Abstract
The incidence of herpes zoster in psoriasis patients is higher than in the general population. However, the association between herpes zoster risk and different systemic therapies, especially biologic agents, remains controversial. This study investigated the association between herpes zoster risk and several systemic antipsoriasis therapies. This prospective open cohort study was conducted using retrospectively collected data from the Taiwan National Health Insurance Research Database. We included 92,374 patients with newly diagnosed psoriasis between January 1, 2001, and December 31, 2013. The exposure of interest was the “on-treatment” effect of systemic antipsoriasis therapies documented by each person-quarter. The outcome was the occurrence of newly diagnosed herpes zoster. During a mean follow-up of 6.8 years, 4834 (5.2%) patients were diagnosed with herpes zoster after the index date. Among the systemic antipsoriasis therapies, etanercept (hazard ratio [HR] 4.78, 95% confidence interval [CI] 1.51–15.17), adalimumab (HR 5.52, 95% CI 1.72–17.71), and methotrexate plus azathioprine (HR 4.17, 95% CI 1.78–9.82) were significantly associated with an increased risk of herpes zoster. By contrast, phototherapy (HR 0.76, 95% CI 0.60–0.96) and acitretin (HR 0.39, 95% CI 0.24–0.64) were associated with a reduced risk of herpes zoster. Overall, this study identified an association of both etanercept and adalimumab with an increased risk of herpes zoster among psoriasis patients. Acitretin and phototherapy were associated with a reduced risk.
Subject terms: Infectious diseases, Skin diseases, Epidemiology
Introduction
Psoriasis is a chronic inflammatory dermatosis whose pathogenesis involves various immune cells and cytokines. The interplay between plasmacytoid and myeloid dendritic cells, helper T cells (Th1, Th17, Th22), and mediators such as tumor necrosis factor (TNF)-α, interleukin (IL)-12, IL-23, and IL-17 provides targets for psoriasis drug development1,2. Several biologic agents have been approved for treating psoriasis, such as anti-TNF-α agents and IL-12/IL-23 antagonists. However, because of the resulting suppression of cell-mediated immunity, addressing the risks of bacterial and viral infections remains of paramount importance3.
Herpes zoster (HZ) is caused by the reactivation of varicella–zoster virus (VZV) from the dorsal root ganglia in individuals who have had a previous varicella infection. VZV-specific CD4+ and CD8+ T cells as well as VZV-specific antibodies participate in the immune response during primary infection and reactivation. Notably, a low number of T cells or low antibody titer has been associated with increased severity of HZ and postherpetic neuralgia4. Although HZ is usually not life-threatening, it has been associated with increased health care utilization and medical expenditure. The pain caused by HZ impairs the quality of life as well5. Tsai et al. reported a higher risk of HZ in psoriasis patients than in the general population. This risk is even higher in patients receiving systemic therapy than in those using topical treatment alone6. Research has yet to identify a significantly different risk of HZ between psoriasis patients treated with biologic agents and those receiving other systemic therapies; nevertheless, the suppression of T cell–mediated immunity leads to the reasonable inference that biologic agents may be associated with an increased risk of HZ7,8.
To determine the risk of HZ in psoriasis patients receiving various medications, we conducted a nationwide population-based cohort study.
Material and methods
Data source
This was a prospective open cohort study that incorporated retrospectively collected data from the Taiwan National Health Insurance Research Database (NHIRD). The NHIRD contains no identifiable personal information, contains data on roughly 99.8% of the 23 million residents of Taiwan, and provides information regarding diagnoses, prescriptions, examinations, surgeries, and expenditures relating to both inpatient and outpatient services since March 1995. Because of the affordability and mandatory enrollment of Taiwan’s National Health Insurance (NHI) program, long-term follow-up is nearly complete. Further information regarding the NHI and NHIRD has been described in previous publications9–11. This study was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital and was conducted in accordance with the principles of the Declaration of Helsinki.
