Abstract
The kappa opioid receptor (KOR)-related ligands have been demonstrated in preclinical studies for several therapeutic potentials. This chapter highlights (1) how non-human primates (NHP) studies facilitate the research and development of ligands targeting the KOR, (2) effects of the endogenous opioid peptide, dynorphin A-(1–17), and its analogs in NHP, and (3) pleiotropic effects and therapeutic applications of KOR-related ligands. In particular, synthetic ligands targeting the KOR have been extensively studied in NHP in three therapeutic areas, i.e., the treatment for itch, pain, and substance use disorders. As the KORs are widely expressed in the peripheral and central nervous systems, pleiotropic effects of KOR-related ligands, such as discriminative stimulus effects, neuroendocrine effects (e.g., prolactin release and stimulation of hypothalamic-pituitary-adrenal axis), and diuresis, in NHP are discussed. Centrally acting KOR agonists are known to produce adverse effects including dysphoria, hallucination, and sedation. Nonetheless, with strategic advances in medicinal chemistry, three classes of KOR-related agonists, i.e., peripherally restricted KOR agonists, mixed KOR/mu opioid receptor partial agonists, and G protein-biased KOR agonists, warrant additional NHP studies to improve our understanding of their functional efficacy, selectivity, and tolerability. Pharmacological studies in NHP which carry high translational significance will facilitate future development of KOR-based medications.
Keywords: Analgesics, Antipruritics, Drug abuse, Itch, Kappa opioid receptor, Macaque, Mu opioid receptor, Neuroendocrine function, Opioids, Pain, Spinal cord
1. The Dynorphin-Kappa Opioid Receptor System
In 1975, Avram Goldstein and his colleagues isolated and purified an endogenous opioid peptide named dynorphin A, a 17 amino acid polypeptide (Cox et al. 1975; Goldstein et al. 1981; Teschemacher et al. 1975). This peptide was described as “extraordinarily potent” (“dyn” from the Greek, dynamis (power) and “orphin” for endogenous morphine peptide) (Goldstein et al. 1981). Almost two decades later, the cognate receptor for this peptide family, i.e., kappa opioid receptor (KOR) (Cox et al. 2015), was cloned by different groups of investigators from rodents and humans (Meng et al. 1993; Yasuda et al. 1993; Zhu et al. 1995). Similar to the mu opioid receptor (MOR), the KOR is coupled to pertussis toxin-sensitive Gi/o proteins which inhibit adenylate cyclase and modulate the conductance of voltage-gated calcium channels and inward rectifying potassium channels (Bruchas and Chavkin 2010; Meng et al. 1993; Yasuda et al. 1993).
In the past few years, Positron Emission Tomography (PET) radiotracers for the KOR have been developed (Kim et al. 2013; Li et al. 2019). Although these newly developed KOR agonist and antagonist tracers do not have ideal selectivity at KOR over MOR and displayed binding discrepancies (Placzek et al. 2019), radiotracers for PET imaging of the KOR are valuable tools to investigate the functional roles of KOR and endogenous dynorphins in humans under different disease states, such as mood disorders and substance abuse disorders (de Laat et al. 2020). The dynorphin-KOR system has been extensively studied in the past four decades. Several articles have provided a comprehensive overview about the biological actions, medicinal chemistry, pharmacology, and therapeutic applications of this ligand-receptor system (Butelman and Kreek 2015; Chavkin and Koob 2016; Cunningham et al. 2011; Tejeda and Bonci 2019). Given a similar distribution of the KOR between human and NHP central nervous system (Peckys and Landwehrmeyer 1999; Sim-Selley et al. 1999; Simonin et al. 1995), this review highlights the functional profiles of KOR-related ligands in non-human primates (NHP). In particular, we discuss the therapeutic potential of KOR-related ligands based on findings from NHP studies which may facilitate the development of KOR-targeted ligands for different therapeutic applications.
