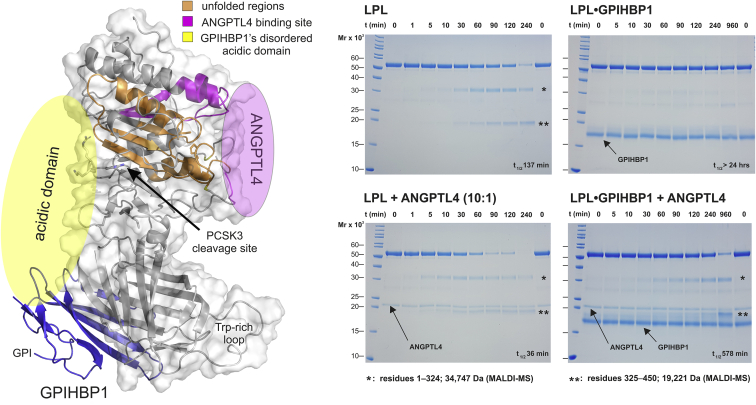
The lipolytic processing of triglyceride-rich lipoproteins within capillaries is mediated by LPL complexed to GPIHBP1, a protein of capillary endothelial cells (1). Angiopoietin-like protein 4 (ANGPTL4) plays an important role in regulating LPL activity, particularly in adipose tissue. Hydrogen-deuterium exchange experiments show that ANGPTL4 binds to sequences adjacent to the catalytic pocket of LPLs. This binding triggers progressive and irreversible unfolding of LPLs catalytic domain (2, 3). Importantly, ANGPTL4 catalyzes this unfolding. Spontaneous unfolding of LPL also occurs in the absence of ANGPTL4, but the kinetics of unfolding is much slower. The binding of GPIHBP1 protects LPL from unfolding. Shown on the left is a cartoon representation of the LPL•GPIHBP1 structure; in purple are the binding regions for ANGPTL4 on LPL (3), in yellow regions where the acidic domain of GPIHBP1s may interact, and in orange the LPL sequences that undergo ANGPTL4-mediated unfolding (3).
In studies of LPL secretion from adipocytes, Dijk et al. (4) observed that ANGPTL4 expression increases PCSK3-mediated cleavage of LPL. The mechanism by which ANGPTL4 promotes PCSK3 cleavage of LPL is unknown. We hypothesized that ANGPTL4-driven LPL unfolding changes the conformation of LPL and increases its susceptibility to PCSK3-mediated cleavage. If this were the case, we predicted that substoichiometric amounts of ANGPTL4 would accelerate the cleavage of LPL, whereas GPIHBP1 would decelerate that cleavage. To test our hypothesis, we incubated purified bovine LPL with PCSK3, alone or in the presence of ANGPTL4 and/or GPIHBP1 at 25°C. The time to reach 50% LPL cleavage (t½) is shown for each condition. Free LPL was cleaved by PCSK3 into its N-terminal (∗) and C-terminal (∗∗) fragments. Substoichiometric amounts of ANGPTL4 greatly accelerated LPL cleavage. GPIHBP1 strongly protected LPL from PCSK3 cleavage, both in the presence and absence of ANGPTL4. Based on these experiments, we propose that ANGPTL4-catalyzed LPL unfolding explains the susceptibility of LPL to PCSK3 cleavage.
REAGENTS: Purified bovine LPL, human ANGPTL41-159, and human GPIHBP11-131 were produced and purified as described (2). A truncated variant of human PSCK3/furin produced by Sf9 cells (>2,000 units/ml) was purchased from Sigma-Aldrich (catalog no. F2677). In these experiments, we incubated purified bovine LPL (20 μM) with PCSK3 (four units), alone or in the presence of ANGPTL4 (2 μM) and/or GPIHBP1 (25 μM) at 25°C in 10 mM MES, 150 mM NaCl, 1 mM CaCl2, and pH 7.0. The cleavage of LPL was monitored over time by SDS-polyacrylamide gels and was quantified by densitometric scanning.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This work was supported by the Lundbeck Foundation (R230-2016-2930), NOVO Nordisk Foundation Grants (NNF17OC0026868, NNF18OC0033864, NNF20OC0063444), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 801481, and the National Heart, Lung, and Blood Institute (HL146358, HL087228, HL139725).
Author contributions
A.-M. L. W. investigation; A. K. investigation and visualization; S. G. Y conceptualization, writing-reviewing and editing as well as funding acquisition; and M. P. conceptualization, writing-reviewing and editing, supervision, as well as funding acquisition.
References
- 1.Young S.G., Fong L.G., Beigneux A.P., Allan C.M., He C., Jiang H., Nakajima K., Meiyappan M., Birrane G., Ploug M. GPIHBP1 and lipoprotein lipase, partners in plasma triglyceride metabolism. Cell Metab. 2019;30:51–65. doi: 10.1016/j.cmet.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mysling S., Kristensen K.K., Larsson M., Kovrov O., Bensadouen A., Jørgensen T.J., Olivecrona G., Young S.G., Ploug M. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. Elife. 2016;5 doi: 10.7554/eLife.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leth-Espensen K.Z., Kristensen K.K., Kumari A., Winther A.L., Young S.G., Jørgensen T.J.D., Ploug M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc. Natl. Acad. Sci. U.S.A. 2021;118 doi: 10.1073/pnas.2026650118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dijk W., Ruppert P.M.M., Oost L.J., Kersten S. Angiopoietin-like 4 promotes the intracellular cleavage of lipoprotein lipase by PCSK3/furin in adipocytes. J. Biol. Chem. 2018;293:14134–14145. doi: 10.1074/jbc.RA118.002426. [DOI] [PMC free article] [PubMed] [Google Scholar]