199–202. doi: 10.1016/j.ijscr.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedrosa I., Saíz A., Arrazola J., Ferreirós J., Pedrosa C.S. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000 May- Jun;20(3):795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 7.Fennira S., Kamoun S., Besbes B., Ben Mrad I., Zairi I., Ben Moussa F., Mzoughi K., Kraiem S. Cardiac hydatid cyst in the interventricular septum: a literature review. Int. J. Infect. Dis. 2019 Nov;88:120–126. doi: 10.1016/j.ijid.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Aljaber N.N., Alshoabi S.A., Qurashi A.A., Daqqaq T.S. Cardiac hydatid cyst in the right ventricle: an unusual case at a rare site. J Taibah Univ Med Sci. 2020 Mar 25;15(3):249–252. doi: 10.1016/j.jtumed.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tejada J.G., Saavedra J., Molina L., Forteza A., Gomez C. Hydatid disease of the interventricular septum causing pericardial effusion. Ann. Thorac. Surg. 2001 Jun;71(6):2034–2035. doi: 10.1016/s0003-4975(00)02269-4. discussion 2035-6. [DOI] [PubMed] [Google Scholar]
- 10.Jerbi S., Romdhani N., Tarmiz A., Kortas C., Mlika S., Khelil N., Belghith M., Limayem F., Ennabli K. Kyste hydatique emboligène du coeur droit [Emboligenous hydatid cyst of the right heart] Ann. Cardiol. Angeiol. 2008 Feb;57(1):62–65. doi: 10.1016/j.ancard.2007.05.007. French. [DOI] [PubMed] [Google Scholar]
- 11.Klodas E., Roger V.L., Miller F.A., Jr., Utz J.P., Danielson G.K., Edwards W.D. Cardiac echinococcosis: case report of unusual echocardiographic appearance. Mayo Clin. Proc. 1995 Jul;70(7):657–661. doi: 10.4065/70.7.657. [DOI] [PubMed] [Google Scholar]
- 12.Lakehal Redha. Kyste hydatique du ventricule droit rompu dans l'artère pulmonaire: a propos d’un cas. Cardiologie Tunisienne. 2017;Volume 13:53–55. N 01 - 1 53 er Trimestre. [Google Scholar]
- 13.Jerbi s, Kortas C., dammak s, Hamida n, ennabli K. Les kystes hydatiques cardiopéricardiques à propos de 19 cas. Tunis. Med. 2004;82(suppl 01):152–157. [PubMed] [Google Scholar]
- 14.Yan F., Huo Q., Abudureheman M., Qiao J., Ma S., Wen H. Surgical treatment and outcome of cardiac cystic echinococcosis. Eur. J. Cardio. Thorac. Surg. 2015 Jun;47(6):1053–1058. doi: 10.1093/ejcts/ezu323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.