Abstract
Diet is a powerful evolutionary force for species adaptation and diversification. Acari is one of the most abundant clades of Arachnida, exhibiting diverse dietary types, while the underlying genetic adaptive mechanisms are not fully understood. Based on comparative analyses of 15 Acari genomes, we found genetic bases for three specialized diets. Herbivores experienced stronger selection pressure than other groups; the olfactory genes and gene families involving metabolizing toxins showed strong adaptive signals. Genes and gene families related to anticoagulation, detoxification, and haemoglobin digestion were found to be under strong selection pressure or significantly expanded in the blood-feeding species. Lipid metabolism genes have a faster evolutionary rate and been subjected to greater selection pressures in fat-feeding species; one positively selected site in the fatty-acid amide hydrolases 2 gene was identified. Our research provides a new perspective for the evolution of Acari and offers potential target loci for novel pesticide development.
Subject terms: Genomics, Evolutionary genetics
Liu et al. present a comparative analysis of 15 genomes from mites and identify genetic signatures for diet specialisation. Different gene families and selective pressures were revealed for herbivorous, haematophagous and fat-feeding species.
Introduction
Diet is one of the most fundamental aspects of an animal’s biology and is a powerful evolutionary force for species adaptation and diversification1–3. Different diets have been acknowledged to generate various physiological, biochemical, and morphological adaptations4. With the development of genome sequencing, the genetic basis of dietary adaptation has been revealed gradually, providing new and valuable insight into evolutionary biology5–8.
The abundant species diversity of mites and ticks (Arachnida: Acari) has inspired acarologists and ecologists to understand their evolutionary processes and ecological characteristics for decades9. Approximately 55,000 species have been described10, and the total species number is estimated to be much larger9,10. It was reported that the diverse lifestyles in Acari (e.g., free-living, symbiotic, and parasitic) serve as an important driver of its species diversity11. The dietary lifestyles of Acari range from predatory to parasitic9. Compared with spiders with a predatory diet, Acari exhibit several new diets, such as herbivory and parasitism (e.g., blood and fat bodies), as well as some transitional states such as the consumption of skin exudates, decomposing biomass, and fungi9,12. These different specialized diets have generated a number of physiological, biochemical, and morphological adaptations4. For example, herbivorous mites have developed a long and stout stylet-like structure for feeding on plant juice13,14. A key mechanism of phenotypic adaptation is genetic adaptation, including changes in functional genes, metabolic and regulatory pathways, and even amino acids5,6,15. However, the genetic mechanisms underlying the dietary adaptation of Acari have not yet been systematically discussed in a comparative manner.
According to the phylogeny of Arachnida16,17, Acari consists of two major clades: Acariformes and Parasitiformes18. In Acariformes, some mites maintained predatory diets, while some obtained transitional, herbivorous (e.g., Tetranychus urticae) or blood sucking (e.g., Leptotrombidium deliense) diets19,20. In Parasitiformes, ticks (Ixodida) became obligate hematophages and some mites also developed parasitic diets, feeding on blood and fat bodies, respectively12,21. The distributions of different diets across the phylogeny of Acari provide an opportunity to understand the genetic mechanisms of dietary adaptation and evolution.
In the current study, we performed comparative analyses of genomes from fifteen Acari species in four major orders and with five kinds of diets. We constructed a well-resolved fossil-calibrated phylogenetic tree and revealed the genomic evolution of Acari. Furthermore, we investigated the genetic adaptation associated with three specialized diets, blood sucking, herbivory, and fat feeding, and found important sites and pathways that underwent adaptive convergent evolution. Our findings, with further experimental validation, can be used as potential targets for drug and pesticide research to control herbivorous and parasitic mites.
Results and discussion
A total of sixteen Arachnida genomes, including those of one tick, fourteen mites, and the velvet spider as an outgroup, were obtained from GenBank (details in “Methods”). The sixteen species displayed all five kinds of diets, including three predacious species, two herbivorous species, three fat-feeding species, three blood-feeding species, and five transitional species (Table 1). Six genomes were reannotated (marked in Table 1) using ab initio prediction and homology prediction methods together (details in “Methods”). To evaluate genome completeness, we examined the genome and genes by Benchmarking Universal Single-Copy Orthologs (BUSCO)22. We observed high BUSCO scores of genome completeness (average 90.9%) and gene completeness (average 79.9%) (Supplementary Table 1). This result suggested that the assemblies of the sixteen genomes were of high quality for downstream comparative analyses.
Table 1.
Genomes information used in this study.
| Latin name | Common name | GenBank AssemblyAccession | Total size of contigs | Contig N50 | Gene number | Diet | Taxonomy |
|---|---|---|---|---|---|---|---|
| Ixodes scapularis74 | Black-legged tick | GCF_002892825.2 | 2,081,329,876 | 835,681 | 24,489 | Blood sucking | Parasitiformes |
| Metaseiulus occidentalis75 | Predatory mite | GCF_000255335.1 | 151,323,873 | 200,706 | 11,603 | Predation | Parasitiformes |
| Dermanyssus gallinae76* | Roost mite | GCA_003439945.1 | 959,010,206 | 278,630 | 42,159 | Blood sucking | Parasitiformes |
| Tropilaelaps mercedesae77 | Honeybee mite | GCA_002081605.1 | 326,213,305 | 13,741 | 15,190 | Fat feeding | Parasitiformes |
| Varroa destructor78 | Honeybee mite | GCF_002443255.1 | 368,670,960 | 201,886 | 10,241 | Fat feeding | Parasitiformes |
| Varroa jacobsoni78 | Honeybee mite | GCF_002532875.1 | 365,177,116 | 96,030 | 10,724 | Fat feeding | Parasitiformes |
| Steganacarus magnus79* | Oribatid mite | GCA_000988885.1 | 112,750,608 | 1727 | 9990 | Transitional | Acariformes |
| Hypochthonius rufulus79* | Soil mite | GCA_000988845.1 | 171,814,378 | 3254 | 6285 | Transitional | Acariformes |
| Sarcoptes scabiei80 | Scabies mite | GCA_000828355.1 | 56,251,741 | 11,383 | 10,644 | Transitional | Acariformes |
| Dermatophagoides farinae81* | House dust mite | GCA_002085665.1 | 91,934,661 | 188,869 | 15,394 | Transitional | Acariformes |
| Psoroptes ovis82* | sheep scab mite | GCA_002943765.1 | 63,214,126 | 2,279,290 | 12,041 | Transitional | Acariformes |
| Leptotrombidium deliense83 | Chigger | GCA_003675905.2 | 117,276,895 | 1422 | 15,096 | Blood sucking | Acariformes |
| Dinothrombium tinctorium83 | Velvet mites | GCA_003675995.1 | 180,156,552 | 16,116 | 19,258 | Predation | Acariformes |
| Brevipalpus yothersi84* | Flat mite | GCA_003956705.1 | 70,567,388 | 56,520 | 8,245 | Herbivory | Acariformes |
| Tetranychus urticae33 | Spider mite | GCF_000239435.1 | 89,613,205 | 212,780 | 18,414 | Herbivory | Acariformes |
| Stegodyphus mimosarum85 | Velvet spider | GCA_000611955.2 | 2,694,371,924 | 46,340 | 27,235 | Predation | Araneae |
Star-tagged species indicate that their genomes have been reannotated
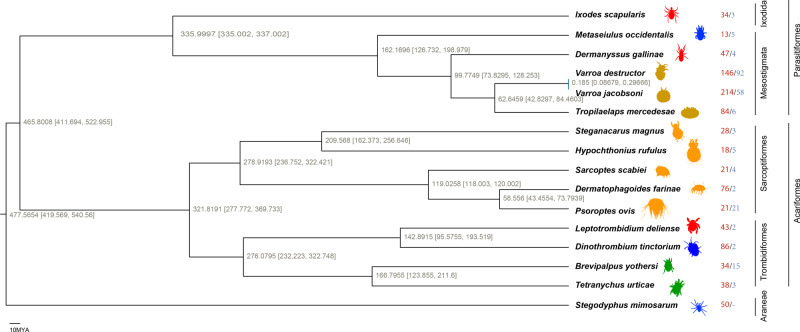
To reveal the genomic signatures of dietary adaptations in Acari, we constructed a genome-wide phylogenetic tree based on 48,831 nucleotides. Based on three fossil calibration points and a relaxed molecular clock, the divergence time between Acari and spiders was estimated to be ~477.6 million years ago (Mya) (Fig. 1). Moreover, the divergence between Sarcoptiformes and Trombidiformes was estimated to be ~321.8 Mya, and the divergence between Ixodida and Mesostigmata occurred ~336 Mya (Fig. 1). The phylogenetic tree and time estimates of key nodes were consistent with those in recent studies16,23–25.
