Abstract
Sila-molecules have recently attracted attention due to their promising applications in medical and industrial fields. Compared with all-carbon parent compounds, the different covalent radius and electronegativity of silicon from carbon generally endow the corresponding sila-analogs with unique biological activity and physicochemical properties. Vinylsilanes feature both silyl-hyperconjugation effect and versatile reactivities, developing vinylsilane-based Smiles rearrangement will therefore provide an efficient platform to assemble complex silacycles. Here we report a practical Ir(III)-catalyzed cycloaromatization of ortho-alkynylaryl vinylsilanes with arylsulfonyl azides for delivering naphthyl-fused benzosiloles under visible-light photoredox conditions. The combination of experiments and density functional theory (DFT) energy profiles reveals the reaction mechanism involving α-silyl radical Smiles rearrangement.
Subject terms: Synthetic chemistry methodology, Photocatalysis
Arene-fused siloles have attracted interest due to their promising applications in electronic and optoelectronic devices. Here, the authors report Ir(III)-catalyzed cycloaromatization of ortho-alkynylaryl vinylsilanes with arylsulfonyl azides via α-silyl radical Smiles rearrangement for accessing naphthyl-fused benzosiloles under visible-light photoredox conditions.
Introduction
Silahydrocarbons are sometimes encountered in pharmaceuticals and material chemistry1–4. Compared with all-carbon parent compounds, Si-element generally endows the corresponding hydrocarbons with unique biological activity and physical–chemical properties5–8, which are mainly determined by the different covalent radius and electronegativity of silicon from carbon. In these regards, arene-fused siloles have especially attracted many concerns due to their promising applications in electronic and optoelectronic devices9–13. In 2012, Xi14 and Chatani15 pioneeringly explored intermolecular coupling-cyclization of alkynes with 2-silylaryl bromides and 2-silylphenylboronic acids to produce 2,3-difunctionalized benzosiloles through Si-C bond cleavage (Fig. 1a). Subsequently, He16 developed rhodium-catalyzed intramolecular silicoamination of ortho-alkynylarylsilanes to construct indole-fused benzosiloles (Fig. 1b). In light of that modifying the π-conjugated system of parent siloles could possibly improve their corresponding photophysical properties. Thus, there has been an ever-increasing demand for the rapid assembly of diversified polycycle-fused siloles17–22.
Fig. 1. Strategies to access benzosiloles.
a Rh/Pd-catalyzed intermolecular cyclovinylation of arylalkylsilanes with alkynes. b Rh(I)-catalyzed intramolecular aminosilylation of ortho-alkynylarylsilanes. c Photocatalyzed carbocyclization of vinylsilanes with arylsulfonyl azides via alpha-silyl radical Smiles rearrangement.
Aryl migration via Smiles rearrangement is a powerful tool for the synthesis of polycyclic arenes23–25. However, the modes of radical Smiles rearrangement are very limited. Up to now, only α-carbonyl radical26–30, β-aminoalkyl radical31–33, N-centered radical34, and ketyl radical35- triggered Smiles rearrangement have been exploited to construct nitrogen-heterocycles, and vinylsilane-based Smiles rearrangement keep unexplored. As is well known, vinylsilanes have proven to be important “alkene” sources in Hiyama coupling, which could efficiently incorporate C=C bond in a particular molecule with the release of silyl moiety36; Meanwhile, the high electronegativity of carbon (2.35) relative to silicon (1.64)37 and silyl-hyperconjugation effect (the so-called β-effect)38–40 generally endow these compounds with the versatile reactivity. For example, Jun41 reported that Rh(I)-catalyzed cross-coupling of aldehydes with vinylsilanes led to the formation of β-acylsilanes via β-silylethylrhodium(III) intermediates. On the contrary, Buchwald42 and Miura43 demonstrated that Cu(I)-catalyzed addition-coupling of vinylsilanes with amines could produce α-aminosilanes. Thus it can be seen that developing vinylsilane-based coupling-cyclization will possibly establish an efficient platform to assemble complex silacycles. Again, azides could be employed as potential nitrogen radical precursors to enable C-H amination44 under photocatalysis systems. Accordingly, visible-light catalyzed coupling of vinylsilanes with arylsulfonylazides could possibly generate α-silyl radicals and initiate the silylalkene Smiles rearrangement.
Here, we show a cycloaromatization of ortho-alkynylarylsilylalkenes with arylsulfonyl azides for rapid assembly of 2,3-naphthyl-fused benzosiloles via a cascade S-N/C-S bond cleavage in the presence of visible light (Fig. 1c).