2. Effects of Dynorphins in Non-human Primates
Dynorphins in different chain lengths have been administered through different delivery routes to characterize their functional roles in NHP. Following intravenous administration, dynorphin A-(1–17) decreased food-maintained operant behavior which was not mediated by KOR. Unlike the prototypical KOR agonist U69,593 producing antinociception, systemic dynorphin A-(1–17) produced mild antinociceptive effects (Butelman et al. 1999c). Nonetheless, both dynorphin A-(1–17) and U69,593 increased serum levels of prolactin and such neuroendocrine effects were antagonized by an opioid antagonist quadazocine (Butelman et al. 1999c). These findings suggest that systemic dynorphin A-(1–17) produced both opioid and non-opioid effects in NHP. On the other hand, dynorphin A-(1–17) coadministered with capsaicin into the tail of the monkey produced peripheral antiallodynic effects, which could be blocked by a KOR antagonist (Ko et al. 2000). This early study provides the first functional evidence that activation of peripheral KORs in primates could be a viable therapeutic target for alleviating peripherally elicited pain. Indeed, KORs are present in rodent and human dorsal root ganglion (DRG) neurons and dynorphin A-(1–17) suppressed evoked Ca2+ transient in human DRG neurons (Ji et al. 1995; Moy et al. 2020; Snyder et al. 2018).
Unlike β-endorphin, intrathecal administration of dynorphin A-(1–17) did not produce antihyperalgesic effects in NHP. Nevertheless, dynorphin A-(1–17) dose-dependently attenuated robust itch scratching responses elicited by intrathecal β-endorphin and gastrin-releasing peptide (Lee and Ko 2015). The inhibitory effect of dynorphin A-(1–17) on centrally elicited scratching supports the notion that patients suffering from chronic itch may have a decreased activity of the endogenous dynorphin-KOR system (Inan and Cowan 2005; Kardon et al. 2014). Importantly, a recent study demonstrates that plasma dynorphin A-(1–17) level inversely correlated with the pruritus severity in patients with chronic liver disease (Moniaga et al. 2019). These findings document a pivotal role of the dynorphin-KOR system in regulating itch sensation.
As noted, the effects of dynorphin A-(1–13) were also studied, but not as extensively as dynorphin A-(1–17). Intravenous administration of dynorphin A-(1–13) produced antinociception which was due to its partial KOR agonist activities (Butelman et al. 1995). Subcutaneous administration of dynorphin A-(1–13) with local capsaicin injection in the NHP tail also produced antiallodynic effects (Ko et al. 1999a). In addition, a synthetic dynorphin A-(1–8) analog, E-2078, was found to be stable without biotransformation products in human and NHP blood samples (Yu et al. 1997). Subcutaneous or intramuscular administration of E-2078 did not produce antinociception in NHP. Following intravenous administration, E-2078 produced non-KOR-mediated antinociceptive effects (Butelman et al. 1999d). Interestingly, E-2078 produced other KOR-mediated effects, including diuresis, sedation, KOR agonist-like discriminative effects, and increased serum prolactin levels (Butelman et al. 2004, 1999b). Overall, dynorphin A-(1–17) and E-2078 did not produce equivalent antinociceptive effects to non-peptidic KOR agonists, except with local administration peripherally. Thus, although dynorphin A-(1–17) and E-2078 produced other KOR-mediated effects, their functional profiles are not identical to prototypical, synthetic KOR agonists, U50,488 and U69,593, across different outcome measures in NHP. Future studies are warranted to determine whether metabolism (e.g., dynorphin A-(2–17)), site of action, degree of CNS exposure, and other mechanisms (e.g., N-methyl-D-aspartate receptor) contribute to non-opioid effects of dynorphin A-(1–17) and E-2078.
Mounting evidence indicates that the dynorphin-KOR system is involved in negative affect derived from pain, drug abuse, and neuropsychiatric disorders (Koob and Volkow 2016; Liu et al. 2019; Massaly et al. 2019; Tejeda and Bonci 2019). It is important to investigate how elevated dynorphin level in the supraspinal regions modulates behavior and mood in humans and NHP. With the advance of surgical techniques, an intrathecal catheter can be implanted and placed in the cisterna magna of NHP for supraspinal drug delivery (Ding et al. 2015). The intracisternal administration of neuropeptides mimics the “volume transmission” of endogenous peptides transported to multiple sites in the brain (Veening et al. 2012). Future NHP studies with intracisternal administration of dynorphin A-(1–17) may improve our understanding of the supraspinal dynorphin-KOR system and the functional efficacy of KOR antagonists for modulating dynorphin-mediated effects in primates under different states.
To our knowledge, synthetic ligands targeting the KOR have been extensively studied in NHP as potential treatments in three therapeutic areas, i.e., for (1) itch (pruritus), (2) pain, and (3) substance use disorders. As the KOR is widely expressed in the peripheral and central nervous systems of humans and NHP (Peckys and Landwehrmeyer 1999; Sim-Selley et al. 1999; Simonin et al. 1995), we also highlight the pleiotropic effects of KOR-related ligands in NHP.