Study population
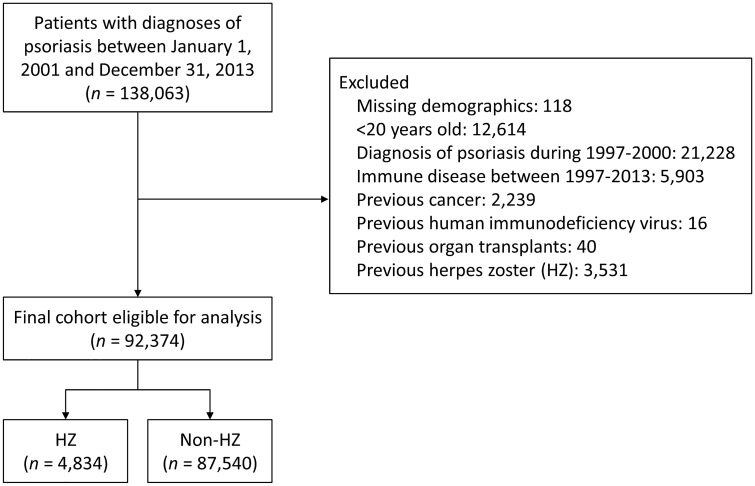
In this study, patients of interest were those diagnosed with psoriasis between January 1, 2001, and December 31, 2013. Psoriasis patients were required to have had three outpatient diagnoses or any inpatient diagnosis based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 6960, 6961, and 6968, as documented in the NHIRD12,13. Patients who had been diagnosed with psoriasis before 2000 were excluded; hence, the analyzed cohort included only new-onset psoriasis patients. Furthermore, the first psoriasis diagnosis date was defined as the index date. We excluded patients with missing demographics (< 0.1%) and those younger than 20 years old. We additionally excluded the following diseases because they were competing indications for several antipsoriatic biologic agents and immunosuppressants: systemic lupus erythematosus (710.xx), rheumatoid arthritis (714.xx), inflammatory bowel disease (Crohn’s disease [555.xx] and ulcerative colitis [556.xx]), ankylosing spondylitis (720.xx), and hidradenitis suppurativa (705.83). Also excluded were severely immunocompromised patients who had a health status involving treatment with an effect that would overshadow that of antipsoriatic therapy: cancer (140–280), human immunodeficiency virus (HIV) infection (042–044), or any organ transplant (V42.xx). Finally, patients who had any diagnosis of HZ before the index date were also excluded (Fig. 1).
Figure 1.
Flowchart of patient inclusion.
Unit of follow-up time and definition of exposures
We divided each calendar year into four quarters for each patient and year after psoriasis diagnosis. The analytic unit was 1 person-quarter. Person-quarters were used because medications for chronic illnesses are refilled for a maximum of 3 months according to the Taiwan NHI reimbursement policy14,15. The covariates and exposures were assessed for each person-quarter.
The following medications or therapies were of interest in the study: methotrexate (MTX), acitretin, cyclosporine, tacrolimus, mycophenolate mofetil, mycophenolic acid, azathioprine, etanercept, adalimumab, ustekinumab, and ultraviolet (UV) phototherapy. In the quarters without incident HZ, the use of oral drugs was defined to include at least 30 prescription days, this includes MTX, which is counted by “days” in the database. The use of UV phototherapy was defined as at least eight sessions in the quarter.
Because of the retrospective nature of this study, the starting date of each patient may differ. Therefore, some patients may receive biologic treatment across two consecutive quarters, but in each quarter, the prescription duration was only two weeks. Considering the different dosing and prescription of biologic agents, we counted these agents with a slightly different way: Ustekinumab, at least one administration in the quarter; at least on two administration of adalimumab in the quarter; etanercept, at least eight administrations in the quarter. If the treatment schedule spanned over two quarters, we count the use by at least one administration of adalimumab and at least four administration of etanercept within 14 days in the starting or ending quarter. Notably, in the quarters with incident HZ, medications of primary interest were identified before the diagnostic date of HZ in which ustekinumab was tracked back to 3 months, whereas the other drugs (including UV phototherapy) were tracked back to 1 month7,16,17.
In addition, we extracted the cumulative prednisone-equivalent dose of oral glucocorticoid during the quarter. Statin use was also extracted during the quarter and was defined as at least 30 prescription days. Information about medications was extracted from the claims data of outpatient visits or pharmacy refills for chronic illness medications by using the Anatomical Therapeutic Chemical codes or the Taiwan NHI reimbursement code.
Covariates and outcome
The covariates in this study were as follows: age at the index date, sex, urbanization level of place of residence, diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, gouty arthritis, psoriatic arthritis, and the Charlson comorbidity index score. Comorbidities were defined as having at least two outpatient diagnoses or an inpatient diagnosis in the previous year. The ICD-9-CM diagnostic codes of the comorbidities are listed in the supplement (Supplemental Table 1). The study outcome was incident HZ, which was defined as the presence of ICD-9-CM diagnostic code 053 with at least two outpatient visits within 14 days or one inpatient diagnosis18–20. All patients were followed from the index date to either the date of incident HZ, the date of withdrawal from the NHI program, or December 31, 2013, whichever came first.