Results
Investigation of reaction conditions
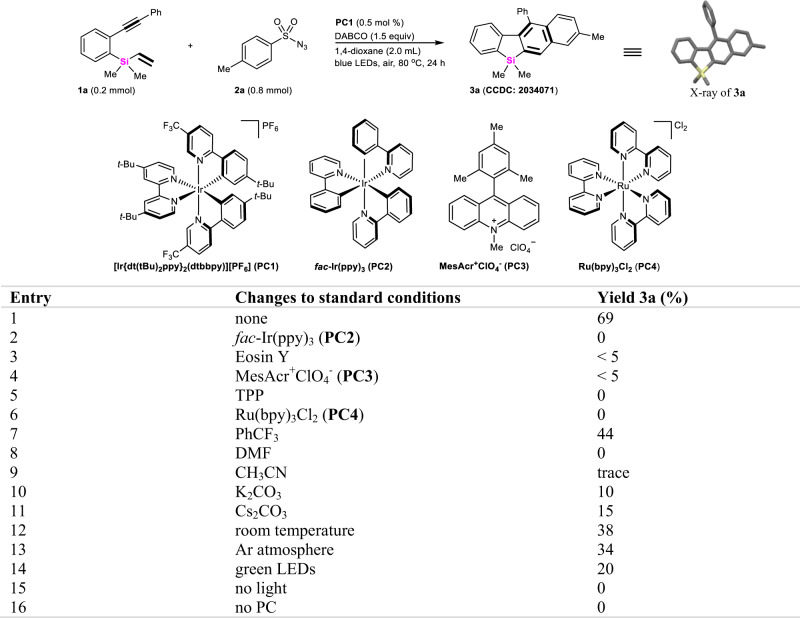
The choice of ortho-alkynylaryl vinylsilane 1a was motivated by the notion that alkynyl and vinyl groups could possibly trap radicals to assemble complex silacycles under blue light-emitting diodes (LEDs) irradiation. After an extensive screening of various reaction parameters, we were pleased to find that the treatment of substrate 1a with TsN3 2a under photocatalyzed system did afford a large π-conjugated benzosilafluorene 3a with an unexpected loss of sulfonylazide (SO2N3) group from TsN3 (Fig. 2). Optimization of the reaction conditions afforded the yield of 3a reaching 69% in the presence of 0.5 mol % of [Ir{dt(tBu)2ppy}2(dtbbpy)][PF6] (PC1) and 1,4-diazabicyclo(2.2.2) octane (DABCO) (1.5 equiv) in 1,4-dioxane at 80 °C for 24 h under air atmosphere (Fig. 2 Table, entry 1). On the contrary, other catalysts such as fac-Ir(ppy)3 (PC2), Eosin Y, MesAcr+ClO4− (PC3), 2,4,6-triphenylpyrylium tetrafluoroborate (TPP), and Ru(bpy)3Cl2 (PC4) did not give the product 3a in more than 5% yield (entries 2–6 vs 1). Meanwhile, utilization of different solvent systems (entries 7–9) or inorganic bases such as K2CO3 and Cs2CO3 (entries 10–11) made this transformation very sluggish. Similarly, running the reaction under room temperature, Ar atmosphere, and green-light irradiation also led to significantly lower yields, respectively (entries 12–14 vs 1). Notably, control experiments under these optimized conditions indicated that 2,3-naphthyl-fused benzosilole 3a could not be observed at all in the absence of light or photocatalysts (PC) (entries 15–16).
Fig. 2. Reaction development.
All the reactions are conducted in sealed tubes, followed by flash chromatography on SiO2. The isolated yields are reported.
Substrate scope
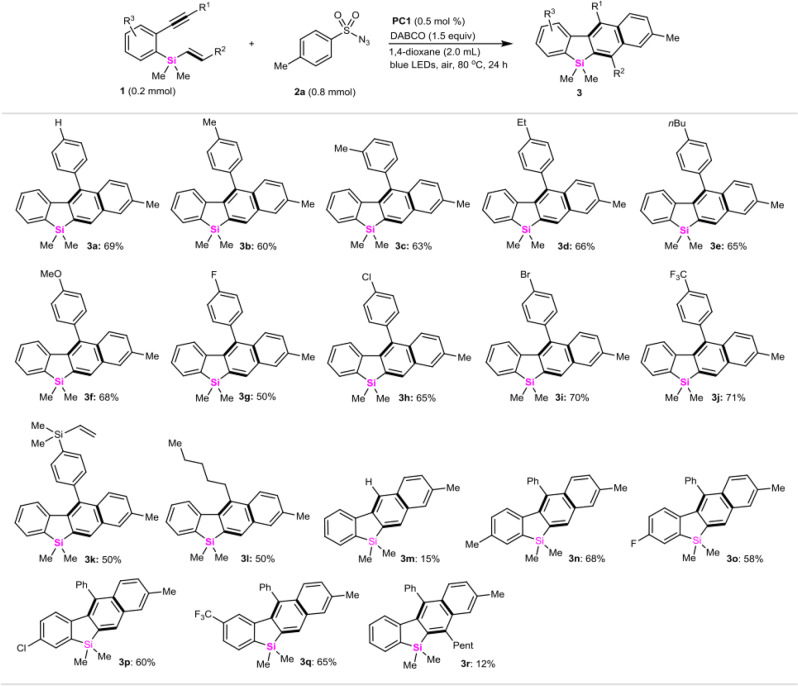
With this optimized protocol, we examined the transformation of various ortho-alkynylaryl vinylsilanes (1) with para-methylphenylsulfonyl azide 2a. As summarized in Fig. 3, different sila-enynes 1 bearing electron-rich (-Me, -Et, -n-Bu, and -MeO), halogen (-F, -Cl and -Br), strong electron-withdrawing (-CF3), and even vinylsilyl substituent at the para-position of alkynylphenyl groups (R1 = phenyl), successfully underwent carbocyclization-aromatization with TsN3 (2a) to afford 4-(4-substituted-phenyl)-2,3-benzosilafluorenes 3a, 3b, 3d–3k in moderate to good yields (50–71%). Likewise, meta-methylphenyl-substituted alkynylaryl vinylsilane could react efficiently to provide 3c in 63% yield. Meanwhile, ortho-(1-heptynyl)-phenyl vinylsilane was also tolerable in this reaction to assemble 4-pentyl-substituted-2,3-benzosilafluorenes 3l (50%). However, when the terminal alkyne-tethered arylvinylsilane was subjected to the reaction system, only a 15% yield of 3m was obtained. The evaluation of the substituent effect (R3) from vinylsilyl benzene ring of 1 indicated that all of the electron-rich or electron-deficient silylarenes could smoothly react with TsN3 (2a) to furnish the desired products 3n–3q with good conversions (58–68% yields), regardless of electronic properties of substituents. It should be noted that hept-1-en-1-yldimethylsilane was not well tolerated under these conditions, only affording 1-pentyl-2,3-benzosilafluorene 3r in 12% yield.
