Abstract
Background:
Two functional measurements (multiple breath washout [MBW] and hyperpolarized 129Xe ventilation magnetic resonance imaging [129Xe MRI]) have been shown to be more sensitive to cystic fibrosis (CF) lung obstruction than traditional spirometry. However, functional techniques may be sensitive to different underlying structural abnormalities. The purpose of this study was to determine relationships between these functional markers, their pathophysiology, and 1-year clinical outcomes.
Methods:
Spirometry, MBW, 129Xe MRI, and ultrashort echo-time (UTE) MRI were obtained in a same-day assessment of 27 pediatric CF patients (ages 11.5±5.0) who had not begun CFTR modulator therapies. UTE MRI was scored for structural abnormalities and functional metrics obtained via spirometry, MBW and 129Xe MRI. 1-year outcomes (ΔFEV1 and pulmonary exacerbations), during which ≈50% initiated modulator therapy, were obtained from the electronic medical record.
Results:
MBW, 129Xe MRI, and UTE MRI detected clinically significant disease in more subjects (>78%) compared to spirometry (<30%). UTE MRI suggests increased odds of bronchial changes when mucus plugging is present in the same lobe. MBW and 129Xe MRI correlated best with mucus plugging, while spirometry correlated best with consolidations. Bronchial abnormalities were associated with future pulmonary exacerbations.
Conclusions:
MBW, 129Xe MRI, and UTE MRI are more sensitive for detection of pediatric CF lung disease when compared to spirometry. MBW and 129Xe MRI correlated with structural abnormalities which occur in early CF disease, suggesting MBW and 129Xe MRI are valuable tools in mild CF lung disease that can guide clinical decision making.
Keywords: Spirometry, Multiple Breath Washout, Xenon, MRI, Structure, Function
Introduction:
Cystic Fibrosis (CF) results in multiple morphological lung abnormalities on computed tomography (CT) examination that are identified as mucus plugging, bronchial wall thickening, bronchiectasis, ground glass opacities, consolidations, and cysts. Significant intra- and inter-subject heterogeneities in abnormalities exist with variability in severity and lobar distribution [1]. The structural abnormalities eventually result in a loss of clinical lung function, which is periodically monitored to follow disease progression and treatment response.
Previous studies have shown the use of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies increased lung function and reduced bronchial wall thickening and mucus plugging [2]. Recently, a triple-combination CFTR) modulator therapy has been shown to be a highly effective treatment, with a greater than 10% improvement in lung function for any patient with a single F508del mutation (~90% of all CF patients) [3]. With highly-effective therapies becoming more widely used, we anticipate a larger fraction of subjects will likely exhibit milder lung disease, both structurally and functionally. Thus, sensitive measures of lung disease progression and outcomes is a growing necessity.
Chest CT is the imaging gold standard in CF care. To provide quantification—primarily in the context of research studies—scoring systems have been developed and validated to quantify extent and severity of the structural abnormalities (e.g., Brody [4], Helbich [5], PRAGMA-CF [6], Eichinger [7]). Since then, ultrashort echo-time 1H magnetic resonance imaging (UTE MRI) of pediatric CF patients [8,9] has been shown to be amenable to CF image scoring [7,9,10], eliminating the need for ionizing-radiation exposure. While intrinsically subjective to some degree, structural image scoring predicts future pulmonary exacerbations [11,12] and lung function loss [13].
Functionally, multiple breath washout (MBW) has been shown to be more sensitive than ubiquitously used spirometry. MBW measures the number of lung turnovers, as defined by the functional residual capacity, to reduce a tracer gas to a concentration of 2.5% of its initial value (lung clearance index; LCI2.5). However, MBW lacks regional specificity needed to fully assess lung dysfunction [14]. To this end, hyperpolarized Xenon-129 ventilation MRI (129Xe MRI) [15] shows promise in bridging the gaps between sensitivity, regionality, structure, and function.
129Xe MRI determines the percent of lung volume which does not receive an adequate amount of gas following an inhalation (ventilation defect percentage, VDP). Both MBW [16,17] and 129Xe MRI [18–21] have demonstrated sensitivity to early CF lung disease and treatment responses. However, each functional measurement may have a potential sensitivity bias towards certain structural abnormalities, which is important when considering clinical prognosis [22].
Thus, we compare functional measurements (spirometry, MBW, and 129Xe MRI) to quantitative structural scores (UTE MRI) to determine the sensitivity of functional measures to structural defects in CF lung disease. We hypothesized both MBW and 129Xe correlate more with mucus plugging and bronchial wall thickening compared to spirometry, as they are more common in mild disease and would lead to early ventilation heterogeneity. Additionally, we hypothesize these more sensitive functional/structural measurements would correlate better with 1-year outcomes (e.g., change in FEV1 and number of pulmonary exacerbations requiring hospitalization) and would be dependent on modulator therapy usage.
Materials and Methods:
This prospective study was performed with approval from the IRB of Cincinnati Children’s Hospital Medical Center and with oversight from the US-FDA under an investigational new drug (IND 123,577) protocol for hyperpolarized 129Xe MRI. The study incorporated same-day assessment using four techniques: spirometry, MBW, 129Xe MRI, and UTE MRI. This was performed in 27 modulator-naïve pediatric CF patients (ages 11.5±5.0; at least one copy of the F508del mutation; 15/12 M/F). Only pediatric subjects were assessed to limit to mild CF lung disease. Following the same-day assessment, patients were followed for 1 year per standard-of-care clinical assessments at our institution, and of the 27 subjects, 26 continued to be seen at our institution. Measured FEV1 (frequency = 5.8±3.5/year) and hospitalizations due to pulmonary exacerbations were recorded from the electronic medical record for short-term outcomes. Average yearly change in FEV1 (ΔFEV1) and number of inpatient hospitalizations for pulmonary exacerbations (determined by a scoring system accounting for symptoms, lung exam findings, and FEV1) were calculated. For ΔFEV1, all clinically-acquired measurements were fit to a linear regression with the slope (in ΔFEV1/year) taken as ΔFEV1. Of the 26 subjects, 14 of the subjects began ivacaftor or double-modulator therapy (lumacaftor/ivacaftor–11 subjects, ivacaftor–2 subjects, and tezacaftor/ivacaftor–1 subject) within the year (mean start of 54 days after same-day assessment).