Statistical analysis
Data were expressed as frequency and percentage for categorical variables and as mean and standard deviation for continuous variables. Each person-quarter was classified into mutually exclusive exposures or control periods (see Table 2). The incidence of “on-treatment,” namely each control period or exposure period, was calculated by dividing the number of HZ cases by net person-years on treatment. We first compared the risk of incident HZ among the exposure or control periods without adjustment of covariates. Next, the associations between covariates, medications of interest, and HZ risk were investigated using a multivariable time-dependent Cox proportional hazards model. The outcome dependency among the multiple person-quarters of each patient was accounted for by using a robust (sandwich) estimator. A two-sided P value of < 0.05 was considered to be statistically significant, and no adjustment of multiple testing (multiplicity) was made. All the statistical analyses were performed using R version 3.6.1 (R Development Core Team) with the ‘rms’ package (Version 5.1-3.1 updated on April 22, 2019).
Table 2.
Crude incidence of herpes zoster per 1000 person-years for each treatment.
| Variable | No. of person-quarter | Net person-year on treatment | No. of herpes zoster | Incidence (95% CI)* | P value versus control period |
|---|---|---|---|---|---|
| Control period | 2,375,268 | 576,853.3 | 4628 | 8.0 (7.8–8.3) | – |
| Methotrexate with any biological agents | 1781 | 440.7 | 3 | 6.8 (− 0.9 to 14.5) | 0.042 |
| Etanercept without Methotrexate | 1063 | 262.9 | 3 | 11.4 (− 1.5 to 24.3) | 0.008 |
| Adalimumab without Methotrexate | 926 | 229.2 | 3 | 13.1 (− 1.7 to 27.9) | 0.001 |
| Ustekinumab without Methotrexate | 249 | 61.7 | 0 | NA | NA |
| Methotrexate with PUVA or UVB | 7093 | 1729.4 | 3 | 1.7 (− 0.2 to 3.7) | 0.027 |
| Methotrexate with Acitretin | 2233 | 545.5 | 2 | 3.7 (− 1.4 to 8.7) | 0.368 |
| Methotrexate with Azathioprine | 1095 | 267.9 | 5 | 18.7 (2.3–35.0) | 0.001 |
| Methotrexate only | 48,839 | 11,799.0 | 79 | 6.7 (5.2–8.2) | 0.522 |
| Acitretin only | 17,484 | 4173.4 | 16 | 3.8 (2.0–5.7) | < 0.001 |
| Acitretin with PUVA or UVB | 4591 | 1113.4 | 4 | 3.6 (0.1–7.1) | 0.092 |
| PUVA or UVB only | 41,144 | 9824.7 | 71 | 7.2 (5.5–8.9) | 0.055 |
| Azathioprine only | 2262 | 550.7 | 7 | 12.7 (3.3–22.1) | 0.060 |
| Other combinations | 4521 | 1101.2 | 10 | 9.1 (3.5–14.7) | 0.056 |
| Total | 2,508,549 | 608,964.3 | 4834 | 7.9 (7.7–8.2) | – |
NA not applicable, PUVA photochemotherapy, UVB ultraviolet B.
*Per 1000 person-years.
Ethics approval and consent to participate
The NHIRD contains no identifiable personal information; the need for informed consent was waived by the Institutional Review Board of Chang Gung Memorial Hospital. because of this study’s retrospective noninterventional design and because patient data confidentiality and privacy were maintained. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital.
Results
A total of 138,063 psoriasis patients were identified between 2001 and 2013. After excluding patients with missing demographics (n = 118), patients younger than 20 years old (n = 12,614) and patients having an autoimmune disease (n = 5903), cancer (n = 2239), HIV infection (n = 16), organ transplant (n = 40), or previous HZ (n = 3531), the remaining 92,374 new-onset psoriasis patients were eligible for the final analysis. During the entire follow-up, 4834 (5.2%) patients were diagnosed with HZ after the index date (Fig. 1).
Table 1 summarizes the patients’ demographic and clinical characteristics. The average age of the patients at baseline was 46.7 years, with a mean follow-up duration of 6.8 years (standard deviation: 3.7 years). Male patients constituted approximately 60%, and the most frequently used therapies were phototherapy, acitretin, and MTX either at baseline or during the entire follow-up (Table 1).
Table 1.