3. Kappa Opioid Receptor Agonists as Antipruritics
3.1. Systemic Effects
The antipruritic effect of KOR agonists was first reported by Alan Cowan in the mid-1980s (Cowan and Gmerek 1986). The KOR seems a prominent therapeutic target for inhibiting itch because a series of rodent studies demonstrate that systemic administration of KOR agonists attenuated pruritogen-elicited scratching behavior (Cowan et al. 2015; Cowan and Ko 2020; Gmerek and Cowan 1984). That this response was consistent across chemical series confirmed that it was a property of KOR agonists and not compound specific.
One key relevant finding was that scratching behavior was a prominent withdrawal sign in NHP treated chronically with and withdrawn from a selective KOR agonist, U50,488 (Gmerek et al. 1987). Many withdrawal symptoms from opioids appear to be opposite to the acute effects of agonist administration (Ding et al. 2016; Ko et al. 2006; Martin and Eades 1964). Excessive scratching activity observed in NHP during withdrawal from the KOR agonist treatment suggests that acute administration of KOR agonists might have antipruritic effects. The first NHP study seemed to support this notion, as systemic administration of U50,488 dose-dependently prevented or attenuated morphine-induced scratching without inducing sedation (Ko et al. 2003b). Other NHP studies further demonstrated that non-antinociceptive doses of KOR agonists, such as nalfurafine, bremazocine, and GR89,696, attenuated intrathecal morphine-induced scratching without interfering with antinociception, supporting the potential clinical use of KOR agonists as antipruritics in the context of spinal opioid analgesia (Ko and Husbands 2009; Wakasa et al. 2004). Interestingly, systemic butorphanol, an opioid partial agonist, effectively blocked morphine-induced itch while maintaining morphine analgesia, through both MOR and KOR partial agonist actions (Lee et al. 2007). These findings encourage the development of opioid partial agonists with dual actions at both MOR and KOR as analgesics with fewer side effects.
More importantly, these animal studies led to a successful clinical trial of nalfurafine in hemodialysis patients suffering from uremic pruritus (Wikstrom et al. 2005). In 2009, nalfurafine was approved for clinical use as an antipruritic in Japan (Kumagai et al. 2012, 2010). However, antipruritic efficacy of a KOR agonist may be compromised by its narrow therapeutic window following systemic administration, i.e., its therapeutic effect could be associated with supraspinal KOR-mediated adverse effects, such as dysphoria and sedation (Butelman et al. 2001; Ko et al. 1999b; Pfeiffer et al. 1986). Recently, a G protein-biased KOR agonist, triazole 1.1, has been demonstrated to suppress scratching behavior without causing sedation and dysphoria in mice (Brust et al. 2016). Systemic triazole 1.1 at a single dose partially attenuated oxycodone-induced scratching without producing sedation and motor-impairing effects in NHP (Huskinson et al. 2020). It is important to have a side-by-side comparison with nalfurafine over a wide dose range to determine to what degree the therapeutic window of triazole 1.1 is wider than nalfurafine across different KOR-mediated effects in NHP.
Another viable strategy is to develop peripherally acting KOR agonists (Cowan et al. 2015). Although such agonists have not been studied in NHP models of itch, a recently completed phase 3 trial report showed that treatment with intravenous CR845 (difelikefalin), a peripherally restricted KOR agonist, for 12 weeks resulted in a marked and rapid reduction in itch intensity and improved itch-related quality of life in hemodialysis patients with chronic kidney disease-associated pruritus, compared with placebo treatment (Fishbane et al. 2020). These treatment outcomes are very encouraging as there were no adverse events of dysphoria and hallucination reported in the difelikefalin group. It is crucial for NHP experiments to investigate and compare the functional efficacy and side-effect profiles of nalfurafine, triazole 1.1, and difelikefalin against peripherally versus centrally elicited itch in a broader context as general antipruritics.