Fig. 1. The genome-wide phylogenetic tree of Arachnida.
The divergence time was estimated based on a maximum likelihood phylogenomic tree of 16 Arachnida species and three fossil calibrations. All nodes have 100% support according to 1,000 bootstraps. The estimated divergence times are displayed with 95% confidence intervals (in square brackets). Blood-sucking species in red; fat-feeding species in olive; herbivorous species in green; predatory species in blue; and others/transitional in orange. The same colour theme is used in the other figures. The numbers to the right of the species indicate the records of gene family expansions and contractions, with red for expansions and blue for contractions.
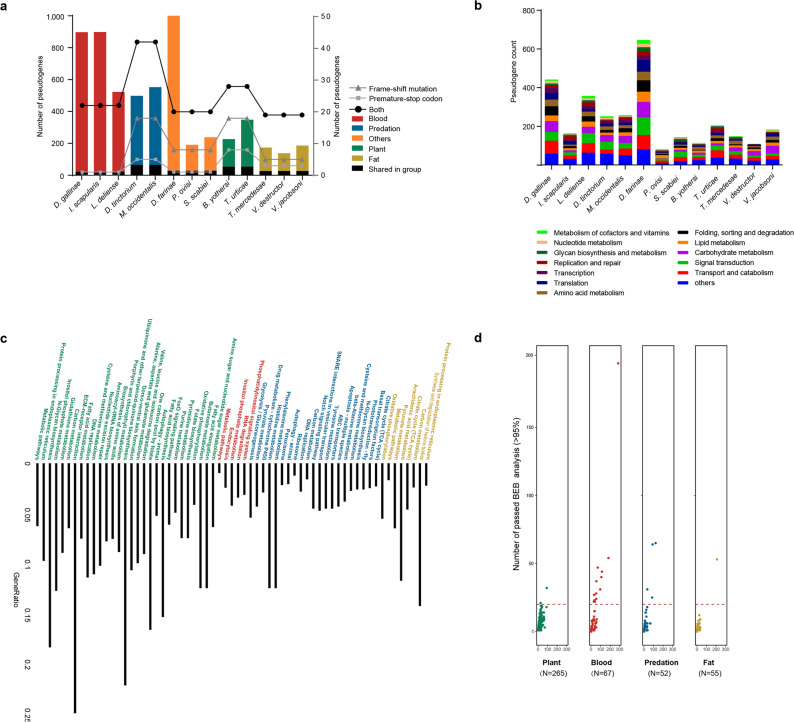
Relaxation of selective constraints on and loss of function of protein-coding genes may occur during dietary shifts6,26. A total of 1,210 pseudogenes were identified across all species (Fig. 2a, Supplementary Data 1). Blood-feeding species with larger genomes had a higher ratio of pseudogenes (Fig. 2a), supporting the idea that pseudogenes act as a determinant of genome size evolution27. The functions of these pseudogenes, most of which were involved in signal transduction, transport, catabolism and so on, were similar across species (Fig. 2b). A total of 65, 23, 54 and 27, and 29 pseudogenes were detected to be common in the groups fed the five diets, predation, blood, plant, fat, and others (transitional type), respectively (Fig. 2a, Supplementary Data 2). Among these pseudogenes, the genes for ribonuclease H were pseudogenized in both the blood-sucking group and fat-feeding group (Supplementary Data 2), which may be related to their parasitic lifestyles. Interestingly, no pseudogenes were shared across all dietary groups, implying that different patterns occurred during the dietary shift (Supplementary Data 2). Since the pseudogenes are nonfunctional, subsequent analyses based on functional genes were carried out after removing the pseudogenes.
Fig. 2. Pseudogene and PSG profiles of different dietary groups.
a The overall numbers of pseudogenes detected in each group are shown in different colours reference to left y-axis, and the numbers of shared pseudogenes in each group are shown in black. The pseudogenes in each group are divided into three types, namely, frame-shift mutation, premature-stop codon, and both, and the ratios of types are displayed by lines reference to right y-axis. b The functional classification of the pseudogenes in each group. c KEGG enrichment analysis of the PSGs. d PSGs generated by the orthologous genes annotated in at least two species in each dietary group. The number in parentheses represents the number of PSGs in the group. The dot plot shows the numbers of positively selected sites across four different dietary groups. The x-axis indicates the number of loci detected as being under selection pressure, and the y-axis indicates the number of positively selected loci with a BEB posterior probability greater than 95% in PAML. PSG positively selected gene, KEGG Kyoto Encyclopedia of Genes and Genomes, BEB Bayesian empirical Bayes.