Demographic and clinical characteristics of patients at baseline and through follow-up.
| Variable | Baseline | Through the follow up |
|---|---|---|
| Age (years; mean ± SD) | 46.7 ± 17.2 | 53.5 ± 17.0* |
| Female sex, n (%) | 36,501 (39.5) | – |
| Urbanization level of patient’s residence, n (%) | ||
| Low | 7282 (7.9) | – |
| Moderate | 28,426 (30.8) | – |
| High | 28,704 (31.1) | – |
| Very High | 27,962 (30.3) | – |
| Comorbidity, n (%) | ||
| Diabetes mellitus | 8577 (9.3) | 18,417 (19.9) |
| Hypertension | 17,260 (18.7) | 33,196 (35.9) |
| Dyslipidemia | 7818 (8.5) | 24,082 (26.1) |
| Chronic kidney disease | 3033 (3.3) | 10,821 (11.7) |
| Gouty arthritis | 4703 (5.1) | 12,383 (13.4) |
| Psoriasis arthritis | 65 (0.1) | 7390 (8.0) |
| Charlson Comorbidity Index score (mean ± SD) | 0.5 ± 1.0 | 0.9 ± 1.7* |
| Medication of primary interest, n (%) | ||
| PUVA or UVB | 3583 (3.9) | 12,728 (13.8) |
| Cyclosporine | 274 (0.3) | 2201 (2.4) |
| Oral retinoids (Acitretin) | 1770 (1.9) | 6496 (7.0) |
| Tacrolimus | 0 (0.0) | 41 (0.04) |
| Hydroxyurea | 7 (0.01) | 102 (0.1) |
| Mycophenolate mofetil | 0 (0.0) | 43 (0.05) |
| Azathioprine | 120 (0.1) | 906 (1.0) |
| Methotrexate without any biological agents | 2921 (3.2) | 12,301 (13.3) |
| Etanercept without methotrexate | 0 (0.0) | 215 (0.2) |
| Adalimumab without methotrexate | 0 (0.0) | 187 (0.2) |
| Ustekinumab without methotrexate | 0 (0.0) | 129 (0.1) |
| Methotrexate with any biological agents | 0 (0.0) | 396 (0.4) |
| Steroid use at diagnosis of psoriasis (prednisone-equivalent dose) | ||
| Never user | 79,909 (86.5) | – |
| < 5 mg/day | 11,722 (12.7) | – |
| ≥ 5 mg/day | 743 (0.8) | – |
| Statin | 2007 (2.2) | 16,771 (18.2) |
SD standard deviation, PUVA photochemotherapy, UVB ultraviolet B.
Data are presented as frequency (percentage) or mean ± standard deviation.
*At the end of the follow-up.
The crude incidence rate of HZ was 8.0 events per 1,000 person-years (Table 2). Table 3 lists the results of multivariable time-dependent Cox analysis. For systemic therapy, we found that etanercept (hazard ratio [HR] 4.78, 95% confidence interval [CI] 1.51–15.17), adalimumab (HR 5.52, 95% CI 1.72–17.71), and MTX plus azathioprine (HR 4.17, 95% CI 1.78–9.82) were significantly associated with an increased risk of HZ. The combination of MTX with any biologic agent (etanercept, adalimumab, or ustekinumab) exhibited a non-significant higher risk of HZ (HR 3.10, 95% CI 0.97–9.92, P = 0.056). By contrast, phototherapy (HR 0.76, 95% CI 0.60–0.96), acitretin (HR 0.39, 95% CI 0.24–0.64), and MTX plus phototherapy (HR 0.29, 95% CI 0.09–0.90) were associated with lowerrisk of HZ.
Table 3.
Associations between covariates, medications, and risk of herpes zoster.