3.2. Intrathecal Effects
Given that the spinal delivery of drugs could minimize the degree of supraspinal KOR-mediated adverse effects, intrathecal administration of KOR-related agonists may change the therapeutic window. Recent studies demonstrate that a subpopulation of spinal interneurons expressing dynorphin A tonically inhibits itch in mice (Kardon et al. 2014). However, intrathecal administration of dynorphin A only partially attenuated scratching activities elicited by intrathecal β-endorphin (Lee and Ko 2015), indicating that there are other ligand-receptor systems in the spinal cord regulates MOR-mediated itch. In general, both NHP and human studies support that mixed MOR/KOR partial agonists are effective in ameliorating spinal opioid-induced itch while maintaining spinal opioid-induced analgesia (Ko 2015).
Despite these exciting findings, the functional efficacy, selectivity, and tolerability of KOR-related agonists as spinal antipruritics in NHP remain unknown. MOR antagonists and gastrin-releasing peptide receptor antagonists are selective antipruritics because both classes of drugs are effective in alleviating central MOR- and gastrin-releasing peptide receptor-mediated itch, respectively (Ding et al. 2015; Lee and Ko 2015). If KOR agonists are found to be effective against peripherally and centrally elicited itch in NHP, such pharmacological evidence will facilitate the development of KOR-related ligands as spinal antipruritics and may benefit a large population of patients affected by different types of itch.
4. Kappa Opioid Receptor Agonists as Analgesics
4.1. Centrally Acting Kappa Opioid Receptor Agonists
Ample evidence indicates that KOR-related agonists exert antinociceptive and anti-hypersensitive effects in rodents against a variety of pain modalities (Vanderah 2010; Zöllner and Stein 2007). The first NHP study documents that KOR agonists such as U50,488 produced antinociceptive effects manifested by increased tail-withdrawal latencies to acute noxious stimulus, 50 °C water (Dykstra et al. 1987). However, these antinociceptive doses of KOR agonists also produced stupor and discriminative stimulus effects, indicating that observed antinociception is accompanied by KOR-mediated interoceptive effects such as dysphoria and psychotomimesis (Chavkin and Koob 2016; Clark and Abi-Dargham 2019; Pfeiffer et al. 1986). Subsequently, numerous NHP studies have reported similar findings, i.e., doses of KOR agonists alleviating acute pain also produced sedation, which were higher than doses that produced discriminative stimulus effects (Butelman et al. 2001, 1993; Ko et al. 1998; Negus et al. 2008). Although KOR agonists are relatively more potent in attenuating capsaicin-induced allodynia and carrageenan- evoked inflammatory pain than acute noxious stimulus (Butelman et al. 2003; Ko et al. 1999a; Sukhtankar et al. 2014), the antiallodynic potency of KOR agonists is similar to their potency in producing discriminative stimulus effects (Butelman et al. 2002; Dykstra et al. 1987). Future studies are warranted to investigate if G protein-biased KOR agonists could display a window between antiallodynic doses and doses eliciting negative interoceptive effects in NHP.
4.2. Peripherally Acting Kappa Opioid Receptor Agonists
The functional efficacy and side-effect profile of peripherally acting KOR agonists have been demonstrated in animal models and in clinical trials (Albert-Vartanian et al. 2016; Little 2013). The most promising peptidic KOR agonist difelikefalin, which is highly hydrophilic, limiting its ability to cross the blood-brain barrier, has shown its analgesic efficacy in hysterectomy and bunionectomy patients without sedation and hallucination. As noted, like any other KOR agonists, difelikefalin increases urine output and prolactin release in humans (Albert-Vartanian et al. 2016). To our knowledge, there is no published NHP study on difelikefalin.
ICI204,448 is the only peripherally acting KOR agonist that has been studied in NHP models. Similar to spiradoline, a centrally acting KOR agonist from the same structural family, ICI204,448 dose-dependently prolonged the food pellet retrieval latency, caused sedation, and increased prolactin levels. These findings suggest that not all the in vivo effects of systemic ICI204,448 are necessarily mediated peripherally in NHP (Butelman et al. 1999b). Nonetheless, it is pivotal to conduct NHP studies to characterize the functional efficacy of peripherally restricted KOR agonists like difelikefalin against different pain modalities and the potential therapeutic window by using well-documented central and peripheral KOR-mediated outcome measures (Butelman et al. 1999b, 2001; Ko et al. 1999b).