Fig. 3. Sila-enyne scope.
All the reactions are conducted in sealed tubes, followed by flash chromatography on SiO2. The isolated yields are reported.
Interestingly, if vinyldimethylsilane 1 was switched to allyldimethylsilane 4, an unexpected [2 + 2 + 3] coupling-cyclization of ortho-alkynyaryl allyldimethylsilane 4 with arylsulfonyl azides 2 occurred (Fig. 4), affording a novel 6/7/7/6-fused silatetracyclic skeleton in good yields (59–65% for 5a–5c) in which SO2 was kept untouched.
Fig. 4. The coupling-cyclization of allylic dimethylsilanes with arylsulfonyl azides.
All the reactions are conducted in sealed tubes, followed by flash chromatography on SiO2. The isolated yields are reported.
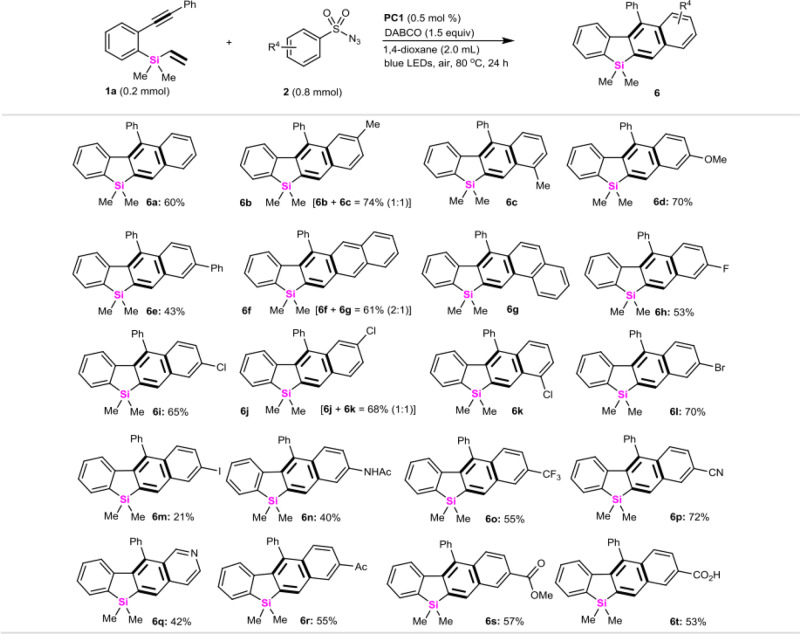
Next, we explored the substrate scope of sulfonyl azides by carrying out the cycloaromatization reaction with vinylsilane 1a (Fig. 5). Compared with electron-neutral phenylsulfonyl azides (60% for 6a), 4-MeO, 4-Ph, 4-halo (F, Cl, Br, I), and 4-acetamido-phenylsulfonyl azides could smoothly react with vinylsilane 1a to produce 2,3-benzosilafluorenes in 21–70% yields (6d, 6e, 6h, 6i, 6l, 6m, 6n). Of noted, 3-methylphenylsulfonyl azide, 3-chlorophenylsulfonyl azide, and even 2-naphthylsulfonyl azide furnished 61–74% overall yields of isomers 6b/6c, 6f/6g, and 6j/6k. Gratifyingly, the protocol was still shown to tolerate strong electron-withdrawing (4-CF3, 4-CN, 4-acyl, 4-CO2Et, 4-CO2H) substituted phenylsulfonyl azides and even 3-pyridylsulfonyl azide, giving 42–72% yields of 6o–6t, respectively.
Fig. 5. Arylsulfonylazide scope.