Spirometry (Koko, nSpire, Longmont, CO) and MBW (Exhalyzer D, EcoMedics, Duernten, Switzerland), were performed according to American Thoracic Society and European Respiratory Society Guidelines [23,24]. MBW incorporated N2 as the tracer gas, and the number of turnovers to reach a lung clearance index of 2.5% (LCI2.5) was recorded.
Immediately following spirometry and MBW, the subjects underwent a single MRI session, during which 129Xe and UTE MR images were acquired using a 3.0T Philips Achieva scanner and a homebuilt dual-loop coil for 129Xe MRI [25] and a 32-channel cardiac coil for UTE MRI. 129Xe images were acquired during a breath hold (~10 seconds, dependent on field of view, maximum of 16 seconds) of hyperpolarized 129Xe gas equivalent to 1/6th the subjects predicted total lung capacity [26]. 129Xe images were acquired as axial slices with a gradient recalled echo MRI sequence (3mm × 3mm × 15mm resolution). RF bias correction was completed by extracting and removing linear inhomogeneity profiles within the lung for all three dimensions. Ventilation defect percentage (VDP) was calculated using the linear binning method [27], with manual lung segmentation (D.J.R.) and a control cohort of healthy age-matched controls (23 subjects; ages 12.1±4.1; 13/10 M/F; ventilation = 0.54 ± 0.15). Structural information was obtained via UTE MRI, acquired with a 3D-radial MRI sequence with rectilinear encoding in the head-to-foot direction (stack-of-stars). The axial images were gated to end-expiration using a pencil-beam navigator (1.5mm × 1.5mm × 4.0mm resolution ≈5 minutes). Additional MRI parameters can be found in Table S1 of the Supplementary Materials. Thus, to acquire both structural and functional MRI images, less than 10 minutes was necessary.
Quantitative structural analysis of the UTE MRI was completed using a simplified Brody score [8]. The reader (M.M.W.), trained and certified for Brody scoring with 3 years of experience, was blinded to the subject/measurements obtained. The lung was divided into 6 lobar-regions (the lingula separated from the left lower lobe, for parallelism), each scored for mucus plugging, bronchial wall thickening, bronchiectasis, ground glass opacities, consolidations, air trapping and cysts. For each abnormality and lobe, a score of 0 to 2 was determined where 0 indicated not present, 1 indicates presence in <1/2 the lobe, and 2 indicates presence in >1/2 the lobe. Thus, the total possible abnormality score is 6 (lobes) × 7 (abnormalities) × 2 (extent) = 84. Additionally, since the Brody score is calculated for each lobe and structure, the distribution of structural abnormalities can be assessed by summing applicable scores.
For sensitivity to mild lung disease, upper/lower limits of normal (normal for 95% of a healthy population: mean ± 1.645×standard deviation or a z-score of ±1.645) were used as clinically-significant abnormality cutoffs and were calculated from the healthy control cohort (spirometry %-predicted values and VDP) or from previously published data (UTE score and LCI2.5), as shown in Table 1 [8,28].
Table 1:
Demographic, same-day assessment results, and short-term outcomes for the cohort.
| Mean | Standard Deviation | Range | Limit of Normal | Percent Abnormal | |
|---|---|---|---|---|---|
| Demographics | |||||
| Gender | 15M/12F | - | - | - | - |
| Age | 11.9 | 4.9 | 6.0–20.5 | - | - |
| Same-day Assessment (N=27) | |||||
| FEV1 %-predicted | 98.7% | 15.3% | 66–131% | >70% | 4% |
| FEV1 GLI z-score | −0.32 | 1.25 | −3.07–2.60 | >−1.645 | 15% |
| FEF25–75 %-predicted | 92.9% | 33.3% | 47–147% | - | - |
| FEF25–75 GLI z-score | −0.55 | 1.41 | −2.96–2.22 | >−1.645 | 30% |
| FEV1/FVC | 84.7% | 8.1% | 70–99% | >73% | 7% |
| FEV1/FVC GLI z-score | −0.54 | 1.19 | −2.52–1.71 | >−1.645 | 22% |
| LCI2.5 | 10.0 | 2.1 | 6.6–14.4 | <7.91 | 78% |
| VDP | 18.5% | 11.0% | 2.6–38.9% | <6.0% | 85% |
| Total Brody Score | 9.7 | 7.9 | 0–32 | <4.6 | 78% |
| Mucus Plugging | 1.4 | 1.9 | 0–8 | - | - |
| Bronchial Wall Thickening | 3.0 | 2.7 | 0–10 | - | - |
| Bronchiectasis | 3.4 | 2.5 | 0–10 | - | - |
| Ground Glass | 0.4 | 0.9 | 0–3 | - | - |
| Consolidation/Atelectasis | 0.6 | 0.9 | 0–3 | - | - |
| Air Trapping | 0.9 | 1.0 | 0–4 | - | - |
| Cysts/Bullae | 0.0 | 0.2 | 0–1 | - | - |
| 1 Year Outcomes (N=26) | |||||
| ΔFEV1 (%/year) | 2.4 | 11.7 | −27.6–19.2 | - | - |
| # Pulmonary Exacerbations | 0.27 | 0.52 | 0–2 | - | - |
Row headings in italics denote structural sub scores which make up the total Brody score. The limits of normal were derived as described in the text. Missing areas represent either not applicable or available value
All functional measures were normally distributed according to the Shapiro-Wilk normality test (P ≥ 0.05); however, structural abnormality scores and number of pulmonary exacerbations were not. Thus, spearman correlations were used to compare structural abnormalities and lung function measurements, as well as their associations with clinical outcomes. A logistic regression was completed to calculated odds ratios. Two-tailed T-tests with unequal variances were implemented for comparisons of outcomes between groups. Statistical significance was determined by a P-value <0.05. Multiple testing correction for significant correlations was completed using the Benjamini-Hochberg method with a Bonferonni α correction allowing for a 0.1 experimental false discovery rate to reduce Type I errors; P-values which are no longer significant are marked by an asterisk (*).