To detect signals of positive selection in Acari with specialized diets, we performed phylogenetic analysis by maximum likelihood (PAML) branch-site model (model = 2, and NSsites = 2) tests for the single-copy orthologous genes. As a result, 530 positively selected genes (PSGs) were identified, and 291, 56, 73, and 110 PSGs were detected for the plant, predation, blood, and fat diets, respectively (Supplementary Data 2). Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis showed that the PSGs were partly correlated with the ability to digest different food types, such as Porphyrin and chlorophyll metabolism in the plant dietary group and Arachidonic acid metabolism in the fat dietary group (Fig. 2c, Supplementary Data 2). Gene ontology (GO) enrichment analysis also revealed biological processes correlated with different diets, such as organonitrogen metabolism in the herbivorous group (Supplementary Fig. 1 and Supplementary Data 2). To compare the overall pressure of natural selection on different food preferences, a total of 439 PSGs in four dietary groups (at least two species for each group) were selected to conduct cross-sectional analysis (Supplementary Data 2). Herbivorous mites were subjected to the strongest natural selection, containing 265 of the 439 PSGs (Fig. 2d). The number of PSGs under intense selection (PSGs with more than 20 positively selected sites) was much larger in the blood-sucking group than in the other groups (Fig. 2d), suggesting that blood-sucking mites were subjected to stronger selection pressure on specific genes during dietary shifts. Some of these intensely selected genes are related to blood digestion, such as carboxylesterase and serine/threonine-protein phosphatase (Supplementary Data 2).
Gene family expansion and contraction are regarded as another key source of adaptive function28,29. Hence, we conducted a protein family (Pfam) domain-based comparative analysis of the sixteen species. Expansion or contraction of a gene family in at least two species in each dietary group was considered a signal of dietary adaptation (details in “Methods”). Fat-feeding mites had more expanded gene families than other dietary groups (Fig. 1); however, no common expanded gene family was found in this group. On the other hand, several gene families related to detoxification and blood coagulation inhibition were expanded in the herbivorous and blooding-feeding mites, respectively (Supplementary Table 2). The detailed genetic basis for each dietary group is presented in each of the following sections.
Adaption to plant-feeding
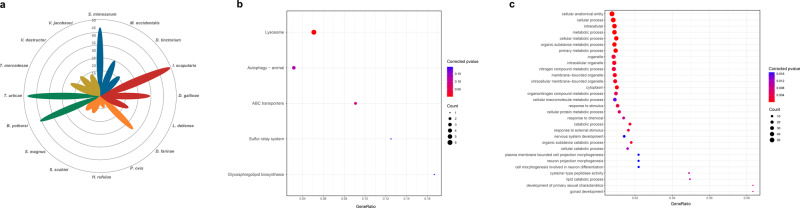
The capability to detoxify plant allelochemicals is considered a key factor driving radiation in herbivorous arthropods9,30. In the Acari, Tetranychidae (spider mites, e.g., T. urticae) and Tenuipalpidae (false spider mites, e.g., Brevipalpus yothersi) are exclusively phytophagous and include major agricultural pests31. T. urticae is also known for its ability to develop rapid resistance to pesticides32. Therefore, we focused on the adaptive traits with a detoxification function in the two herbivorous mites. Consistent with previous reports in several plant-feeding species33, we observed significant expansion of Cyp18a1, a cytochrome P450 enzyme, at the root of the herbivorous clade (OG0000061, P < 0.01) (Fig. 3a, Supplementary Table 2). To confirm this signal, we rescanned all Cyp18a1 genes from our sixteen genomes based on the amino acid sequences of the P450 family and performed manual filtering. The copy number of Cyp18a1 in herbivorous mites, especially in T. urticae, was twice as high as that in other mites (Supplementary Data 2). We further collected sixteen RNAseq datasets from four populations of T. urticae from GenBank to check whether detoxification-related genes had increased expression (details in “Methods”). As expected, nearly half of the 50 most highly expressed genes were involved in cellular transport and ion binding, which are essential for toxin metabolism34,35 (Supplementary Fig. 2). Additionally, KEGG pathway enrichment analysis of the 200 most highly expressed genes (Supplementary Data 3) revealed significant enrichment in ATP-binding cassette (ABC) transporters, which are major detoxification families, including the Carboxyl/Cholinesterase (CCE), Cytochrome P450, and Glutathione-S-Transferase (GST) families36–38 (Fig. 3b; Supplementary Data 2). The GO item “response to external stimulus”, which was enriched in KEGG pathway enrichment analysis, is related to stress responses and handling toxin stimuli (Fig. 3c; Supplementary Data 2). In summary, gene family expansion, as well as gene expression analyses, suggest that herbivorous mites are prominent in their detoxification ability, which may largely contribute to their tolerance to plant allelochemicals and insensitivity to chemical insecticides39.
Fig. 3. Adaptation of herbivorous mites.
a Gene counts related to the P450 gene family of sixteen species. b KEGG enrichment of the 200 most highly expressed genes in herbivorous mites. c GO enrichment of the 200 most highly expressed genes in herbivorous mites. KEGG Kyoto Encyclopedia of Genes and Genomes, GO Gene Ontology.
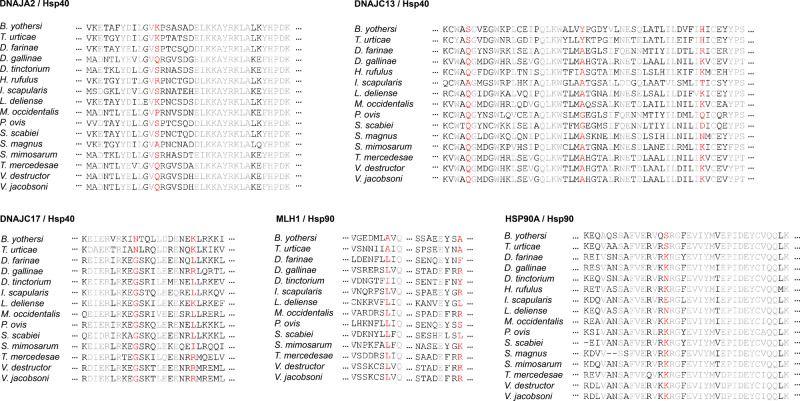
Herbivorous mites are known to use odours to select fresh food, locate mates, avoid interspecific competitors and escape predators40,41. Among the PSGs found in the herbivorous mites, three genes of the type III heat-shock protein-40 family (Hsp40), namely, DNAJA2, DNAJC13, and DNAJC17, and two genes of Hsp90, namely, MLH1 and HSP90A, displayed the same amino acid changes (Fig. 4). The expression of Hsp25 and Hsp70 was found to increase when exposed to environmental odourants, such as food odour42,43. Hsp40 and Hsp90 are often coexpressed with Hsp70, playing a major chaperone role42,44,45. Changes in amino acids in Hsp40 and Hsp90 may adjust the expression and/or function of these proteins and make the olfactory response system more conductive. In addition, the expanded P450 superfamily found in the gene family analysis is also involved in the degradation of odour molecules in addition to detoxification46. Sensitive olfaction and strong detoxification abilities may provide a strong genetic basis for herbivorous mite feeding on angiosperms.
Fig. 4. The positively selected sites in HSP40 and HSP90 genes.
Red indicates convergence sites.