| Variable | HR (95%CI) | P |
|---|---|---|
| Age, per year* | 1.036 (1.03–1.04) | < 0.001 |
| Female sex | 1.10 (1.04–1.17) | 0.001 |
| Urbanization level | ||
| Low | Reference | – |
| Moderate | 1.02 (0.92–1.14) | 0.666 |
| High | 1.00 (0.89–1.11) | 0.939 |
| Very high | 1.04 (0.93–1.15) | 0.527 |
| Comorbid conditions* | ||
| Diabetes mellitus | 0.92 (0.84–1.01) | 0.068 |
| Hypertension | 1.11 (1.03–1.19) | 0.004 |
| Dyslipidemia | 1.18 (1.08–1.29) | < 0.001 |
| Chronic kidney disease | 0.95 (0.84–1.08) | 0.451 |
| Gouty arthritis | 0.92 (0.82–1.03) | 0.141 |
| Psoriasis arthritis | 1.25 (1.01–1.53) | 0.035 |
| Charlson Comorbidity Index score* | 1.18 (1.15–1.22) | < 0.001 |
| Medication of primary interest* | ||
| Control period | Reference | – |
| Methotrexate with any biological agents | 3.10 (0.97–9.92) | 0.056 |
| Etanercept without Methotrexate | 4.78 (1.51–15.17) | 0.008 |
| Adalimumab without Methotrexate | 5.52 (1.72–17.71) | 0.004 |
| Ustekinumab without Methotrexate | NA | NA |
| Methotrexate with PUVA or UVB | 0.29 (0.09–0.90) | 0.032 |
| Methotrexate with Acitretin | 0.58 (0.15–2.32) | 0.442 |
| Methotrexate with Azathioprine | 4.17 (1.78–9.82) | 0.001 |
| Methotrexate only | 1.01 (0.80–1.27) | 0.958 |
| Acitretin only | 0.39 (0.24–0.64) | < 0.001 |
| Acitretin with PUVA or UVB | 0.44 (0.17–1.18) | 0.104 |
| PUVA or UVB only | 0.76 (0.60–0.96) | 0.023 |
| Azathioprine only | 1.59 (0.76–3.33) | 0.218 |
| Other combinations | 1.50 (0.78–2.85) | 0.222 |
| Medication not of primary interest | ||
| Steroid (prednisone-equivalent dose)* | ||
| Never user | Reference | – |
| < 5 mg/day | 0.99 (0.91–1.08) | 0.786 |
| ≥ 5 mg/day | 1.68 (1.24–2.27) | 0.001 |
| Statin* | 1.54 (1.38–1.71) | < 0.001 |
PUVA photochemotherapy, UVB ultraviolet B, HR hazard ratio, CI confidence interval, NA not applicable.
*Time-varying covariates that may change in each person-quartile.
In addition, older age, female sex, hypertension, dyslipidemia, psoriatic arthritis, and a relatively high Charlson comorbidity index score were observed to be associated with an increased risk of HZ. Finally, concurrent exposure to a steroid at an equivalent dose higher than a 5-mg prednisolone per day (HR 1.68, 95% CI 1.24–2.27) or statin (HR 1.54, 95% CI 1.38–1.71) was associated with higher risk of HZ (Table 3). In the alternative model, each medication was treated as a covariate rather than pre-specified combinations of therapy and the results showed that adalimumab, etanercept and mycophenolate mofetil/mycophenolic acid were associated with higher risks, whereas phototherapy and acitretin were associated with lower risks. (Supplemental Table 2).
Discussion
This nationwide population-based cohort study yielded real-world evidence of HZ risk in psoriasis patients receiving systemic therapy. We observed that treatment with etanercept, adalimumab, and MTX plus AZA were significantly associated with higher risk of HZ. Furthermore, MTX combined with any biologic agent non-significantly associated with HZ risk. By contrast, acitretin and phototherapy were associated with lower risk of HZ. The patients’ characteristics associated with increased HZ include age, female sex, steroid use, hypertension, dyslipidemia, and psoriatic arthritis.
The risk of HZ was higher in patients with immunocompromised statuses, such as those with HIV, cancer, and allogeneic solid organ or hematopoietic stem cell transplants21–23. Associations between HZ and autoimmune diseases, including systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease, and between immunosuppressant use and HZ have been reported16,24–27. Data pooled both from the meta-analysis of clinical trials and from observational studies show that biologic agents against rheumatic disease increased the risk of HZ28. However, the association between HZ risk and biologic agents against psoriasis is not clear. For example, Dreiher et al. compared various systemic treatments with control period measurements and found that only steroid use was associated with a higher risk of HZ29. Shalom et al. reported an increased risk of HZ in patients receiving a combination of MTX plus biologic agents; however, in a large cohort, they observed no increased risk in patients receiving biologic agents alone7. Shalom et al. subsequently demonstrated that therapy with biologic agents and MTX did not entail a higher risk of HZ than the risk in the composite reference group, which included patients treated with phototherapy, oral steroids, topical therapy, and immunomodulators other than MTX (HR 2.22 [95% CI 0.82–5.97; P = 0.116] for TNF-α inhibitors, 2.73 [0.98–7.58; P = 0.054] for ustekinumab, and 1.04 [0.20–5.41; P = 0.966] for MTX)8. This is the first large cohort study to demonstrate an increased risk of HZ among psoriasis patients receiving etanercept or adalimumab. We observed no occurrence of HZ after exposure to ustekinumab, which may be attributed to limited exposure time due to the late approval of ustekinumab by the Taiwan Food and Drug Administration in 2011.