Results:
A summary of the data can be seen in Table 1 for all subjects and in Table S2 for modulator and modulator-naïve cohorts. Additionally, example results for 3 subjects can be seen in Figure 1. From spirometry, the average FEV1 %-predicted of the cohort was 98.7±15.3% with only 3 subjects (11%) below 80%, the typical clinical abnormal function threshold, with one below our healthy cohort-derived lower limit of 70%. Similarly, the FEV1/FVC ratio detected significant abnormality in 7%. Moving from percent predicted to GLI z-scores, spirometry results were more sensitive with 15%, 30%, and 22% of subjects having significantly abnormal lung function according to FEV1, FEF25–75, and FEV1/FVC, respectively. Thus, GLI z-scores will be used for future analysis. MBW found a mean LCI2.5 of 10.0±2.1 with an upper limit of normal (7.91) indicating abnormal lung function in 21 (78%) subjects. 129Xe MRI analysis showed a VDP = 18.5±11.0% (ventilation = 0.42 ± 0.18) with an upper limit of normal (6.0%) indicating abnormal lung function in 23 (85%) subjects. The structural UTE MR images had a mean total score of 9.7±7.9 with the mean abnormality sub-scores ranging from 0.0 (cysts/bullae) up to 3.4 (bronchiectasis). The upper limit of 4.6 for the total score indicated 21 (78%) of the subjects had significant visible structural abnormalities.
Figure 1:
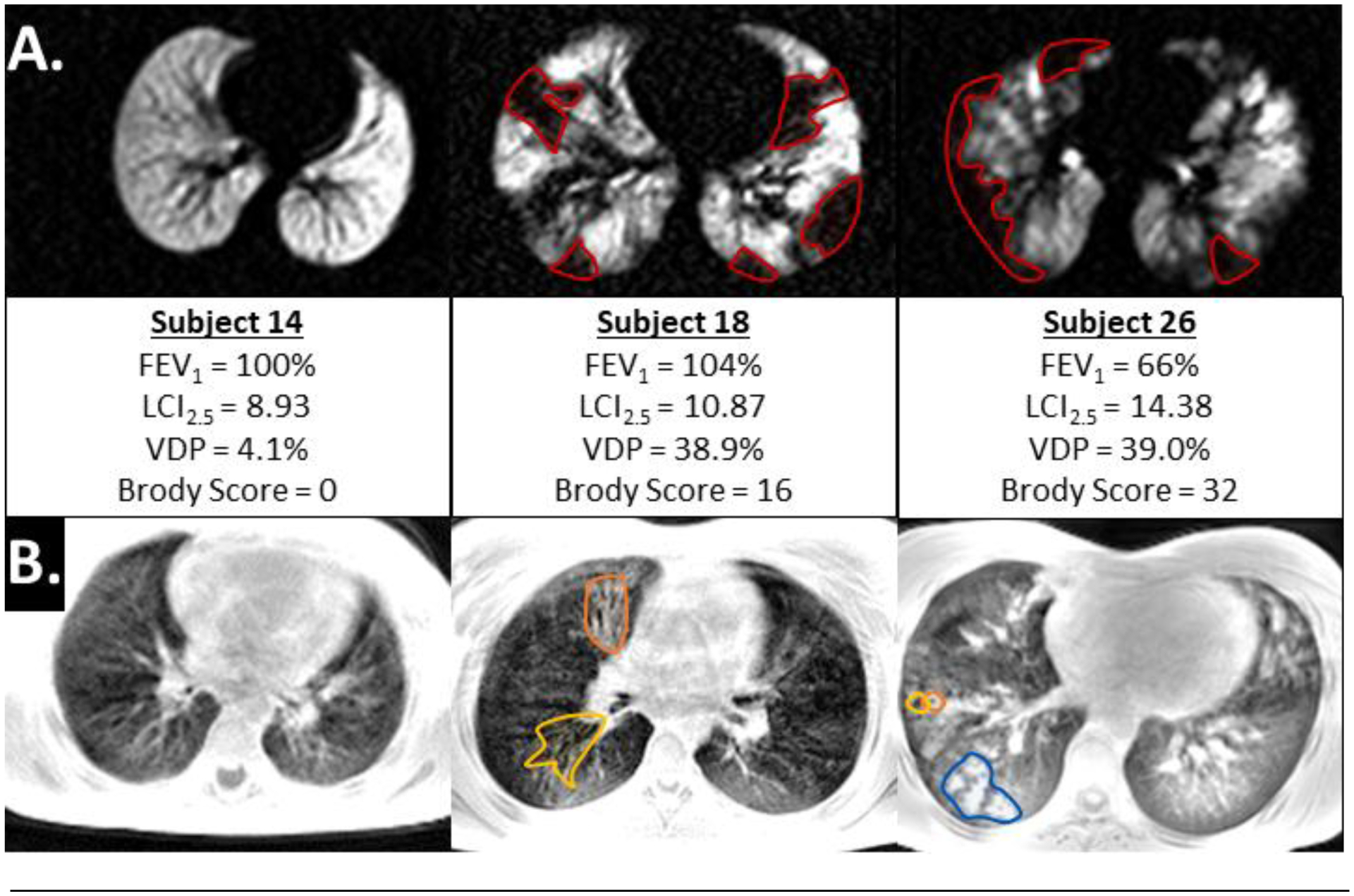
Example image slices for 129Xe (a.) and UTE (b.) images in CF subjects. Subject 14 (left) is an example of a subject a relatively healthy subject with only LCI2.5 being slightly elevated. Subject 18 (middle) is an example of a subject with elevated LCI2.5, VDP and structural abnormalities despite normal FEV1. Subject 26 (right) is an example subject who also had an abnormal FEV1 %-predicted. In (a.), regions circled in red illustrate examples of ventilation defects and in (b.), example regions are circled in orange for combined bronchiectasis/wall thickening, yellow for only bronchiectasis and blue for mucus plugging. Each image provides the subject’s FEV1 %-predicted, LCI2.5, VDP, and Brody score.