Adaptations to blood-sucking
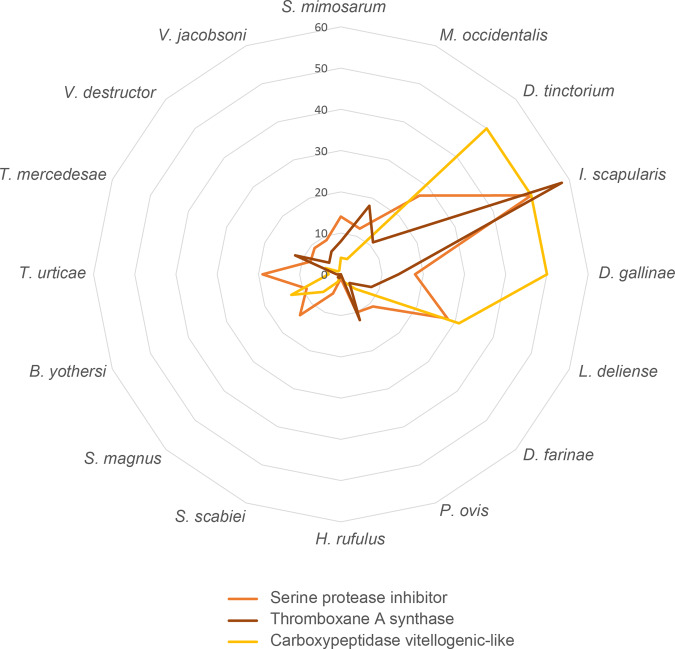
Blood feeding has evolved several times in Acari (Fig. 1). Adaptive phenotypic convergence has been found in blood-feeding Acari, such as stylet-shaped piercing proboscides and teeth-like mouthparts47. Our genomic convergence analysis revealed that nine gene families have undergone significant expansion, including serine protease inhibitor (serpin family member), thromboxane A (TxA) synthase, and carboxypeptidase vitellogenic-like (CPVL) (Fig. 5; Supplementary Table 2). The serpin family directly inhibits blood coagulation factor XIa or enhances the inhibition of blood coagulation factor Xa via Protein Z48, which is highly relevant to the blood-sucking process. To quantify the number of genes in the serpin family in each species, we rescanned all serpin family genes from the sixteen genomes based on the amino acid sequences of the serpin domain and performed manual filtering. As expected, blood-sucking species had more serpin family genes than the other species (Fig. 5; Supplementary Data 2), and expansion was observed for all serpin family genes (Supplementary Fig. 3). TxA is an enzyme producing thromboxane A2 (TXA2), which can cause vessel constriction and platelet activation and aggregation49. In addition, TxA can combine cytokines and inflammatory mediators to activate the coagulation cascade50. The seemingly antagonistic functions of the serpin family (anticoagulant) and TxA (coagulation) may thus provide a fine control system for blood-feeding diets.
Fig. 5. Gene family expansion in blood-sucking mites.
Gene counts related to three haematophagous traits of sixteen species.
Other important features in the blood-sucking process include haemoglobin digestion and detoxification of xenobiotic factors. The expanded CPVL gene family is regarded as a member of the serine carboxypeptidases, which are characterized as the main enzymes acting in blood digestion51–54. Among the PSGs, PAQR5 (OG0000879, P = 0.02) and CaMKII (OG0000260, P = 0.05), which are involved in blood pressure management, digestion, and absorption of vascular contents55–57, were found to be under strong positive selection (Supplementary Data 2). There was one gene, Carboxylesterase (CES2), with more than 200 sites under positive selection (Fig. 2d, Supplementary Data 2). CES2 is recognized as a highly conserved metabolic pathway involved in the metabolism of endogenous and exogenous compounds, which is the key to detoxification during blood feeding58,59. The high number of nucleotide sites under selection in CES2 probably modifies the activity of the coded enzyme, significantly increasing the detoxification abilities in these blood-feeding Acari60.
Adaption to fat-feeding
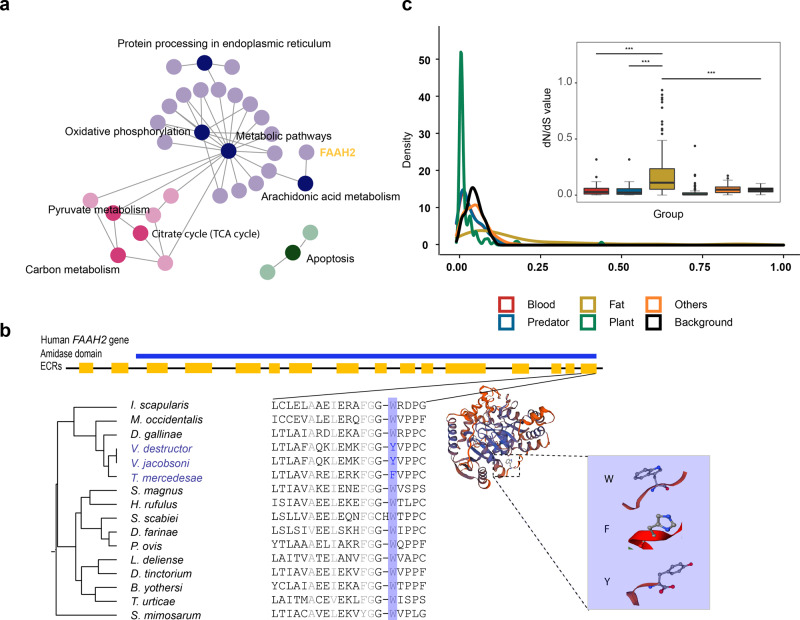
Another major dietary shift in Acari occurs in honeybee mites, which have attracted attention due to their damage to the beekeeping industry worldwide21. The identification of the genetic basis underlying fat-feeding habits could contribute to more effective measures for pest prevention, such as the development of new insecticides. KEGG enrichment analysis showed that the 110 PSGs found on the honeybee mite branch were significantly enriched in the Arachidonic acid metabolism pathway (dme00590, P = 0.036) (Fig. 6a, Supplementary Data 2). One positively selected site, W516F/Y, in the fatty-acid amide hydrolases 2 (FAAH2) gene was TTT(F) or TAT(Y) in fat-eating honeybee mites, different from the TGG(W) in other mites (Fig. 6b). The amino acid substitution in FAAH2 occurs in the amidase signature (AS) domain region (Fig. 6b) and may lead to a change in hydrolase activity to influence the ability of FAAH2 to generate free arachidonate acid (AA) from anandamide. AA is indispensable for the development of the nervous and immune systems, and anandamide is one kind of enriched lipid in honey bees61. Compared with the absence of FAAH2 in some mammals such as rats and low-fat-diet mice62, the change in W516F/Y may enhance the generation of AA from an anandamide-enriched diet. Further experimental validation is needed to confirm this relationship.