All the reactions are conducted in sealed tubes, followed by flash chromatography on SiO2. The isolated yields are reported and ratios of isomers are shown in parenthesis.
Application
To evaluate the potentiality of 2,3-naphthyl-fused benzosiloles in developing organic optoelectronic materials, 8-bromo-5,5-dimethyl-11-phenyl-5H-benzo[b]naphtho[2,3-d]silole 6l was employed to react with anthracene-9,10-diyldiboronic acid 7 in the presence of Pd-catalysts, and highly π-conjugated teranthracene-tethered 1-phenyl-2,3-benzosilafluorene 8 which contains three anthracenyl units, could be obtained in 14% yield (Fig. 6a) (it should be noted that this transformation easily led to the protonation of anthracene-9,10-diyldiboronic acid 7, affording 53% yield of anthracence 9 (see Supplementary Information, pg S26)). Photophysical properties of benzosilole derivative 8 were investigated by ultraviolet-visible (UV-vis) absorption and photoluminescence (PL) spectra in diluted toluene solution (10−5 M) (Fig. 6b). The absorption bands in the long-wavelength region (350–400 nm) can be attributed to the π–π* transition of the anthracenyl units45. Both PL spectra at r.t. and 77 K exhibited a 0–0 peak at 415 nm, a 0–1 sub-peak at 433 nm, and a 0–2 shoulder at 465 nm. Meanwhile, a PL quantum yield of 51% was achieved, indicating that this molecular skeleton could be a promising building block for deep-blue luminescent materials.
Fig. 6. Synthetic applications of this method.
a Synthesis of π-conjugated teranthracene-tethered 1-phenyl-2,3-benzosilafluorene 8. b UV-vis absorption and PL spectra of benzosilafluorene 8 measured in diluted toluene solution (10−5 M) (a.u. refers to the arbitrary unit. Fluo@298K refers to the fluorescence spectra measured at 298 K. Fluo@77K refers to the fluorescence spectra measured at 77 K. Phos refers to the phosphorescence spectra.).
Mechanistic investigations
Sulfonyl azides have been rarely converted into sulfonyl radicals in chemical transformation. Up to now, only Konig reported an example that sulfonyl azides were employed as precursors of nitrenes in visible-light photocatalysis46. More recently, Lam found that arylsulfonyl azides could be converted into arylsulfonyl radicals under the photocatalytic system, in which hydrogen abstraction from THF was involved47. However, our solvent screening indicated that this cycloaromatization could also be allowed in PhCF3 (Fig. 2, entry 7), in which hydrogen abstraction from PhCF3 is very difficult. The observation implied that sulfonyl azides could possibly produce sulfonyl radical via unstable arylsulfonyldiimide intermediates (ArSO2N=NSO2Ar)48, which is derived from the nitrene radical dimerization and protonation (this transformation was performed under air conditions, in which the synergistic cooperation of O2, and alkylamines (DABCO), etc. could possibly provide proton sources)49. Given that disulfonylhydrazines could produce disulfonyldiimines via single electron transfer (SET) followed by denitrogenation to afford sulfonyl radicals50,51, we utilized sulfonylhydrazine 10 to react with 1a (Fig. 7a), the desired product 3a (30%) could be obtained, this control experiment implied that disulfonyldiimines were possibly involved in this cycloaromatization.
Fig. 7. Preliminary mechanism studies.
a Photocatalyzed cycloaromatization of vinylsilane 1a with sulfonylhydrazine 10. b Radical intermediate trapping reaction by TEMPO. c Photocatalyzed coupling-cyclization of vinylether 1u with arylsulfonylazide 2h. d Photocatalyzed coupling-cyclization of vinylsilane 1a with ortho-methylphenylsulfonyl azide 2s.
In addition, when 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) (2.0 equiv) was applied to the reaction of vinylsilane 1a and TsN3, it was found that TEMPO completely inhibited the cycloaromatization of 1a, and sulfonyl ester 11 (HR-MS: 334.1448, Supplementary Fig. 3) (see Supplementary Information for the HR-MS) could be detected (Fig. 7b), confirming that sulfonyl radicals were involved in this transformation. Interestingly, changing vinylsilane (1a) to vinylether (1u) produced a seven-membered ring compound 12 (CCDC 2034125) instead of cycloaromatization product 13 (Fig. 7c). This experiment demonstrated that Si/O switch significantly affected the reaction pathway of vinylsilanes, in which sulfonyl radicals derived from sulfonyl azides preferred to attack carbon-carbon double bonds instead of carbon-carbon triple bonds. Furthermore, compared to para- or meta-methylphenylsulfonyl azides (3a in Fig. 3, 6b/6c in Fig. 5), the coupling-cyclization of ortho-methylphenylsulfonyl azide 2s with vinylsilane 1a only afforded a 16% yield of non-aromatized silacycle 14 (CCDC 2064550), and the aromatized silacycle 6u was not obtained possibly due to that steric hindrance of ortho-methyl substituent of 2s inhibited a potential structural arrangement and subsequent aromatization (Fig. 7d).