5. Kappa Opioid Receptor-Related Ligands for the Treatment of Substance Use Disorders
5.1. Effects of Kappa Opioid Receptor-Related Agonists
Given that KORs are expressed at numerous sites in the reward neurocircuitry and activation of KORs inhibited dopamine release in the nucleus accumbens (Darcq and Kieffer 2018; Spanagel et al. 1992) and medial prefrontal cortex (Tejeda et al. 2013), KOR agonists are expected to attenuate the rewarding and reinforcing effects of abused drugs. Indeed, across different operant schedules of reinforcement, KOR agonists reduced self-administration of drug and non-drug reinforcers in NHP (Cosgrove and Carroll 2002; Negus et al. 1997). However, food-maintained responding was usually decreased at doses that decreased drug self-administration, indicating lack of selectivity between drugs of abuse and natural rewards (Cosgrove and Carroll 2002; Mello and Negus 1998).
Both MOR and KOR are dynamically involved in drug abuse, dependence, and relapse (Darcq and Kieffer 2018; Karkhanis et al. 2017). Given that MOR and KOR agonists produce opposing effects on dopaminergic neurons (e.g., euphoria versus dysphoria) (Darcq and Kieffer 2018; Freeman et al. 2014; Negus et al. 2008), a viable strategy is to develop compounds with mixed KOR/MOR agonist activities as a treatment option for substance use disorders (Bidlack and Knapp 2013; Greedy et al. 2013). A cyclazocine analog, 8-carboxamidocyclazocine, with mixed KOR/MOR agonist actions, produced only mild sedation, but it did not show improved selectivity for inhibiting cocaine-versus food-maintained responding in NHP (Stevenson et al. 2004). However, another study reported that other mixed KOR/MOR agonists such as MCL-101 produced selective and sustained decreases in cocaine self-administration (Bowen et al. 2003). The efficacies of these mixed KOR/MOR agonists at the MOR relative to buprenorphine are not clear. Nonetheless, it is known that the mild-to-moderate reinforcing effects of buprenorphine, a low-efficacy partial MOR agonist, can be decreased by activating additional receptors such as nociceptin/orphanin FQ peptide receptors (NOR) (Ding et al. 2016). Compounds with mixed NOR/MOR low-efficacy partial agonist activities display improved side-effect profiles and effectively block drug abuse-related effects in NHP (Ding et al. 2018; Flynn et al. 2019; Kiguchi et al. 2019). In a similar approach, future studies using compounds with mixed KOR/MOR low-efficacy partial agonist activities in different ratios of efficacy at KOR versus MOR will advance our understanding of the functional role of KOR in modulating reinforcing effects of abused drugs.
Another viable strategy is to develop G protein signaling-biased KOR agonists. Effects of triazole 1.1 (Brust et al. 2016) alone and against the reinforcing effects of abused drugs have not been studied in NHP yet. As both MOR and KOR oppositely modulate dopaminergic neurons (Darcq and Kieffer 2018; Spanagel et al. 1992), it is important to know if G protein-biased MOR and KOR agonists have decreased euphoric and dysphoric effects, respectively. Two reported G protein-biased MOR agonists such as TRV130 and PZM21 produced oxycodone-like reinforcing effects in rodents and NHP (Ding et al. 2020; Zamarripa et al. 2018), indicating that biasing an agonist towards G protein signaling pathways does not change MOR-dopamine receptor-mediated interoceptive effects. As noted, the in vitro assay amplification and the degree of biased agonism may confound how investigators determine which ligands are biased enough (Gillis et al. 2020). Whether such G protein-biased signaling would change KOR-dopamine receptor-mediated interoceptive effects remains to be determined. Intriguingly, low intrinsic efficacy for G protein activation may also contribute to an improved side-effect profile of G protein-biased MOR agonists (Azevedo-Neto et al. 2020; Gillis et al. 2020). It is pivotal to further investigate the similarities and differences between triazole 1.1 and KOR agonists with partial versus full efficacy in NHP.