Fig. 6. PSG and dN/dS values of the lipid metabolism system.
a KEGG pathway enrichment of the PSGs in the fat-feeding dietary group reveals that FAAH2 is involved in arachidonic acid metabolism. b Structural domains of the FAAH2 protein and comparison of the positively selected amino acid substitutions among the genomes. The site is W516F/Y, and all groups except the fat-feeding group show strict conservation of W amino acids. The structural domain annotation was derived from the NCBI database. c Density of dN/dS values of the lipid metabolism gene set in five dietary groups and the background. A total of 122 genes related to the development of lipid metabolism were retrieved from the KEGG, and we calculated the dN/dS ratios on the branches connected to fat-feeding, blood-feeding, herbivorous, predatory, and other dietary groups with the two-ratio branch model (model 2) of PAML. Background dN/dS ratios were evaluated with a one-ratio branch model (model 1) of PAML. The distribution density and a box plot of dN/dS values (dN/dS<1) in different dietary groups are displayed. The mean dN/dS value on the branch of the fat-feeding group was significantly higher than that of the background and other groups (***P < 0.01). Error bars represent standard deviation (SD). KEGG Kyoto Encyclopedia of Genes and Genomes, PSG positively selected gene, PAML phylogenetic analysis by maximum likelihood.
To detect the evolutionary pressure on the lipid metabolism system, we retrieved 122 genes related to fat metabolism from the KEGG database and calculated the ratios between the nonsynonymous substitution rates (dN) and synonymous substitution rates (dS) of genes on branches with different diets. Significantly higher dN/dS ratios were observed for the fat-feeding branches (Student’s t test, P < 0.01) (Fig. 6c, Supplementary Data 2), which indicates that lipid metabolism genes have evolved at a faster rate and been subjected to greater selection pressures in lipophilic species.
Instead of gene family expansion, we found that three common gene families contracted in fat-feeding mites, including TxA synthase, Serine carboxypeptidase, and Serine protease inhibitor, which were found to be expanded in blood-sucking mites (Supplementary Table 2). This phenomenon suggests a significant difference in dietary adaptation between honeybee mites and blood-feeding mites and provides genetic evidence for the controversy over whether honeybee mites eat blood or lipids12.
Conclusion
In the current research, we carried out comparative genomic analyses of sixteen Arachnida species with five different dietary types to explore their genetic evolution and adaptation. We found different genetic bases underlying different diets, mainly related to the need to handle different food types, including increased abilities to find (olfaction), prepare (detoxification) and digest (metabolism) food. Several candidate genes, with further experimental validation, could be used as target loci for novel pesticide development, especially for controlling herbivorous mites and honeybee mites. Future studies on the dietary evolution of the Acari will be important for improving pest control and elucidating host-parasite coevolution.
Methods
Genome generation and gene annotation
All the Acari genomes analyzed in our study are available as public resources from the NCBI (ftp://ftp.ncbi.nlm.nih.gov/). The steps for inclusion were as follows: (1) All Arachnida species in the database (before 2020.06) were searched as candidates; (2) the genome with the best completeness score was selected as the representative if there were two or more genomes for one species; (3) species with genome completeness less than 80% were eliminated; (4) gene prediction was conducted for genomes lacking gene annotation information; and (5) contigs less than 1 kb in length were excluded from the whole analysis. The repetitive elements in the genome sequence were identified by RepeatMasker63 (version 4.0.7) with Repbase (version 16.02). For gene prediction, the BRAKER pipeline64 (version 1.9) was used, and the protein sequences of the velvet spider, scabies mite, honeybee mite, chigger, velvet mite, spider mite, predatory mite, dust mite, and tick genome were applied as references for homology-based gene prediction. A link to the annotated results is provided in the Data Availability section.
Pseudogene detection
To reduce the heterogeneity of background data, we selected the three genomes with the best quality (those of D. farina, P. ovis, and S. scabiei) in the transitional group as representatives. GeneWise (version 2.4.1) was used for gene alignment, while the protein-coding sequences of a spider (Stegodyphus mimosarum) were used as references. The minimal sequence identity was set to 80%, and the cut-off E-value was set to 10−5. The genes with frame-shift mutations (SNPs or indels) or premature-stop codons were annotated as pseudogenes.
Phylogenetic tree construction
Protein sequences with a short length (<50 amino acids) or premature-stop codons were excluded. Orthologous genes were inferred by OrthoFinder (version 2.3.8) with DIAMOND software (version 0.9.24). An expectation value was set to 10−5. Single-copy orthologous genes were generated from the OrthoFinder result, and conserved blocks of each gene were extracted by Gblocks (version 0.91b). Then, we combined all the conserved regions of every orthologous gene. A total of 50,502 sites were concatenated to build one supergene sequence for each species, which was adopted to construct the phylogenetic tree. The supergene phylogeny was constructed with RAxML65 (version 8.2.12) under the GTRGAMMA model. A total of 200 bootstrap replicates were conducted, and the tree with the highest likelihood score was picked as a standard tree. Based on the topology of the standard tree, the branch length of each gene was estimated with its sequence. Then, genes with extremely biased branch lengths were excluded. After removing all biased genes, the species tree was reconstructed based on 48,831 conserved sites by RAxML with 1,000 bootstrap replicates following the command raxmlHPC-PTHREADS-SSE3 -x 12345 -p 12345 -# 1000 -m GTRGAMMA.
Divergence time estimation
MCMCTREE in PAML66 (version 4.9i) was used to calibrate divergence time via the Markov chain Monte Carlo method. Fossil time (Steganacarus magnus versus Hypochthonius rufulus: 394 - 571 Mya; Dermatophagoides pteronyssinus versus Sarcoptes scabiei: 119 Mya; and Ixodes scapularis versus Dermanyssus gallinae: 336 Mya) was determined from the Time Tree database (http://www.timetree.org). A relaxed-clock model (clock = 2) was established, and MCMC was performed (burnin = 4,000,000; sampfreq = 100; nsample = 100,000). Other parameters were set to the default, and the Baseml program was run in duplicate to check for convergence.
Gene family analysis
The protein sequences of sixteen Arachnida genomes were generated from the annotated results. We removed the proteins with premature-stop codons or a length less than 50 amino acids. The remaining proteins were used to perform protein clustering by OrthoFinder with the all-to-all DIAMOND method and an E-value = 10−5. A dataset of 39,474 clusters was generated, and some of the clustered were removed due to a low annotation ratio of less than 50 percent of all species. Then 5,718 remaining gene families were subjected to expansion and contraction analysis using CAFÉ67 (version 4.2.1), and a stochastic birth-and-death model was adopted. The phylogenetic tree with divergence time was obtained with MCMCTREE analysis in PAML.