Structurally, the right lung experienced more severe structural scores (average combined abnormality score of 5.9±4.4) than the left lung (3.9±3.8, P < 0.01). Additionally, the upper lobes displayed more severe structural disease (4.1±3.4) than the middle lobe/lingula (2.8±2.7, P < 0.01) and lower lobes (3.0±2.9, P < 0.01), in agreement with previous CT findings [4,29]. Notably, lobes with mucus plugs were strongly correlation with the occurrence of bronchiectasis and/or bronchial wall thickening. Eighty-nine percent of lobes with mucus plugging also exhibited more permanent structural airway changes, while only 37% of lobes lacking mucus plugging exhibited the bronchial abnormalities. This results in an odds ratio of 12.9 for more permanent airway structural changes given mucus plugging is visibly present in the same lobe (confidence interval = 4.3–39.0; P<0.0001).
Significant correlations between the functional measures (Table S3) were found (all P < 0.003). Variance between FEV1 z-score, FEF25–75 z-score, and FEV1/FVC z-score was minimal (R2 > 0.5) as was the same for LCI2.5 and VDP. However, correlations between spirometry and the newer functional measures all had an R2 below 0.5.
Correlations of structural changes/severity with functional measurements were investigated to investigate which structural abnormalities most-influenced each functional metric (Table 2 and Figure 2). FEV1/FVC z-score, LCI2.5 and VDP correlated with the total structural abnormality score while FEV1 and FEF25–75 z-scores did not. FEV1 and FEF25–75 z-scores only correlated with ground glass opacities and consolidation/atelectasis abnormalities. FEV1/FVC z-score correlated with mucus plugging and consolidation/atelectasis. LCI2.5 and VDP significantly correlated with mucus plugging while LCI2.5 also correlated with bronchial wall thickening.
Table 2:
Spearman r and P-value for correlations between structural abnormality scores from UTE MRI and functional measures from spirometry, MBW, and 129Xe MRI.
| FEV1 z-score | FEF25–75 z-score | FEV1/FVC z-score | LCI2.5 | VDP | |
|---|---|---|---|---|---|
| Total Brody Score | r = −0.31 | r = −0.35 | r = −0.44 | r = 0.47 | r = 0.41 |
| (P = 0.12) | (P = 0.07) | (P = 0.02) | (P = 0.01) | (P = 0.03*) | |
| Mucus Plugging | r = −0.23 | r = −0.33 | r = −0.45 | r = 0.51 | r = 0.53 |
| (P = 0.23) | (P = 0.10) | (P = 0.02) | (P < 0.01) | (P <0.01) | |
| Bronchial Wall Thickening | r = −0.16 | r = −0.17 | r = −0.27 | r = 0.39 | r = 0.32 |
| (P = 0.43) | (P = 0.40) | (P = 0.17) | (P =0.05*) | (P = 0.10) | |
| Bronchiectasis | r = −0.14 | r = −0.14 | r = −0.21 | r = 0.36 | r = 0.33 |
| (P = 0.50) | (P = 0.49) | (P = 0.30) | (P = 0.07) | (P = 0.09) | |
| Ground Glass | r = −0.44 | r = −0.47 | r = −0.30 | r = 0.09 | r = −0.00 |
| (P = 0.02) | (P = 0.01) | (P = 0.12) | (P = 0.66) | (P = 0.98) | |
| Consolidation/Atelectasis | r = −0.46 | r = −0.45 | r = −0.46 | r = 0.35 | r = 0.34 |
| (P = 0.02) | (P = 0.02) | (P = 0.02) | (P = 0.07) | (P = 0.08) | |
| Air Trapping | r = −0.10 | r = −0.19 | r = −0.27 | r = 0.28 | r = 0.12 |
| (P = 0.55) | (P = 0.33) | (P = 0.17) | (P = 0.16) | (P = 0.95) | |
| Cysts/Bullae | r = 0.09 | r = 0.16 | r = 0.18 | r = −0.03 | r = 0.01 |
| (P = 0.66) | (P = 0.41) | (P = 0.38) | (P = 0.90) | (P = 0.95) |
Statistically significant correlations are in bold.
indicates no longer significant after correction for multiple tests.