5.2. Effects of Kappa Opioid Receptor Antagonists
Mounting evidence shows that enhanced KOR signaling during drug dependency and withdrawal may contribute to the anhedonic component of the addiction process, indicating that KOR antagonists may show greater therapeutic effects than agonist-based treatments (Chavkin and Koob 2016; Karkhanis et al. 2017). To our knowledge, there are only two NHP studies conducted to examine the effectiveness of the KOR antagonist, nor-binaltorphimine (norBNI), against drug abuse-related effects. Acute injection of norBNI at a single dose of 3 mg/kg (30 min before the session) reduced ethanol-reinforced responding and ethanol intake (Williams and Woods 1998). However, on the next day, ethanol intake returned to levels similar to those at baseline or after saline pretreatment (Williams and Woods 1998). Given the long duration of KOR antagonism by norBNI extending to several weeks in NHP (Butelman et al. 1998; Ko et al. 1999b), its acute attenuation of ethanol intake may not be mediated via KOR blockade. The other NHP study shows that norBNI did not alter cocaine choice or extended-access cocaine intake (Hutsell et al. 2016). In the past decade, several short-acting selective KOR antagonists have been synthesized and characterized (Carroll and Carlezon Jr. 2013; Guerrero et al. 2019). Currently, there are numerous human studies initiated to investigate the therapeutic potential of KOR antagonists. A recent human study shows that an orally active KOR antagonist, CERC-501 (also called LY-2456302, JNJ-67953964, and aticaprant), did not affect cigarette craving, nicotine withdrawal, and subjective effects of smoking, indicating ineffectiveness of CERC-501 in the treatment of nicotine use disorder (Jones et al. 2020). To date, there are no positive findings from NHP or human studies regarding the functional efficacy of KOR antagonists in the context of substance use disorder-related endpoints. Nonetheless, future studies may explore the functional efficacy of newly developed KOR antagonists in NHP under different states (e.g., withdrawal and relapse) (Gerak et al. 2016; Kiguchi et al. 2020; Ko et al. 2006).
6. Pleiotropic Effects of Kappa Opioid Receptor-Related Ligands
As the KOR is widely expressed in the central and peripheral nervous systems (Ko et al. 2003a; Peckys and Landwehrmeyer 1999; Sim-Selley et al. 1999), it is not surprising that KOR agonists and antagonists produce pleiotropic effects in NHP and humans. Other than the abovementioned antipruritic and analgesic effects and as a potential treatment for substance use disorders, a few KOR-mediated effects in NHP are briefly discussed herein.
I. Discriminative Stimulus Effects
Early drug discrimination studies have provided convincing evidence that KOR and MOR possess distinct interoceptive effects (i.e., dysphoric/hallucinogenic versus euphoric subjective effects) in NHP (Dykstra et al. 1987; Herling and Woods 1981; Pfeiffer et al. 1986). Interestingly, in the drug discrimination assay, NHP trained to discriminate salvinorin A generalized to centrally acting KOR agonists and such effects were mediated by KORs, not serotonergic 5HT2 receptors (Butelman et al. 2010). Salvinorin A is unique pharmacologically and chemically as it represents the first non-nitrogenous, naturally occurring KOR-selective agonist and the only known non-alkaloidal hallucinogen (Roth et al. 2002). Salvinorin A-containing products have been widely used for non-medical purposes and its related analogs in a new scaffold may lead to future development for KOR-based pharmacotherapy (Butelman and Kreek 2015; Roach and Shenvi 2018).
II. Sedation
Early NHP studies also find that sedation is a common adverse effect associated with KOR agonists (Dykstra et al. 1987). KOR agonist-induced sedation was mediated by supraspinal KORs, as intracisternal pretreatment with a long-acting KOR antagonist norBNI fully blocked such an effect for more than 4 weeks (Ko et al. 1999b). Centrally acting KOR agonists generally produce more robust sedation than peripherally acting KOR agonists, mixed KOR/MOR partial agonists, and agonists selective for other opioid receptor subtypes (Butelman et al. 1999b; Lee et al. 2007; Podlesnik et al. 2011; Sukhtankar et al. 2014). It will be important to determine and compare the electroencephalographic profiles at analgesic doses derived from different classes of opioid analgesics in NHP and humans (Malver et al. 2014).
III. Neuroendocrine Effects
Prolactin release from the anterior pituitary is under tonic inhibition by hypothalamic dopaminergic systems and KOR agonists increase prolactin levels by suppressing these dopaminergic neurons (Durham et al. 1996; Ur et al. 1997). Importantly, NHP studies find that increased serum prolactin level is a sensitive and quantitative neuroendocrine endpoint for the apparent efficacy of KOR agonists (Butelman et al. 1999a). As its site of action may be outside of the blood- brain barrier, prolactin release could be sensitive to the action of peripherally restricted KOR agonists (Butelman et al. 1999b). Indeed, all KOR-targeted agonists (i.e., peptides, centrally penetrating, and peripherally restricted agonists) increased the serum prolactin levels in NHP and humans (Albert-Vartanian et al. 2016; Butelman et al. 2001, 2002, 2004).