Identification of positively selected genes
A total of 9,306 gene families were identified by OrthoFinder, and 4,546 single-copy orthologous genes were generated to perform positive selection analysis, which annotated at least 60% of the sixteen genomes. The sequences of orthologous genes were aligned by MAFFT software (version 7.455), and a gene tree was generated from the species tree based on the remaining species. The branch-site model in PAML was used to detect PSGs along specific lineages with model A (model = 2 and NSsites = 2). A likelihood ratio test (LRT) was performed to compare model A (sites under positive selection in the foreground; fix_omega = 0) with the null model (sites might evolve neutrally/under purifying selection; fix_omega = 1 and omega = 1) by the codeml program in PAML. The P values from the LRTs were evaluated via chi-square statistics, a false discovery rate (FDR) was calculated, and the cut-off was set to 0.1. Sites with a Bayesian empirical Bayes (BEB) posterior probability greater than 95% were selected as positively selected sites in PAML. KEGG and GO enrichment analyses of PSGs were performed by KOBAS 3.068 with cut-offs of a P value < 0.05 in Fisher’s exact tests and an FDR-corrected P value < 0.1. Drosophila melanogaster genes were selected as the background set. Bubble diagrams of significantly enriched KEGG and GO terms were generated using the R ggplot package.
Evolutionary rate of fat metabolism
To investigate the evolutionary rate of fat metabolism among mites with different diets, 122 genes involved in lipid metabolism from the KEGG database (http://www.genome.jp/kegg/catalog/org_list.html) were included for analysis. The branches were divided into five groups with different dietary types. The dN/dS ratios of selected groups were calculated by the codeml program with the two-ratio branch model (model = 2) in PAML, and the dN/dS values of the background were detected with the one-ratio model (model = 0, NSsites = 0). The density distribution of dN/dS values and a violin plot of dN/dS values for different dietary groups were generated. The dN/dS ratios were compared with that of background data with Student’s t test.
Gene expression analysis
Sixteen samples from four populations of spider mites were downloaded from the SRA database (BioProject: PRJNA610897)69, and Trimmomatic (version 0.36) was applied to perform quality control, including cutting adaptor sequences and removing low-quality reads, with the parameters “LEADING:3 TRAILING:3 SLIDINGWINDOW:4:20 MINLEN:45”. The qualified reads were mapped to the spider mite reference using HISAT2 (version 2.1.0). Fragments per kilobase of transcript per million mapped reads (FPKM) values were calculated by Cufflinks70 (version 2.2.1). Then, gene expression in all sixteen samples was sorted according to the median FPKM values. The 200 and 50 most highly expressed genes were selected and analyzed. A heatmap was drawn based on the 50 most highly expressed genes using Heatmapper71. KEGG and GO enrichment analyses were performed by KOBAS 3.0 with cut-offs of a P value < 0.05 in Fisher’s exact tests and an FDR-corrected P value < 0.1. Bubble diagrams of significantly enriched KEGG and GO terms were generated using the R ggplot package.
Serpin family analysis
The serpin family was manually annotated using methods described in previous studies72. The serpin protein sequences of Ixodes scapularis, Metaseiulus occidentalis, Dermanyssus gallinae, Tropilaelaps mercedesae, Varroa destructor, Varroa jacobsoni, Sarcoptes scabiei, Psoroptes ovis, Leptotrombidium delicense, Dinothrombium tinctorium, Tetranychus urticae, and Stegodyphus mimosarum from the NCBI database were used as query sequences. Then, the reference sequences were aligned to the sixteen genomes using BLASTP (version 2.2.29+). Blast hits belonging to the same query protein were combined into one predicted gene from each genome. Then, gene structure was predicted by GeneWise, and domains of sequences were estimated based on the Pfam database. Finally, a total of 2,748 positions in the serpin genes of sixteen species were aligned with ClustalW and then applied to construct a maximum likelihood tree in MEGA73 (version 10.0.1) with 1,000 bootstrap replicates. The resulting tree was visualized in Figtree (version 1.4.3).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31601839). We thank Chunqiao Liu, Yu Liu and Bin Zou for their help during data analysis, and Dr. Shenghan Gao for his advice in the earlier version of the manuscript. We also thank editors and three anonymous reviewers whose feedback helped to improve the manuscript substantially.
Author contributions
L.W. and D.C. conceived the study; Q.L. supervised the overall data collection and analysis; Y.H.D. and A.S. supervised and performed the comparative genomics pipeline; Y.F.X. provides key discussion; L.Q. and D.C. drafted and revised the manuscript. All authors read and approved the final version of the manuscript.
Data availability
The final dataset is available at Mendeley Data (https://data.mendeley.com/datasets/xm23f9mkdx/1).
Code availability
Analysis code is accessible through github (https://github.com/offlinecat/mite_diet).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
De Chen, Email: chende@bnu.edu.cn.
Lai Wei, Email: weil9@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02173-3.
References
- 1.McKenna DD, et al. The evolution and genomic basis of beetle diversity. Proc. Natl Acad. Sci. USA. 2019;116:24729–24737. doi: 10.1073/pnas.1909655116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roman-Palacios C, Scholl JP, Wiens JJ. Evolution of diet across the animal tree of life. Evol. Lett. 2019;3:339–347. doi: 10.1002/evl3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burin G, Kissling WD, Guimaraes PR, Jr., Sekercioglu CH, Quental TB. Omnivory in birds is a macroevolutionary sink. Nat. Commun. 2016;7:11250. doi: 10.1038/ncomms11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enrico de Lillo ADP, Giorgio Nuzzaci. Morphological adaptations of mite chelicerae to different trophic activities (Acari) Entomologica. 2001;35:125–180. [Google Scholar]
- 5.Kim S, et al. Comparison of carnivore, omnivore, and herbivore mammalian genomes with a new leopard assembly. Genome Biol. 2016;17:211. doi: 10.1186/s13059-016-1071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, et al. Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc. Natl Acad. Sci. USA. 2017;114:1081–1086. doi: 10.1073/pnas.1613870114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallmark B, et al. Genomic evidence of local adaptation to climate and diet in indigenous Siberians. Mol. Biol. Evol. 2019;36:315–327. doi: 10.1093/molbev/msy211. [DOI] [PubMed] [Google Scholar]
- 8.Axelsson E, et al. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495:360–364. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- 9.Walter, D. E. & Proctor, H. Mites: Ecology, Evolution & Behaviour: Life at a Microscale 2nd edn, 172–184 (Springer, 2013).
- 10.Zhang Z. Animal biodiversity: an introduction to higher-level classification and taxonomic richness. Zootaxa. 2011;3148:7–12. doi: 10.11646/zootaxa.3148.1.3. [DOI] [PubMed] [Google Scholar]
- 11.Skoracka A, Magalhaes S, Rector BG, Kuczynski L. Cryptic speciation in the Acari: a function of species lifestyles or our ability to separate species? Exp. Appl Acarol. 2015;67:165–182. doi: 10.1007/s10493-015-9954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsey SD, et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl Acad. Sci. USA. 2019;116:1792–1801. doi: 10.1073/pnas.1818371116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Palma A, Nuzzaci Giorgio, Alberti Gerd. Morphological, ultrastructural and functional adaptations of the mouthparts in cheyletid mites (Acari: Actinedida: Cheyletidae) Int. J. Acarol. 2009;35.6:521–532. doi: 10.1080/01647950903468265. [DOI] [Google Scholar]
- 14.Krantz, G. W. & Walter, D. E. A Manual of Acarology. 3rd edn, 1–807 (Tech University Press, 2009).