Phosphorescence spectra of the diluted toluene solutions detected at 77 K with a delay time of 0.05 ms were employed to determine the triplet energies of PC1 (2.58 eV) and TsN3 (3.10 eV) (Supplementary Fig. 2), demonstrating that an energy transfer (EnT) process between the excited state *Ir(III)-catalyst PC1 and TsN3 could not occur. Meanwhile, the reduction potential difference (~0.39 V) between PC1 (EIII/IV: −1.45 V versus Ag/AgNO3 in CH3CN) and TsN3 (Ered: −1.06 V vs Ag/AgNO3 in CH3CN) (see Supplementary Information for the cyclic voltammetry (CV) of TsN3 and Ir(III)-catalyst PC1) means that the electron transfer between them possibly occurred under heating conditions (Supplementary Figs. 4 and 5). Moreover, the fluorescence quenching (Supplementary Figs. 6 and 7) further suggested that a possible SET process between the excited state *Ir(III)-catalyst and TsN3 led to the formation of sulfonyl radicals via nitrene radicals52 and arylsulfonyldiimide48. Calibrated with DFT calculations, the possible reaction mechanism is shown in Fig. 8. In Path a, the addition of sulfonyl radical to the carbon-carbon double bond in substrate 1a results in α-silyl carbon radical A (5.6 kcal/mol), which could further undergo the radical cyclization with carbon-carbon triple bond to afford vinylradical B (−11.6 kcal/mol). Intramolecular cyclization of B at the benzene ring followed by the Smiles rearrangement involved a spirocyclic intermediate C (−12.7 kcal/mol) and a subsequent desulfonylation to produce beta-silyl radical D (−29.9 kcal/mol). Again, the cascade radical cyclization to E (−41.4 kcal/mol), SET, and aromatization give the cycloaromatization product 3a. In contrast, Ts• radical could attack the carbon-carbon triple bond of 1a in Path b, leading to ethylenic radical G (8.0 kcal/mol), which then forms radical intermediate H (−2.0 kcal/mol) and I (−4.8 kcal/mol) through cascade cyclization. Although intermediate I could be converted to E by desulfonylation, control experiments and DFT calculations both suggest Path a to be the more plausible mechanism. Because of that ortho-methylphenylsulfonyl azide 2s was subjected to the standard photocatalyzed system, although ortho-methylphenylsulfonyl radical could attack the carbon-carbon double bond of 1a, the steric hindrance from ortho-methyl substituent of 2s possibly inhibited the formation of the spirocyclic intermediate C (Path a), resulting in very poor yield (16%) of non-aromatized silacycle 14 (see Supplementary Information for the HMBC, NOE spectra and single crystal structure (Supplementary Figs. 8, 9, and 15) of compound 14) through cascade radical cyclization and desulfonylation. This control experiment (Fig. 7d) indirectly implied that the cycloaromatization between vinylsilanes and arylsulfonyl azides via Path b (Fig. 8) was not possible.
Fig. 8. Proposed reaction mechanism.
Cycloaromatization of vinylsilanes with arylsulfonylazides via Path a and Path b.
Discussion
In conclusion, we have reported a photoredox-catalyzed cycloaromatization of ortho-alkynylaryl vinylsilanes and arylsulfonyl azides, furnishing naphthyl-fused benzosilole skeletons with wide functional group tolerance. This protocol features a unique combination of cascade S-N/C-S bond cleavages and α-silyl radical Smiles rearrangement. These silaarenes show promising potential in luminescent materials, and further application studies of these highly π-conjugated siloles in luminescent materials are undergoing in our lab.
Methods
Procedure for the photocatalyzed cycloaromatization of vinylsilanes with arylsulfonylazides
To a 10 mL vial equipped with a magnetic stir bar, was added vinylsilanes 1 (0.2 mmol), arylsulfonylazides 2 (0.8 mmol), DABCO (33 mg, 0.3 mmol), [Ir{dt(tBu)2ppy}2(dtbbpy)][PF6] PC1 (1.3 mg, 0.5 mol %) and 1,4-dioxane (2.0 mL) under air conditions. The vial was equipped with a Teflon septum and stirred at 80 °C under blue LED irradiation with two Kessil LEDs (30 W, 456 nm, ~3 cm away from the reaction mixture) for 24 h. The solvent was removed in vacuo and the residue was purified by flash column chromatography on silica gel to yield the desired products.
Supplementary information
Acknowledgements
We thank NKRDPC (No. 2016YFA0602900), the NSFC (Nos. 21871097, 51625301, 21973113), KARDPGP (No. 2020B010188001), NSFGP (Nos. 2018B030308007, 2019A1515011790), and STPG (No. 201904010113) for financial support.
Source data
Author contributions
W.Z. conceived and directed the project. F.C. performed the experiments. M.L. and C.Y. prepared some substrates. H.J. joined the discussion about this project. Y.S. performed the DFT calculations. Z.K. directed the DFT calculations. S.-J.S. directed the photophysical experiments. W.Z. wrote the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the article as well as from the authors upon reasonable request. The X-ray crystallographic coordinates for structures 3a, 5c, 12, and 14 reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under CCDC 2034071, CCDC 2034073, CCDC 2034125, and CCDC 2064550, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data˗request/cif. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fengjuan Chen, Youxiang Shao, Mengke Li.