Figure 2:
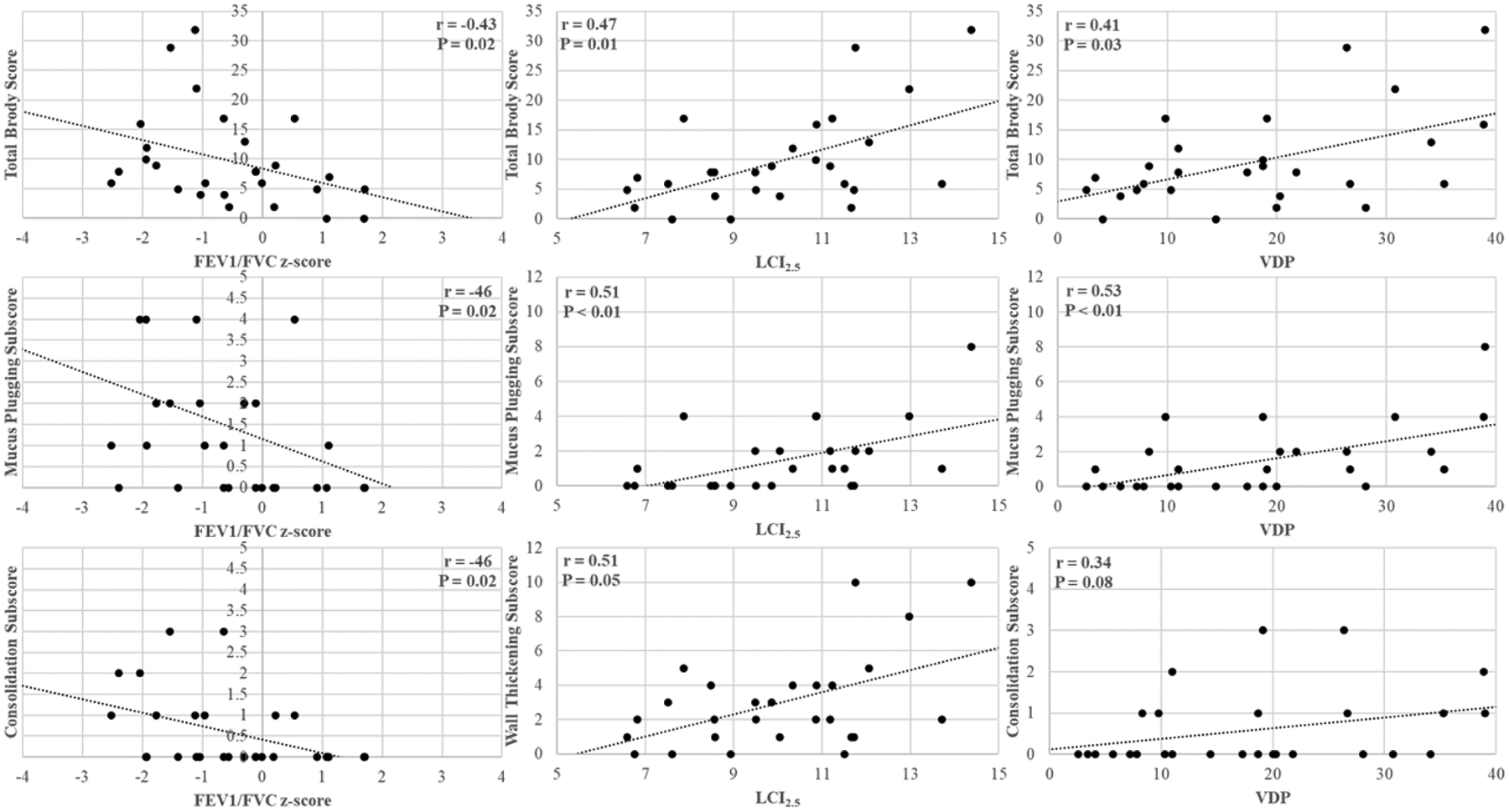
Select correlations between structural scores and the functional measurements - FEV1/FVC z-score (left column), LCI (middle column), and VDP (right column). Dots correspond to subjects and the dashed line shows the linear regression.
Correlations between the same-day assessment metrics and outcomes are shown in Table 3. Seven pulmonary exacerbations in 6 subjects were reported, with 4 occurring in subjects who started modulator therapy (P = 0.87). ΔFEV1 was only associated with VDP (P = 0.05*). For pulmonary exacerbations, the only significant correlation was bronchial wall-thickening (P=0.05*). However, there is a difference when considering modulator usage. For the subjects who began modulator therapy, we found three significant correlations (bronchial wall thickening, P < 0.01; bronchiectasis, P=0.01; and total Brody score, P = 0.03*).
Table 3:
Spearman r and P-value for correlations between quantitative functional and structural scores with outcomes including the yearly change in FEV1 and number of pulmonary exacerbations for the year following the same-day assessment.
| Cohort | ΔFEV1 | # Pulmonary Exacerbations | |
|---|---|---|---|
| FEV1 z-score | All | r = −0.18 (P = 0.38) | r = −0.23 (P = 0.25) |
| Modulator | r = −0.36 (P = 0.20) | r = −0.16 (P = 0.59) | |
| No Modulator | r = 0.10 (P = 0.74) | r = −0.42 (P = 0.18) | |
| FEF25–75 z-score | All | r = −0.21 (P = 0.30) | r = −0.21 (P = 0.30) |
| Modulator | r = −0.36 (P = 0.20) | r = −0.26 (P = 0.38) | |
| No Modulator | r = 0.04 (P = 0.91) | r = −0.25 (P = 0.44) | |
| FEV1/FVC z-score | All | r = −0.08 (P = 0.68) | r = −0.13 (P = 0.51) |
| Modulator | r = −0.20 (P = 0.48) | r = −0.27 (P = 0.34) | |
| No Modulator | r = 0.12 (P = 0.71) | r = −0.02 (P = 0.96) | |
| LCI2.5 | All | r = 0.12 (P = 0.57) | r = 0.19 (P = 0.36) |
| Modulator | r = 0.46 (P = 0.09) | r = 0.04 (P = 0.89) | |
| No Modulator | r = −0.34 (P = 0.29) | r = 0.35 (P = 0.26) | |
| VDP | All | r = −0.39 (P = 0.05*) | r = 0.28 (P = 0.17) |
| Modulator | r = −0.44 (P = 0.12) | r = 0.27 (P = 0.34) | |
| No Modulator | r = −0.44 (P = 0.15) | r = 0.35 (P = 0.26) | |
| Total Brody Score | All | r = −0.03 (P = 0.90) | r = 0.30 (P = 0.13) |
| Modulator | r = 0.25 (P = 0.39) | r = 0.57 (P = 0.03*) | |
| No Modulator | r = −0.33 (P = 0.30) | r = 0.06 (P = 0.85) | |
| Mucus Plugging | All | r = −0.10 (P = 0.61) | r = 0.08 (P = 0.70) |
| Modulator | r = 0.35 (P = 0.23) | r = 0.15 (P = 0.60) | |
| No Modulator | r = −0.42 (P = 0.17) | r = 0.15(P = 0.63) | |
| Bronchial Wall Thickening | All | r = −0.12 (P = 0.56) | r = 0.39 (P = 0.05*) |
| Modulator | r = 0.10 (P = 0.74) | r = 0.71 (P < 0.01) | |
| No Modulator | r = −0.31 (P = 0.33) | r = 0.08 (P = 0.79) | |
| Bronchiectasis | All | r = −0.17 (P = 0.40) | r = 0.34 (P = 0.09) |
| Modulator | r = 0.08 (P = 0.80) | r = 0.65 (P = 0.01) | |
| No Modulator | r = −0.44 (P = 0.15) | r = 0.06 (P = 0.85) | |
| Ground Glass | All | r = 0.34 (P = 0.09) | r = 0.24 (P = 0.25) |
| Modulator | r = 0.46 (P = 0.10) | r = 0.23 (P = 0.44) | |
| No Modulator | r = 0.32 (P = 0.32) | r = 0.32 (P = 0.31) | |
| Consolidation/Atelectasis | All | r = 0.07 (P = 0.73) | r = 0.15 (P = 0.46) |
| Modulator | r = 0.17 (P = 0.57) | r = 0.11 (P = 0.71) | |
| No Modulator | r = −0.18 (P = 0.57) | r = 0.20 (P = 0.52) | |
| Air Trapping | All | r = 0.06 (P = 0.75) | r = −0.31 (P = 0.13) |
| Modulator | r = 0.23 (P = 0.43) | r = −0.21 (P = 0.46) | |
| No Modulator | r = −0.29 (P = 0.36) | r = −0.42 (P = 0.17) | |
| Cysts/Bullae | All | r = 0.01 (P = 0.95) | r = 0.35 (P = 0.08) |
| Modulator | r = −0.03 (P = 0.91) | r = 0.44 (P = 0.12) | |
| No Modulator | N/A* | N/A* |
Statistics are given for the entire cohort, those who started a modulator therapy the following year (n=14), and those who did not (n=12). Statistically significant correlations are in bold.
indicates no longer significant after correction for multiple tests.
N/A indicates inability to calculate due to all subjects having the same value (0, not present).
Discussion:
In this study, we assessed multiple functional measurements in a group of pediatric CF patients and compared to same-day structural MRI findings. Additionally, clinical outcomes were analyzed to determine the relationship between structural/functional measurements to future ΔFEV1 and number of pulmonary exacerbations. This improves the ability for clinicians to select the appropriate test, interpret the results, and determine the best treatment/intervention in a personalized manner.