In this study, acitretin was associated with reduced risk of HZ, which was similarly reported by Shalom et al. Acitretin is a mediator of cell differentiation and immune function modulation whose use may not be associated with profound immunosuppression. Retinoic acid–inducible gene-I (RIG-I), a pattern recognition receptor that identifies viral RNA transcripts, is upregulated by retinoic acid and its intracellular receptors30. RIG-I participates in the immune response against herpes simplex virus and VZV4,31,32. Additional evidence has emerged from a study of hematopoietic stem cell transplant patients. High et al. showed that low plasma retinol concentration was associated with increased HZ during the peri-transplantation period33. From a clinical perspective, psoriasis patients who achieve disease control with an acitretin-only regimen may experience less immune suppression compared with those receiving stronger immunosuppressive agents.
Next, we observed that UV phototherapy reduced the risk of HZ. UV light exerts immunomodulatory effects such as inducing a shift from Th1- to Th2-mediated response, increasing regulatory T cell function, augmenting macrophage differentiation, and inhibiting plasma cell differentiation34. Therefore, UV phototherapy is widely used as a systemic therapy for psoriasis. Although the effect of UV light on the skin is mainly anti-inflammatory, this may not imply an overt immunosuppressive effect. Upon exposure to UV light, the human skin generates vitamin D, a vital nutrient for skeletal health and a well-known immunoregulator. Vitamin D can upregulate several antimicrobial peptides, facilitate macrophage function, and modulate inflammation elicited by T cells and B cells. Although certain immunosuppressive effects of high-dose vitamin D on lymphocytes have been reported in animal and cell studies, vitamin D supplements can be used to enhance T cell–mediated immunity in vitamin D–deficient patients35. Chao et al. reported that vitamin D–deficient hemodialysis patients had lower immunity against VZV and that active vitamin D therapy lowered the risk of HZ36,37. Patients with psoriasis are also susceptible to vitamin D deficiency, which may be attributable to chronic inflammation and steroid exposure. Moreover, Vahavihu et al. demonstrated an improved vitamin D serum level and cutaneous expression of cathelicidin in patients with concurrent psoriasis and vitamin D deficiency38. We contend that the protective effect of UV phototherapy is due to an increased vitamin D level associated with scheduled and carefully monitored UV exposure.
Similar to previous studies on general population or on diabetic patients, the use of statin is associated with higher risk of HZ. The actual mechanisms of these association have not fully understood, but may involve the effect of statin on T cell function39–43.
Our study has the strength of being a cohort study that used a time-dependent design to assess the risk of HZ associated with different treatment exposures. This study, however, has limitations. First, ustekinumab was approved and became available in Taiwan in 2011; therefore, the duration of follow-up for this drug was shorter than that of other medications. Second, several biologic agents, including infliximab, efalizumab, and alefacept, are not approved in Taiwan for treating psoriasis. Therefore, the number of patients exposed to biologic agents will be relatively small, which could lead to the risk estimates underpowered. Third, because the NHIRD data did not include psoriasis severity or laboratory findings, we were unable to adjust for these parameters in our model. Previous studies of psoriasis using NHIRD used the presence of systemic therapies or psoriatic arthritis to classify patients into “moderate-to-severe” category as compared to the “mild” category. This classification could reflect long-term morbidities in psoriasis patients12,13,44–46. In addition, the changes in disease severity are accompanied with medication adjustment, which could be adjusted in our study because they were time-varying covariates. Therefore, this study design may partially mitigate the lack of severity score.
In conclusion, this study found that patients with moderate to severe psoriasis who were treated with certain biologic agents had a higher risk of HZ, whereas those treated with UV phototherapy or acitretin were associated with lower risk of HZ.
Supplementary Information
Acknowledgements
We thank two biostatisticians, Mr. Alfred Hsing-Fen Lin and Mr. Ben Yu-Lin Chou, for the consultation in the statistical analysis during the completion of the manuscript.
Author contributions
Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. Research idea and study design: S.W.T., S.Y.T.; data acquisition: Y.S.L., M.S.L.; data analysis and interpretation: M.S.L., Y.S.L., G.K.; statistical analysis: S.W.T., S.Y.T.; supervision or mentorship: G.K. S.W.T and S.Y.T. contributed equally to this work.
Data availability
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sze-Wen Ting and Sze-Ya Ting.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91356-3.