Contributor Information
Shi-Jian Su, Email: mssjsu@scut.edu.cn.
Zhuofeng Ke, Email: kezhf3@mail.sysu.edu.cn.
Wei Zeng, Email: zengwei@scut.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-23326-2.
References
- 1.Tacke R, Metz S. Odorant design based on the carbon/silicon switch strategy. Chem. Biodivers. 2008;5:920–941. doi: 10.1002/cbdv.200890105. [DOI] [PubMed] [Google Scholar]
- 2.Friedrich J, Dorrich S, Berkefeld A, Kraft P, Tacke R. Synthesis and olfactory characterization of sila-methyl pamplemousse and related odorants with a 2,2,5-trimethyl-2-silahex-4-ene skeleton. Organometallics. 2014;33:796–803. doi: 10.1021/om401181h. [DOI] [Google Scholar]
- 3.Driess M, Oestreich M. New frontiers and challenges in silicon chemistry: ISOS XVII in berlin. Chem. Eur. J. 2014;20:9144–9145. doi: 10.1002/chem.201404080. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu M, Hiyama T. Silicon-bridged biaryls: molecular design, new synthesis, and luminescence control. Synlett. 2012;23:973–989. doi: 10.1055/s-0031-1290566. [DOI] [Google Scholar]
- 5.Förster B, Bertermann R, Kraft P, Tacke R. Sila-rhubafuran and derivatives: synthesis and olfactory characterization of novel silicon-containing odorants. Organometallics. 2014;33:338–346. doi: 10.1021/om401070c. [DOI] [Google Scholar]
- 6.Pujals S, et al. Replacement of a proline with silaproline causes a 20-fold increase in the cellular uptake of a pro-rich peptide. J. Am. Chem. Soc. 2006;128:8479–8483. doi: 10.1021/ja060036c. [DOI] [PubMed] [Google Scholar]
- 7.Lukevics E, Germane S, Segal I, Zablotskaya A. Silyl modification of biologically active compounds. Chem. Heterocycl. Compd. 1997;33:234–238. doi: 10.1007/BF02256766. [DOI] [Google Scholar]
- 8.Lippert W-P, et al. Silicon analogues of the RXR-selective retinoid agonist SR11237 (BMS649): chemistry and biology. ChemMedChem. 2009;4:1143–1152. doi: 10.1002/cmdc.200900090. [DOI] [PubMed] [Google Scholar]
- 9.Kumagai T, Itsuno S. Asymmetric allylation polymerization of bis(allylsilane) and dialdehyde containing arylsilane structure. Macromolecules. 2002;35:5323–5325. doi: 10.1021/ma012023j. [DOI] [Google Scholar]
- 10.Bai D, Han S, Lu Z-H, Wang S. Bright blue luminescent pyrenyl-containing organosilicon compounds with contrasting charge transport functionality-SiPh2(p-C6H4-pyrenyl)(p-C6H4-N-benzimidazolyl) and SiPh2(p-C6H4-pyrenyl)[p-C6H4-NPh(1-naph)] Can. J. Chem. 2008;86:230–237. doi: 10.1139/v07-151. [DOI] [Google Scholar]
- 11.Shirota Y, Kageyama H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007;107:953–1010. doi: 10.1021/cr050143+. [DOI] [PubMed] [Google Scholar]
- 12.Cui Y-M, Lin Y, Xu L-W. Catalytic synthesis of chiral organoheteroatom compounds of silicon, phosphorus, and sulfur via asymmetric transition metal-catalyzed C–H functionalization. Coord. Chem. Rev. 2017;330:37–52. doi: 10.1016/j.ccr.2016.09.011. [DOI] [Google Scholar]
- 13.Ilies L, Sato Y, Mitsui C, Tsuji H, Nakamura E. Modular synthesis of polybenzo[b]silole compounds for hole-blocking material in phosphorescent organic light emitting diodes. Chem. Asian J. 2010;5:1376–1381. doi: 10.1002/asia.201000112. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Geng W-Z, Wei J-N, Xi Z-F. Palladium-catalyzed intermolecular coupling of 2-silylaryl bromides with alkynes: synthesis of benzosiloles and heteroarene-fused siloles by catalytic cleavage of the C(sp3)–Si bond. Angew. Chem. Int. Ed. 2012;51:1934–1937. doi: 10.1002/anie.201108154. [DOI] [PubMed] [Google Scholar]
- 15.Onoe M, et al. Rhodium-catalyzed carbon–silicon bond activation for synthesis of benzosilole derivatives. J. Am. Chem. Soc. 2012;134:19477–19488. doi: 10.1021/ja3096174. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q-W, An K, He W. Rhodium-catalyzed tandem cyclization/Si–C activation reaction for the synthesis of siloles. Angew. Chem. Int. Ed. 2014;53:5667–5671. doi: 10.1002/anie.201400828. [DOI] [PubMed] [Google Scholar]
- 17.Minami Y, Noguchi Y, Hiyama T. Synthesis of benzosiloles by intramolecular anti-hydroarylation via ortho-C–H activation of aryloxyethynyl silanes. J. Am. Chem. Soc. 2017;139:14013–14016. doi: 10.1021/jacs.7b08055. [DOI] [PubMed] [Google Scholar]