From the regional correlations between mucus plugging and bronchial changes, the odds ratio of 12.9 illustrates a likely causative relationship between mucus plugging and permanent airway structural changes and suggests that identifying and treating early mucus plugging with effective airway clearance and pharmaceutic interventions may yield long-term benefits. Additionally, while we used a simplified Brody scoring system, we suspect any validated CF scoring system [30] would show similar results. Moreover, while the structural scoring allowed for prognostic power with the ability to discriminate different abnormalities, it is currently a time-consuming process.
In agreement with previous publications, LCI2.5 and VDP were more sensitive than FEV1 at detecting mild disease [18–21]. This is demonstrated by LCI2.5 and VDP detecting functional abnormalities in over 78% of the subjects despite mostly normal FEV1 z-scores. This may be due to the sensitivity of LCI2.5 and VDP to mucus plugging and bronchial abnormalities.
Additionally, an upper limit of normal for VDP is not well established and can vary depending on the cohort, acquisition methods, and precise quantitative analysis methods. Thus, the normal limit listed here is subject to change as this technique becomes more developed/standardized. Future refinements in analysis of 129Xe MRI, will likely decrease variation within healthy subjects, lowering the limit of normal and increasing sensitivity. Thus, future studies should ensure consistent acquisition and analysis protocols. Lastly, previous studies showed moderate correlation between FEV1 and total abnormality scores, but it was only significant when including subjects with abnormal spirometry [4]. Thus, the mild severity of disease in this cohort further emphasizes the sensitivity of LCI2.5 and VDP compared to FEV1.
There were some disagreements between the structural findings and the functional measurements. For example, subject 4 had the 7th highest VDP and 2nd highest LCI2.5 but the structural score was average (16th highest). This is potentially due to the sensitivity of these functional scores to certain structural abnormalities as shown here. Subject 4’s abnormalities were primarily present in mucus plugging and bronchial abnormalities with no ground glass, cysts, or air trapping observed which would explain the disagreement between VDP/LCI2.5 and the structural scores. Additionally, the location of defects could lead to a disproportionate functional decline. For example, a mucus plug in a lobar bronchus would lead to a larger functional decline than one in a subsegmental bronchus.
While most of the variation in the functional measurements were not explained by structural measurements (max R2 = 0.28), there was also noticeable variation in the correlations between functional measures (such as FEF25–75 z-score and VDP with an R2 of 0.30). Since the correlations were not perfect between the functional measures, a potential sensitivity bias for each functional measure towards different structural abnormalities is likely, as supported by the functional measures being correlated with different structural abnormalities.
Transitioning onto modulator therapy was an effect modifier when investigating the correlations with pulmonary outcomes. None of the measurements acquired during the initial assessment (functional/structural scores) were different (all P > 0.05) between the modulator (n=14) and non-modulator (n=12) groups. Thus, prior to the 1-year observation, all structural and functional metrics were similar for the two cohorts. There is an expected reversal of non-permanent structural findings (i.e., mucus plugging), leaving only more permanent structural changes (i.e., bronchial damage) once modulator therapy is initiated. Thus, outcome associations are likely dependent on the abnormalities each technique is most sensitive. This effect modification resulted in the functional measurements (spirometry, LCI2.5, and VDP) being poorly associated with 1 year outcomes. Associations of outcomes with UTE MRI scoring was also affected by modulator therapy, but since different types of abnormalities could be discriminated, we could observe that the more-permanent abnormalities were correlated for those who did initiate modulator therapy.