KOR agonists are also known to increase adrenocorticotropic hormone (ACTH) and cortisol levels in humans (Ur et al. 1997). A selective KOR agonist, U50,488, dose-dependently stimulates ACTH and cortisol release in both male and female NHP (Pascoe et al. 2008). This study demonstrates only KOR agonists, not MOR or delta opioid receptor agonists, can stimulate the hypothalamic-pituitary-adrenal axis activity. Unexpectedly, a KOR antagonist, norBNI, caused mild-to-moderate increases in ACTH and cortisol with unknown receptor mechanisms in NHP (Williams et al. 2003). As the stimulation of hypothalamic-pituitary-adrenal axis is highly associated with stress-related disorders (Ehlert et al. 2001; Stephens and Wand 2012), future NHP and human studies are warranted to investigate the potential adverse consequences from repeated use of KOR-related ligands including both agonists and antagonists.
IV. Diuresis
Human studies have documented the diuretic effects of KOR agonists (Albert-Vartanian et al. 2016; Peters et al. 1987; Reece et al. 1994). Similarly, KOR agonists potently increased the urine output in NHP (Butelman et al. 2001, 1999d; Dykstra et al. 1987). Pretreatment with intracisternal norBNI significantly blocked KOR agonist-induced diuresis in NHP for 20 weeks, indicating central KOR-mediated diuresis (Ko et al. 2003c). Further evidence suggests the sites of KOR-mediated diuresis could be both inside and outside of the blood-brain barrier, as more peripherally restricted KOR agonists also produce diuretic effects (Albert-Vartanian et al. 2016; Butelman et al. 1999d).
V. Other Effects
KOR agonists have other therapeutic applications such as cardio-protection, anti-inflammation, neuroprotection, and potential treatment for multiple sclerosis (Beck et al. 2019). In addition, KOR antagonists have been proposed and developed as potential therapeutics for neuropsychiatric disorders such as depression and schizophrenia (Clark and Abi-Dargham 2019; Jacobson et al. 2020; Zhang et al. 2007). Although NHP researchers did not study these listed effects, such effects illustrate a vast diversity of potential therapeutics from KOR-related ligands.
7. Conclusion
Taken together, pharmacological profiles of KOR-related agonists in NHP have shown therapeutic potentials for treating itch, pain, and drug abuse. Figure 1 illustrates the functional profiles of four different classes of KOR-related ligands based on NHP and human studies. NHP models offer the most phylogenetically appropriate evaluation of opioid and non-opioid receptor functions and drug effects (Chen et al. 2013; Lin and Ko 2013; Phillips et al. 2014). We have often seen that exciting findings from rodents cannot be translated to primates. For example, a newly discovered G protein signaling-biased MOR agonist, PZM21, did not exert rewarding effects in mice (Manglik et al. 2016), while others found it to induce respiratory depression and develop tolerance to its analgesic effects (Hill et al. 2018). However, PZM21 produced oxycodone-like reinforcing effects and strength, i.e., the same degree of abuse liability, in NHP (Ding et al. 2020). With recent strategic advances in medicinal chemistry, three classes of KOR-related ligands, i.e., G protein-biased KOR agonists, mixed KOR/MOR partial agonists, and peripherally restricted KOR agonists, warrant additional NHP studies to improve our understanding of their functional efficacy, selectivity, and tolerability. Pharmacological studies in NHP will continue to provide a translational bridge and facilitate future drug development of KOR-based medications.
Fig. 1.