- 18.Shintani R, Takano R, Nozaki K. Rhodium-catalyzed asymmetric synthesis of silicon-stereogenic silicon-bridged arylpyridinones. Chem. Sci. 2016;7:1205–1211. doi: 10.1039/C5SC03767K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocanu A, et al. Synthesis, optical, and redox properties of regioisomeric benzoheterocycles-fused pyrene. J. Org. Chem. 2019;84:957–962. doi: 10.1021/acs.joc.8b02884. [DOI] [PubMed] [Google Scholar]
- 20.Leifert D, Studer A. 9-Silafluorenes via base-promoted homolytic aromatic substitution (BHAS)—the electron as a catalyst. Org. Lett. 2015;17:386–389. doi: 10.1021/ol503574k. [DOI] [PubMed] [Google Scholar]
- 21.Yang C, et al. Visible-light induced radical silylation for the synthesis of dibenzosiloles via dehydrogenative cyclization. Adv. Synth. Catal. 2018;360:3049–3054. doi: 10.1002/adsc.201800417. [DOI] [Google Scholar]
- 22.Zhang QW, et al. Rhodium-catalyzed intramolecular C−H silylation by silacyclobutanes. Angew. Chem. Int. Ed. 2016;55:6319–6323. doi: 10.1002/anie.201602376. [DOI] [PubMed] [Google Scholar]
- 23.Snape T-J. A truce on the smiles rearrangement: revisiting an old reaction-the truce-smiles rearrangement. Chem. Soc. Rev. 2008;37:2452–2458. doi: 10.1039/b808960d. [DOI] [PubMed] [Google Scholar]
- 24.Holden C-M, Greaney M-F. Modern aspects of the smiles rearrangement. Chem. Eur. J. 2017;23:8992–9008. doi: 10.1002/chem.201700353. [DOI] [PubMed] [Google Scholar]
- 25.Ramazani A, Moradnia F, Aghahosseini H, Abdolmaleki I. Several species of nucleophiles in the smiles rearrangement. Curr. Org. Chem. 2017;21:1612–1625. [Google Scholar]
- 26.Fuentes N, Kong W-Q, Fernández – Sánchez L, Merino E, Nevado C. Cyclization cascades via N-amidyl radicals toward highly functionalized heterocyclic scaffolds. J. Am. Chem. Soc. 2015;137:964–973. doi: 10.1021/ja5115858. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, et al. Metal-free cascade construction of C–C bonds by activation of inert C(sp3)–H bonds. Chem. Commun. 2015;51:1320–1322. doi: 10.1039/C4CC08629E. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Li Y-J. Sustainable difluoroalkylation cyclization cascades of 1,8-enynes. J. Org. Chem. 2017;82:4449–4457. doi: 10.1021/acs.joc.7b00350. [DOI] [PubMed] [Google Scholar]
- 29.Kong W, Fuentes N, García–Domínguez A, Merino E, Nevado C. Stereoselective synthesis of highly functionalized indanes and dibenzocycloheptadienes through complex radical cascade reactions. Angew. Chem. Int. Ed. 2015;54:2487–2491. doi: 10.1002/anie.201409659. [DOI] [PubMed] [Google Scholar]
- 30.Tan F-L, Song R-J, Hu M, Li J-H. Metal-free oxidative 1,2-arylmethylation cascades of N-(arylsulfonyl)acrylamides using peroxides as the methyl resource. Org. Lett. 2016;18:3198–3201. doi: 10.1021/acs.orglett.6b01419. [DOI] [PubMed] [Google Scholar]
- 31.Monos T-M, McAtee R-C, Stephenson CRJ. Ary-sulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science. 2018;361:1369–1373. doi: 10.1126/science.aat2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpers D, Cole K-P, Stephenson CRJ. Visible light mediated aryl migration by homolytic C−N cleavage of aryl amines. Angew. Chem. Int. Ed. 2018;57:12167–12170. doi: 10.1002/anie.201806659. [DOI] [PubMed] [Google Scholar]
- 33.Whalley D-M, Duong H-A, Greaney M-F. Alkene carboarylation through catalyst–free, visible light-mediated smiles rearrangement. Chem. Eur. J. 2019;25:1927–1930. doi: 10.1002/chem.201805712. [DOI] [PubMed] [Google Scholar]
- 34.Brachet E, Marzo L, Selkti M, Kӧnig B, Belmont P. Visible light amination/Smiles cascade:access to phthalazine derivatives. Chem. Sci. 2016;7:5002–5006. doi: 10.1039/C6SC01095D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z-S, et al. Ynamide smiles rearrangement triggered by visible-light-mediated regioselective ketyl-ynamide coupling: rapid access to functionalized indoles and isoquinolines. J. Am. Chem. Soc. 2020;142:3636–3644. doi: 10.1021/jacs.9b13975. [DOI] [PubMed] [Google Scholar]