Another limitation of this study is the small number of pulmonary exacerbations in this cohort, likely due to the mild lung disease present in the pediatric CF population. While this increases the type II errors, the goal was to identify the most strongly correlated relationships between these measures. Despite this, statistically significant correlations were found but many more correlations likely exist. Future studies should consider including larger cohorts for an increased number of exacerbations or more sensitive outcome measures for improved sensitivity to smaller effects missed in this lower-powered study. Lastly, one of the modulator subjects who experienced a pulmonary exacerbation in the year following did not start their modulator until 286 days post-assessment (the last subject to initiate modulator therapy) and the exacerbation occurred before the start of the modulator therapy. However, adjusting for this does not change any conclusions within this study.
Our study suggests that LCI2.5 and VDP may provide clinicians with earlier indicators of lung abnormalities. Furthermore, the association of mucus plugging with more permanent bronchial abnormalities suggests clinical interventions to prevent mucus plugs may prevent progression of lung disease. As highly effective modulators become available to the broader CF population and approved for younger patients, we anticipate these sensitive tests will be important clinical tools to screen for pulmonary abnormalities and/or monitor lung function.
Conclusions:
LCI2.5 from MBW and VDP from 129Xe MRI were more sensitive to mild CF lung disease than spirometry—similar to that shown by others using 3He MRI [20]. Reader scores of structural UTE MRI demonstrated the presence of mucus plugs in mild disease that correlated with structural bronchial changes (and irreversible lung-function declines). The significant correlations for MBW and 129Xe MRI with mucus plugging—more common in earlier CF lung disease—are probable reasons for increased sensitivity to mild lung function decline. More permanent abnormalities (bronchiectasis and bronchial wall thickening) are associated with exacerbations for those who began modulator therapy. These findings suggest functional imaging could be used for screening, due to its sensitivity, and structural imaging can also be used in determination of interventions/treatments. Thus, the use of tomographic imaging to visualize both structural (UTE MRI or CT) and functional (129Xe MRI) abnormalities will likely benefit future studies/clinical decisions in the coming age of milder lung disease in cystic fibrosis.
Supplementary Material
Highlights:
LCI2.5, 129Xe VDP, and UTE more sensitive to pediatric CF disease than spirometry
1H MRI suggests increased odds for airway damage in lobes with mucus plugging
Most spirometry measures correlated with consolidations and ground glass
LCI2.5 and 129Xe VDP correlated with mucus plugging
Increased exacerbations most strongly correlated with mucus plugs and airway damage
Acknowledgments:
Funding: This work was supported by the National Institutes of Health [grant numbers R01 HL131012, R01 HL143011, R44 HL123299, K99 HL138255, and T32 HL007752]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
J. Woods: grants from Vertex Pharmaceuticals, personal fees from Polarean, Inc, outside the submitted work. M. Willmering, D. Roach, E. Kramer, L. Walkup, and Z. Cleveland have no conflicts of interest to report.
Declarations of interest: none
References:
- [1].Calder AD, Bush A, Brody AS, Owens CM. Scoring of chest CT in children with cystic fibrosis: state of the art. Pediatr Radiol 2014;44:1496–506. 10.1007/s00247-013-2867-y. [DOI] [PubMed] [Google Scholar]
- [2].Ronan NJ, Einarsson GG, Twomey M, Mooney D, Mullane D, NiChroinin M, et al. CORK Study in Cystic Fibrosis: Sustained Improvements in Ultra-Low-Dose Chest CT Scores After CFTR Modulation With Ivacaftor. Chest 2018;153:395–403. 10.1016/j.chest.2017.10.005. [DOI] [PubMed] [Google Scholar]
- [3].Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G, et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res 2019;5:00082–2019. 10.1183/23120541.00082-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW. High-resolution computed tomography in young patients with cystic fibrosis: Distribution of abnormalities and correlation with pulmonary function tests. J Pediatr 2004;145:32–8. 10.1016/j.jpeds.2004.02.038. [DOI] [PubMed] [Google Scholar]
- [5].Helbich TH, Heinz-Peer G, Eichler I, Wunderbaldinger P, Götz M, Wojnarowski C, et al. Cystic Fibrosis: CT Assessment of Lung Involvement in Children and Adults. Radiology 1999;213:537–44. 10.1148/radiology.213.2.r99nv04537. [DOI] [PubMed] [Google Scholar]
- [6].Rosenow T, Oudraad MCJ, Murray CP, Turkovic L, Kuo W, de Bruijne M, et al. PRAGMA-CF. A Quantitative Structural Lung Disease Computed Tomography Outcome in Young Children with Cystic Fibrosis. Am J Respir Crit Care Med 2015;191:1158–65. 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- [7].Eichinger M, Optazaite D-E, Kopp-Schneider A, Hintze C, Biederer J, Niemann A, et al. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. Eur J Radiol 2012;81:1321–9. 10.1016/j.ejrad.2011.02.045. [DOI] [PubMed] [Google Scholar]
- [8].Roach DJ, Crémillieux Y, Fleck RJ, Brody AS, Serai SD, Szczesniak RD, et al. Ultrashort Echo-Time Magnetic Resonance Imaging Is a Sensitive Method for the Evaluation of Early Cystic Fibrosis Lung Disease. Ann Am Thorac Soc 2016;13:1923–31. 10.1513/AnnalsATS.201603-203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dournes G, Yazbek J, Benhassen W, Benlala I, Blanchard E, Truchetet ME, et al. 3D ultrashort echo time MRI of the lung using stack-of-spirals and spherical k-Space coverages: Evaluation in healthy volunteers and parenchymal diseases. J Magn Reson Imaging 2018;48:1489–97. 10.1002/jmri.26212. [DOI] [PubMed] [Google Scholar]