References
- 1.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017;140:645–653. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann. Rheum. Dis. 2018;77:175–187. doi: 10.1136/annrheumdis-2017-211555. [DOI] [PubMed] [Google Scholar]
- 3.Chiu YM, et al. A real-world risk analysis of biological treatment (adalimumab and etanercept) in a country with a high prevalence of tuberculosis and chronic liver disease: a nationwide population-based study. Scand. J. Rheumatol. 2017;46:236–240. doi: 10.1080/03009742.2016.1202318. [DOI] [PubMed] [Google Scholar]
- 4.Laing KJ, Ouwendijk WJD, Koelle DM, Verjans G. Immunobiology of varicella-zoster virus infection. J. Infect. Dis. 2018;218:S68–s74. doi: 10.1093/infdis/jiy403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu WH, Lin CW, Wang CY, Chen LK, Hsiao FY. Epidemiology and long-term disease burden of herpes zoster and postherpetic neuralgia in Taiwan: a population-based, propensity score-matched cohort study. BMC Public Health. 2018;18:369. doi: 10.1186/s12889-018-5247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SY, et al. Increased risk of herpes zoster in patients with psoriasis: a population-based retrospective cohort study. PLoS ONE. 2017;12:e0179447. doi: 10.1371/journal.pone.0179447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalom G, et al. Systemic therapy for psoriasis and the risk of herpes zoster: a 500,000 person-year study. JAMA Dermatol. 2015;151:533–538. doi: 10.1001/jamadermatol.2014.4956. [DOI] [PubMed] [Google Scholar]
- 8.Shalom G, et al. Biological treatment for psoriasis and the risk of herpes zoster: results from the psoriasis longitudinal assessment and registry (PSOLAR) J. Dermatol. Treat. 2019;30:534–539. doi: 10.1080/09546634.2018.1445193. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh CY, et al. Taiwan's national health insurance research database: past and future. Clin. Epidemiol. 2019;11:349–358. doi: 10.2147/clep.s196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan national health insurance research database. JAMA Intern. Med. 2015;175:1527–1529. doi: 10.1001/jamainternmed.2015.3540. [DOI] [PubMed] [Google Scholar]
- 11.Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the national health insurance research database (NHIRD) Epidemiol. Health. 2018;40:e2018062. doi: 10.4178/epih.e2018062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SH, Wang J, Lin YS, Tung TH, Chi CC. Increased risk for incident thyroid diseases in people with psoriatic disease: a cohort study. J. Am. Acad. Dermatol. 2018 doi: 10.1016/j.jaad.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 13.Chi CC, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135:415–422. doi: 10.1001/jamaophthalmol.2017.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang SH, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA. 2017;318:1250–1259. doi: 10.1001/jama.2017.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romley JA, et al. Association between use of warfarin with common sulfonylureas and serious hypoglycemic events: retrospective cohort analysis. BMJ. 2015;351:h6223. doi: 10.1136/bmj.h6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun H, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease-modifying therapy. Arthritis Care Res. (Hoboken) 2015;67:731–736. doi: 10.1002/acr.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon WG, et al. EULAR points to consider when establishing, analysing and reporting safety data of biologics registers in rheumatology. Ann. Rheum. Dis. 2010;69:1596–1602. doi: 10.1136/ard.2009.125526. [DOI] [PubMed] [Google Scholar]
- 18.Wu MY, Hsu YH, Su CL, Lin YF, Lin HW. Risk of herpes zoster in CKD: a matched-cohort study based on administrative data. Am. J. Kidney Dis. 2012;60:548–552. doi: 10.1053/j.ajkd.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Hu SC, et al. Immunosuppressive medication use and risk of herpes zoster (HZ) in patients with systemic lupus erythematosus (SLE): a nationwide case-control study. J. Am. Acad. Dermatol. 2016;75:49–58. doi: 10.1016/j.jaad.2015.12.059. [DOI] [PubMed] [Google Scholar]
- 20.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 21.Pergam SA, Limaye AP. Varicella zoster virus in solid organ transplantation: guidelines from the american society of transplantation infectious diseases community of practice. Clin. Transplant. 2019 doi: 10.1111/ctr.13622. [DOI] [PubMed] [Google Scholar]