- 15.Pan S, et al. Convergent genomic signatures of flight loss in birds suggest a switch of main fuel. Nat. Commun. 2019;10:2756. doi: 10.1038/s41467-019-10682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue XF, Dong Y, Deng W, Hong XY, Shao R. The phylogenetic position of eriophyoid mites (superfamily Eriophyoidea) in Acariformes inferred from the sequences of mitochondrial genomes and nuclear small subunit (18S) rRNA gene. Mol. Phylogenet Evol. 2017;109:271–282. doi: 10.1016/j.ympev.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Ballesteros JA, Sharma PP. A critical appraisal of the placement of Xiphosura (Chelicerata) with account of known sources of phylogenetic error. Syst. Biol. 2019;68:896–917. doi: 10.1093/sysbio/syz011. [DOI] [PubMed] [Google Scholar]
- 18.Dunlop JA, Alberti G. The affinities of mites and ticks: a review. J. Zool. Syst. Evolut. Res. 2008;46.1:1–18. [Google Scholar]
- 19.Shih C-iT, Sidney LPoe, Harvey L. Cromroy. Biology, life table, and intrinsic rate of increase of Tetranychus urticae. Ann. Entomological Soc. Am. 1976;69.2:362–364. doi: 10.1093/aesa/69.2.362. [DOI] [Google Scholar]
- 20.Bronswijk VJA. Food preference of pyroglyphid house-dust mites (Acari) Neth. J. Zool. 1971;22:335–340. doi: 10.1163/002829672X00130. [DOI] [Google Scholar]
- 21.Sammataro D, Gerson U, Needham G. Parasitic mites of honey bees: life history, implications, and impact. Annu Rev. Entomol. 2000;45:519–548. doi: 10.1146/annurev.ento.45.1.519. [DOI] [PubMed] [Google Scholar]
- 22.Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YX, et al. Genomic insights into mite phylogeny, fitness, development, and reproduction. BMC Genomics. 2019;20:954. doi: 10.1186/s12864-019-6281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabert M, Witalinski W, Kazmierski A, Olszanowski Z, Dabert J. Molecular phylogeny of acariform mites (Acari, Arachnida): strong conflict between phylogenetic signal and long-branch attraction artifacts. Mol. Phylogenet Evol. 2010;56:222–241. doi: 10.1016/j.ympev.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Van Dam MH, Trautwein M, Spicer GS, Esposito L. Advancing mite phylogenomics: designing ultraconserved elements for Acari phylogeny. Mol. Ecol. Resour. 2019;19:465–475. doi: 10.1111/1755-0998.12962. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Thomas SD, Zhang J. Relaxation of selective constraint and loss of function in the evolution of human bitter taste receptor genes. Hum. Mol. Genet. 2004;13:2671–2678. doi: 10.1093/hmg/ddh289. [DOI] [PubMed] [Google Scholar]
- 27.Petrov DA, Sangster TA, Johnston JS, Hartl DL, Shaw KL. Evidence for DNA loss as a determinant of genome size. Science. 2000;287:1060–1062. doi: 10.1126/science.287.5455.1060. [DOI] [PubMed] [Google Scholar]
- 28.Treangen TJ, Rocha EP. Horizontal transfer, not duplication, drives the expansion of protein families in prokaryotes. PLoS Genet. 2011;7:e1001284. doi: 10.1371/journal.pgen.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blomme T, et al. The gain and loss of genes during 600 million years of vertebrate evolution. Genome Biol. 2006;7:R43. doi: 10.1186/gb-2006-7-5-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krantz GW, Lindquist EE. Evolution of phytophagous mites (Acari) Annu. Rev. Entomol. 1979;24.1:121–158. doi: 10.1146/annurev.en.24.010179.001005. [DOI] [Google Scholar]
- 31.Bensoussan N, et al. Plant-Herbivore Interaction: dissection of the cellular pattern of Tetranychus urticae feeding on the host plant. Front Plant Sci. 2016;7:1105. doi: 10.3389/fpls.2016.01105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem. Mol. Biol. 2010;40:563–572. doi: 10.1016/j.ibmb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Grbic M, et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature. 2011;479:487–492. doi: 10.1038/nature10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roesijadi G. Metal transfer as a mechanism for metallothionein-mediated metal detoxification. Cell Mol. Biol. (Noisy-le.-Gd.) 2000;46:393–405. [PubMed] [Google Scholar]
- 35.Nishito Y, et al. Direct comparison of manganese detoxification/efflux proteins and molecular characterization of ZnT10 protein as a manganese transporter. J. Biol. Chem. 2016;291:14773–14787. doi: 10.1074/jbc.M116.728014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feyereisen R, Dermauw W, Van Leeuwen T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic. Biochem. Physiol. 2015;121:61–77. doi: 10.1016/j.pestbp.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Nelson DR. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta Proteins Proteom. 2018;1866:141–154. doi: 10.1016/j.bbapap.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traverso L, et al. Comparative and functional triatomine genomics reveals reductions and expansions in insecticide resistance-related gene families. PLoS Negl. Trop. Dis. 2017;11:e0005313. doi: 10.1371/journal.pntd.0005313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dermauw W, et al. A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proc. Natl Acad. Sci. USA. 2013;110:E113–E122. doi: 10.1073/pnas.1213214110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pallini AJA, Sabelis MW. Spider mites avoid plants with predators. Exp. Appl Acarol. 1999;23:803–815. doi: 10.1023/A:1006266232714. [DOI] [Google Scholar]
- 41.Pallini A, Janssen A, Sabelis MW. Odour-mediated responses of phytophagous mites to conspecific and heterospecific competitors. Oecologia. 1997;110:179–185. doi: 10.1007/s004420050147. [DOI] [PubMed] [Google Scholar]
- 42.Carr VM. Induced and constitutive heat shock protein expression in the olfactory system–a review, new findings, and some perspectives. J. Neurocytol. 2005;34:269–293. doi: 10.1007/s11068-005-8358-9. [DOI] [PubMed] [Google Scholar]
- 43.Frenkel L, Dimant B, Suarez LD, Portiansky EL, Delorenzi A. Food odor, visual danger stimulus, and retrieval of an aversive memory trigger heat shock protein HSP70 expression in the olfactory lobe of the crab Chasmagnathus granulatus. Neuroscience. 2012;201:239–251. doi: 10.1016/j.neuroscience.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 44.Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94:73–82. doi: 10.1016/S0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 45.Carr VM, Menco BP, Yankova MP, Morimoto RI, Farbman AI. Odorants as cell-type specific activators of a heat shock response in the rat olfactory mucosa. J. Comp. Neurol. 2001;432:425–439. doi: 10.1002/cne.1112. [DOI] [PubMed] [Google Scholar]