- [10].Dournes G, Menut F, Macey J, Fayon M, Chateil J, Salel M, et al. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol 2016;26:3811–20. 10.1007/s00330-016-4218-5. [DOI] [PubMed] [Google Scholar]
- [11].Brody AS, Sucharew H, Campbell JD, Millard SP, Molina PL, Klein JS, et al. Computed tomography correlates with pulmonary exacerbations in children with cystic fibrosis. Am J Respir Crit Care Med 2005;172:1128–32. 10.1164/rccm.200407-989OC. [DOI] [PubMed] [Google Scholar]
- [12].Sanders DB, Li Z, Brody AS. Chest computed tomography predicts the frequency of pulmonary exacerbations in children with cystic fibrosis. Ann Am Thorac Soc 2015;12:64–9. 10.1513/AnnalsATS.201407-338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schaefer JF, Hector A, Schmidt K, Teufel M, Fleischer S, Graepler-Mainka U, et al. A semiquantitative MRI-Score can predict loss of lung function in patients with cystic fibrosis: Preliminary results. Eur Radiol 2018;28:74–84. 10.1007/s00330-017-4870-4. [DOI] [PubMed] [Google Scholar]
- [14].Subbarao P, Milla C, Aurora P, Davies JC, Davis SD, Hall GL, et al. Multiple-Breath Washout as a Lung Function Test in Cystic Fibrosis. A Cystic Fibrosis Foundation Workshop Report. Ann Am Thorac Soc 2015;12:932–9. 10.1513/AnnalsATS.201501-021FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Couch MJ, Thomen R, Kanhere N, Hu R, Ratjen F, Woods J, et al. A two-center analysis of hyperpolarized 129Xe lung MRI in stable pediatric cystic fibrosis: Potential as a biomarker for multi-site trials. J Cyst Fibros 2019;18:728–33. 10.1016/j.jcf.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Amin R, Stanojevic S, Kane M, Webster H, Ratjen F. A randomized controlled trial to evaluate the lung clearance index as an outcome measure for early phase studies in patients with cystic fibrosis. Respir Med 2016;112:59–64. 10.1016/j.rmed.2016.01.020. [DOI] [PubMed] [Google Scholar]
- [17].Subbarao P, Stanojevic S, Brown M, Jensen R, Rosenfeld M, Davis S, et al. Lung Clearance Index as an Outcome Measure for Clinical Trials in Young Children with Cystic Fibrosis. A Pilot Study Using Inhaled Hypertonic Saline. Am J Respir Crit Care Med 2013;188:456–60. 10.1164/rccm.201302-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Svenningsen S, Kirby M, Starr D, Leary D, Wheatley A, Maksym GN, et al. Hyperpolarized 3He and 129Xe MRI: Differences in asthma before bronchodilation. J Magn Reson Imaging 2013;38:1521–30. 10.1002/jmri.24111. [DOI] [PubMed] [Google Scholar]
- [19].Thomen RP, Sheshadri A, Quirk JD, Kozlowski J, Ellison HD, Szczesniak RD, et al. Regional Ventilation Changes in Severe Asthma after Bronchial Thermoplasty with 3He MR Imaging and CT. Radiology 2015;274:250–9. 10.1148/radiol.14140080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marshall H, Horsley A, Taylor CJ, Smith L, Hughes D, Horn FC, et al. Detection of early subclinical lung disease in children with cystic fibrosis by lung ventilation imaging with hyperpolarised gas MRI. Thorax 2017;72. 10.1136/thoraxjnl-2016-208948. [DOI] [PubMed] [Google Scholar]
- [21].Rayment JH, Couch MJ, McDonald N, Kanhere N, Manson D, Santyr G, et al. Hyperpolarised 129 Xe magnetic resonance imaging to monitor treatment response in children with cystic fibrosis. Eur Respir J 2019;53:1802188. 10.1183/13993003.02188-2018. [DOI] [PubMed] [Google Scholar]
- [22].Thomen RP, Walkup LL, Roach DJ, Higano N, Schapiro A, Brody A, et al. Regional Structure-function in Cystic Fibrosis Lung Disease Using Hyperpolarized 129Xe and Ultrashort Echo Magnetic Resonance Imaging. Am J Respir Crit Care Med 2020:rccm.202001–0031LE. 10.1164/rccm.202001-0031LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–38. 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- [24].Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J 2013;41:507–22. 10.1183/09031936.00069712. [DOI] [PubMed] [Google Scholar]
- [25].Loew W, Thomen RP, Giaquinto R, Pratt R, Cleveland ZI, Walkup LL, et al. A dual loop T/R- xenon coil for homogenous excitation with improved comfort and size. Proc. 24th Annu. Meet ISMRM, Singapore, 2016. [Google Scholar]
- [26].Stocks J, Quanjer PH. Reference values for residual volume, functional residual capacity and total lung capacity: ATS Workshop on Lung Volume Measurements Official Statement of the European Respiratory Society. Eur Respir J 1995;8:492–506. 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- [27].He M, Driehuys B, Que LG, Huang YCT. Using Hyperpolarized 129Xe MRI to Quantify the Pulmonary Ventilation Distribution. Acad Radiol 2016;23:1521–31. 10.1016/j.acra.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Anagnostopoulou P, Latzin P, Jensen R, Stahl M, Harper A, Yammine S, et al. Normative data for multiple breath washout outcomes in school-aged Caucasian children. Eur Respir J 2020;55. 10.1183/13993003.01302-2019. [DOI] [PubMed] [Google Scholar]
- [29].Li Z, Sanders DB, Rock MJ, Kosorok MR, Collins J, Green CG, et al. Regional differences in the evolution of lung disease in children with cystic fibrosis. Pediatr Pulmonol 2012;47:635–40. 10.1002/ppul.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].De Jong PA, Tiddens HAWM. Cystic fibrosis-specific computed tomography scoring. Proc Am Thorac Soc 2007;4:338–42. 10.1513/pats.200611-175HT. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