- 36.Denmark S-E, Sweis R-F. Design and implementation of new, silicon-based, cross-coupling reactions: Importance of silicon–oxygen bonds. Acc. Chem. Res. 2002;35:835–846. doi: 10.1021/ar020001r. [DOI] [PubMed] [Google Scholar]
- 37.Simons G, Zandler M-E, Talaty E-R. Nonempirical electronegativity scale. J. Am. Chem. Soc. 1976;98:7869–7870. doi: 10.1021/ja00440a093. [DOI] [Google Scholar]
- 38.Hanstein W, Berwin H-J, Traylor T-G. Modes of carbonium ion stabilization. Evidence from charge-transfer spectra. J. Am. Chem. Soc. 1970;92:829–836. doi: 10.1021/ja00707a016. [DOI] [Google Scholar]
- 39.Wierschke S-G, Chandrasekha J, Jorgensen W-L. Magnitude and origin of the beta-silicon effect on carbenium ions. J. Am. Chem. Soc. 1985;107:1496–1500. doi: 10.1021/ja00292a008. [DOI] [Google Scholar]
- 40.Blumenkopf T-A, Overman L-E. Vinylsilan- and alkynylsilane-terminated cyclization reactions. Chem. Rev. 1986;86:857–873. doi: 10.1021/cr00075a009. [DOI] [Google Scholar]
- 41.Park J-W, Jun C-H. Transition metal-catalyzed immobilization of organic functional groups onto solid supports through vinylsilane coupling reactions. J. Am. Chem. Soc. 2010;132:7268–7269. doi: 10.1021/ja102741k. [DOI] [PubMed] [Google Scholar]
- 42.Niljianskul N, Zhu S-L, Buchwald S-L. Enantioselective synthesis of α-aminosilanes by copper-catalyzed hydroamination of vinylsilanes. Angew. Chem. Int. Ed. 2015;54:1638–1641. doi: 10.1002/anie.201410326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kato K, Hirano K, Miura M. Synthesis of β-boryl-α-aminosilanes by copper-catalyzed aminoboration of vinylsilanes. Angew. Chem. Int. Ed. 2016;55:14400–14404. doi: 10.1002/anie.201608139. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Webster RD, Harms K, Meggers E. Asymmetric catalysis with organic azides and diazo compounds initiated by photoinduced electron transfer. J. Am. Chem. Soc. 2016;138:12636–12642. doi: 10.1021/jacs.6b07692. [DOI] [PubMed] [Google Scholar]
- 45.Park H, et al. Highly rigid and twisted anthracene derivatives: a strategy for deep blue OLED materials with theoretical limit efficiency. J. Mater. Chem. 2012;22:2695–2700. doi: 10.1039/C2JM16056K. [DOI] [Google Scholar]
- 46.Brachet E, Ghosh T, Ghosh I, Konig B. Visible light C–H amidation of heteroarenes with benzoyl azides. Chem. Sci. 2015;6:987–992. doi: 10.1039/C4SC02365J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu S, et al. Sulfonylative and azidosulfonylative cyclizations by visible-light-photosensitization of sulfonyl azides in THF. Chem. Eur. J. 2017;23:17598–17604. doi: 10.1002/chem.201704380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maioli A-T, Anselme J-P. Oxidation of 1,2–bis–(benzenesulfonyl)hydrazine to 1,2– diphenyldisulfone. Tetrahedron Lett. 1995;36:1221–1222. doi: 10.1016/0040-4039(95)00038-E. [DOI] [Google Scholar]
- 49.Yilmaz, O., Oderinde, M. S. & Emmert, M. H. Photoredox-catalyzed Cα−H cyanation of unactivated secondary and tertiary aliphatic amines: late-stage functionalization and mechanistic studies. J. Org. Chem.83, 11089–11100 (2018). [DOI] [PubMed]
- 50.Luo D, et al. N,N–disulfonylhydrazines: new sulfonylating reagents for highly efficient synthesis of (E)–vinyl sulfones at room temperature. Tetrahedron. 2020;76:131019. doi: 10.1016/j.tet.2020.131019. [DOI] [Google Scholar]
- 51.Liang T, et al. Transition metal–free C5 tosyloxylation of 8-aminoquinolines with phenyliodine bistrifluoroacetate and substituted 1,2–disulfonyl hydrazides. Eur. J. Org. Chem. 2019;2019:2513–2519. doi: 10.1002/ejoc.201900092. [DOI] [Google Scholar]
- 52.Singha K, Mondal A, Ghosh SC, Panda AB. Visible-light-driven efficient photocatalytic reduction of organic azides to amines over CdS sheet-rGO nanocomposite. Chem. Asian J. 2018;13:255–260. doi: 10.1002/asia.201701614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article as well as from the authors upon reasonable request. The X-ray crystallographic coordinates for structures 3a, 5c, 12, and 14 reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under CCDC 2034071, CCDC 2034073, CCDC 2034125, and CCDC 2064550, respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data˗request/cif. Source data are provided with this paper.