- 22.Dadwal SS. Herpes virus infections other than cytomegalovirus in the recipients of hematopoietic stem cell transplantation. Infect. Dis. Clin. N. Am. 2019;33:467–484. doi: 10.1016/j.idc.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Jansen K, et al. Incidence and risk factors of herpes zoster among hiv-positive patients in the german competence network for HIV/AIDS (KompNet): a cohort study analysis. BMC Infect. Dis. 2013;13:372. doi: 10.1186/1471-2334-13-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang K, et al. Increased risk of herpes zoster infection in patients with inflammatory bowel diseases in Korea. Clin. Gastroenterol. Hepatol. 2018;16:1928–1936.e1922. doi: 10.1016/j.cgh.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the Medical Board of the National Psoriasis Foundation. J. Am. Acad. Dermatol. 2019;81:102–110. doi: 10.1016/j.jaad.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Schroder C, Enders D, Schink T, Riedel O. Incidence of herpes zoster amongst adults varies by severity of immunosuppression. J. Infect. 2017;75:207–215. doi: 10.1016/j.jinf.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin. Proc. 2017;92:1806–1821. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Marra F, Lo E, Kalashnikov V, Richardson K. Risk of herpes zoster in individuals on biologics, disease-modifying antirheumatic drugs, and/or corticosteroids for autoimmune diseases: a systematic review and meta-analysis. Open Forum Infect. Dis. 2016;3:ofw205. doi: 10.1093/ofid/ofw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreiher J, Kresch FS, Comaneshter D, Cohen AD. Risk of Herpes zoster in patients with psoriasis treated with biologic drugs. J. Eur. Acad. Dermatol. Venereol. 2012;26:1127–1132. doi: 10.1111/j.1468-3083.2011.04230.x. [DOI] [PubMed] [Google Scholar]
- 30.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 31.Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 2011;11:143–154. doi: 10.1038/nri2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen SB, et al. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene- like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 2009;90:74–78. doi: 10.1099/vir.0.005389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.High KP, et al. Low plasma concentrations of retinol and alpha-tocopherol in hematopoietic stem cell transplant recipients: the effect of mucositis and the risk of infection. Am. J. Clin. Nutr. 2002;76:1358–1366. doi: 10.1093/ajcn/76.6.1358. [DOI] [PubMed] [Google Scholar]
- 34.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat. Rev. Immunol. 2011;11:584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 35.Konijeti GG, et al. Vitamin D supplementation modulates T cell-mediated immunity in humans: results from a randomized control trial. J. Clin. Endocrinol. Metab. 2016;101:533–538. doi: 10.1210/jc.2015-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao CT, Lai CF, Huang JW. Risk factors for herpes zoster reactivation in maintenance hemodialysis patients. Eur. J. Intern. Med. 2012;23:711–715. doi: 10.1016/j.ejim.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Chao CT, et al. Serum vitamin D levels are positively associated with varicella zoster immunity in chronic dialysis patients. Sci. Rep. 2014;4:7371. doi: 10.1038/srep07371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahavihu K, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br. J. Dermatol. 2010;163:321–328. doi: 10.1111/j.1365-2133.2010.09767.x. [DOI] [PubMed] [Google Scholar]
- 39.Antoniou T, et al. Statins and the risk of herpes zoster: a population-based cohort study. Clin. Infect. Dis. 2014;58:350–356. doi: 10.1093/cid/cit745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews A, et al. Statin use and the risk of herpes zoster: a nested case-control study using primary care data from the U.K. clinical research practice datalink. Br. J. Dermatol. 2016;175:1183–1194. doi: 10.1111/bjd.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim MC, et al. Statins increase the risk of herpes zoster: a propensity score-matched analysis. PLoS ONE. 2018;13:e0198263. doi: 10.1371/journal.pone.0198263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan L, Wang Y, Liu X, Guan X. Association between statin use and herpes zoster: systematic review and meta-analysis. BMJ Open. 2019;9:e022897. doi: 10.1136/bmjopen-2018-022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan SW, et al. Opposite effects of statins on the risk of tuberculosis and herpes zoster in patients with diabetes: a population-based cohort study. Br. J. Clin. Pharmacol. 2020;86:569–579. doi: 10.1111/bcp.14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang YT, et al. Epidemiological study of psoriasis in the national health insurance database in Taiwan. Acta Derm. Venereol. 2009;89:262–266. doi: 10.2340/00015555-0642. [DOI] [PubMed] [Google Scholar]
- 45.Chen YJ, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J. Am. Acad. Dermatol. 2011;65:84–91. doi: 10.1016/j.jaad.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 46.Dai YX, et al. The risk of mortality among psoriatic patients with varying severity: a nationwide population-based cohort study in Taiwan. Int. J. Environ. Res. Public Health. 2018 doi: 10.3390/ijerph15122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).