- 46.Younus F, et al. Identification of candidate odorant degrading gene/enzyme systems in the antennal transcriptome of Drosophila melanogaster. Insect Biochem. Mol. Biol. 2014;53:30–43. doi: 10.1016/j.ibmb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Krenn HW, Aspock H. Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Struct. Dev. 2012;41:101–118. doi: 10.1016/j.asd.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Girard TJ, Lasky NM, Tuley EA, Broze GJ., Jr. Protein Z, protein Z-dependent protease inhibitor (serpinA10), and the acute-phase response. J. Thromb. Haemost. 2013;11:375–378. doi: 10.1111/jth.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nie D, et al. Differential expression of thromboxane synthase in prostate carcinoma: role in tumor cell motility. Am. J. Pathol. 2004;164:429–439. doi: 10.1016/S0002-9440(10)63133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rico-Mesa JS, et al. The role of anticoagulation in COVID-19-induced hypercoagulability. Curr. Cardiol. Rep. 2020;22:53. doi: 10.1007/s11886-020-01328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Motobu M, et al. Molecular characterization of a blood-induced serine carboxypeptidase from the ixodid tick Haemaphysalis longicornis. FEBS J. 2007;274:3299–3312. doi: 10.1111/j.1742-4658.2007.05852.x. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Valle M, et al. Transcriptome and toxin family analysis of the paralysis tick, Ixodes holocyclus. Int. J. Parasitol. 2018;48:71–82. doi: 10.1016/j.ijpara.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Horn M, et al. Hemoglobin digestion in blood-feeding ticks: mapping a multipeptidase pathway by functional proteomics. Chem. Biol. 2009;16:1053–1063. doi: 10.1016/j.chembiol.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahoney JA, Ntolosi B, DaSilva RP, Gordon S, McKnight AJ. Cloning and characterization of CPVL, a novel serine carboxypeptidase, from human macrophages. Genomics. 2001;72:243–251. doi: 10.1006/geno.2000.6484. [DOI] [PubMed] [Google Scholar]
- 55.Goupille O, et al. Characterization of Pax3-expressing cells from adult blood vessels. J. Cell Sci. 2011;124:3980–3988. doi: 10.1242/jcs.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang M, et al. PAQR3 modulates blood cholesterol level by facilitating interaction between LDLR and PCSK9. Metabolism. 2019;94:88–95. doi: 10.1016/j.metabol.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Saura M, et al. Oral administration of bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014;28:4719–4728. doi: 10.1096/fj.14-252460. [DOI] [PubMed] [Google Scholar]
- 58.Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem. Biol. Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Hosokawa M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules. 2008;13:412–431. doi: 10.3390/molecules13020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen F, Zhang B, Parker RB, Laizure SC. Clinical implications of genetic variation in carboxylesterase drug metabolism. Expert Opin. Drug Metab. Toxicol. 2018;14:131–142. doi: 10.1080/17425255.2018.1420164. [DOI] [PubMed] [Google Scholar]
- 61.Perry, R. M. Jr. The Detection and Quantitative Analysis of Endocannabinoids and Endogenous Fatty Acid Amides in Apis Mellifera and Tribolium Castaneum. M.S. thesis, University of South Florida (2015).
- 62.Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006;281:36569–36578. doi: 10.1074/jbc.M606646200. [DOI] [PubMed] [Google Scholar]
- 63.Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics Chapter 4, Unit 4 10 (2004). [DOI] [PubMed]
- 64.Hoff KJ, Lange S, Lomsadze A, Borodovsky M, Stanke M. BRAKER1: unsupervised RNA-Seq-based genome annotation with GeneMark-ET and AUGUSTUS. Bioinformatics. 2016;32:767–769. doi: 10.1093/bioinformatics/btv661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 67.De Bie T, Cristianini N, Demuth JP, Hahn MW. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006;22:1269–1271. doi: 10.1093/bioinformatics/btl097. [DOI] [PubMed] [Google Scholar]
- 68.Xie C, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–W322. doi: 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xue W, et al. Geographical distribution and molecular insights into abamectin and milbemectin cross-resistance in European field populations of Tetranychus urticae. Pest Manag. Sci. 2020;76:2569–2581. doi: 10.1002/ps.5831. [DOI] [PubMed] [Google Scholar]
- 70.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Babicki S, et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 2016;44:W147–W153. doi: 10.1093/nar/gkw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keeling CI, et al. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 2013;14:R27. doi: 10.1186/gb-2013-14-3-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller JR, et al. A draft genome sequence for the Ixodes scapularis cell line. ISE6. F1000Res. 2018;7:297. doi: 10.12688/f1000research.13635.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoy MA, et al. Genome sequencing of the phytoseiid predatory mite metaseiulus occidentalis reveals completely atomized hox genes and superdynamic intron evolution. Genome Biol. Evol. 2016;8:1762–1775. doi: 10.1093/gbe/evw048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burgess STG, et al. Draft genome assembly of the poultry red mite, Dermanyssus gallinae. Microbiol. Resour. Announc. 2018;7:e01221–01218. doi: 10.1128/MRA.01221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong X, et al. Draft genome of the honey bee ectoparasitic mite, Tropilaelaps mercedesae, is shaped by the parasitic life history. Gigascience. 2017;6:1–17. doi: 10.1093/gigascience/gix008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Techer MA, et al. Divergent evolutionary trajectories following speciation in two ectoparasitic honey bee mites. Commun. Biol. 2019;2:357. doi: 10.1038/s42003-019-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bast J, et al. No accumulation of transposable elements in asexual arthropods. Mol. Biol. Evol. 2016;33:697–706. doi: 10.1093/molbev/msv261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rider SD, Jr., Morgan MS, Arlian LG. Draft genome of the scabies mite. Parasit. Vectors. 2015;8:585. doi: 10.1186/s13071-015-1198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mondal M, Klimov P, Flynt AS. Rewired RNAi-mediated genome surveillance in house dust mites. PLoS Genet. 2018;14:e1007183. doi: 10.1371/journal.pgen.1007183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burgess STG, et al. Draft genome assembly of the sheep scab mite, Psoroptes ovis. Genome Announc. 2018;6:e00265–00218. doi: 10.1128/genomeA.00265-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong X, et al. Genomes of trombidid mites reveal novel predicted allergens and laterally transferred genes associated with secondary metabolism. Gigascience. 2018;7:giy127. doi: 10.1093/gigascience/giy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Navia D, et al. Draft genome assembly of the false spider mite Brevipalpus yothersi. Microbiol. Resour. Announc. 2019;8:e01563–01518. doi: 10.1128/MRA.01563-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanggaard KW, et al. Spider genomes provide insight into composition and evolution of venom and silk. Nat. Commun. 2014;5:3765. doi: 10.1038/ncomms4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The final dataset is available at Mendeley Data (https://data.mendeley.com/datasets/xm23f9mkdx/1).
Analysis code is accessible through github (https://github.com/offlinecat/mite_diet).