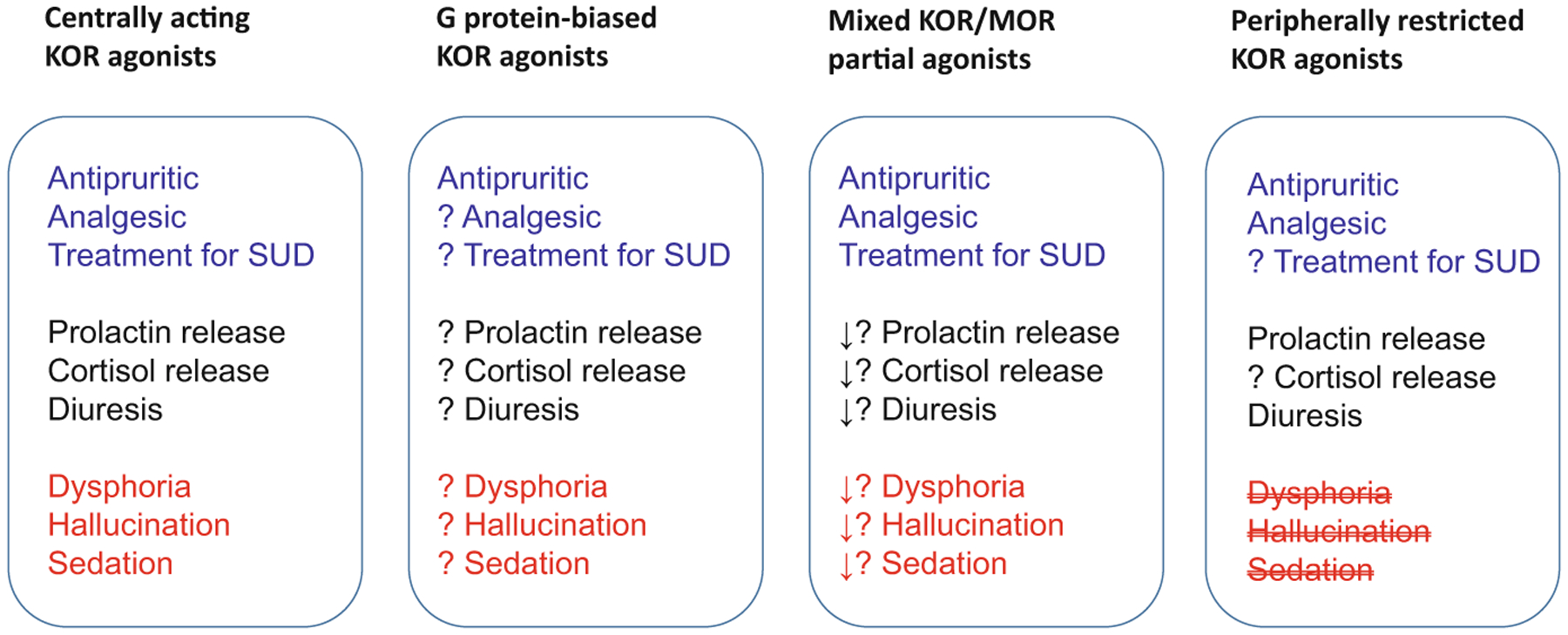
Simplified scheme to compare functional profiles of kappa opioid receptor-related ligands based on NHP and human studies. Noted, ? to be determined, ↓ decreased effect
Acknowledgments
Funding by the US National Institutes of Health, National Institute on Drug Abuse (DA023281, DA044775, DA044450, and DA049580), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR069861) is gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US federal agencies.
Footnotes
Conflict of Interest M.C.K. and S.M.H. declare that there is no conflict of interest.
Contributor Information
Mei-Chuan Ko, Department of Physiology and Pharmacology, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Stephen M. Husbands, Department of Pharmacy & Pharmacology, University of Bath, Bath, UK
References
- Albert-Vartanian A, Boyd MR, Hall AL, Morgado SJ, Nguyen E, Nguyen VP, Patel SP, Russo LJ, Shao AJ, Raffa RB (2016) Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther 41:371–382 [DOI] [PubMed] [Google Scholar]
- Azevedo-Neto J, Costanzini A, De Giorgio R, Lambert DG, Ruzza C, Calò G (2020) Biased versus partial agonism in the search for safer opioid analgesics. Molecules 25:3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck TC, Hapstack MA, Beck KR, Dix TA (2019) Therapeutic potential of kappa opioid agonists. Pharmaceuticals (Basel) 12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack JM, Knapp BI (2013) Mixed mu/kappa opioid agonists. In: Ko MC, Husbands SM (eds) Research and development of opioid-related ligands. American Chemical Society, Washington, pp 257–272 [Google Scholar]
- Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK (2003) Effects of mixed-action kappa/mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology 28:1125–1139 [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology 210:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aube J, Jones SR, Martin TJ, Bohn LM (2016) Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci Signal 9:ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Kreek MJ (2015) Salvinorin A, a kappa-opioid receptor agonist hallucinogen: pharmacology and potential template for novel pharmacotherapeutic agents in neuropsychiatric disorders. Front Pharmacol 6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH (1993) Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther 267:1269–1276 [PubMed] [Google Scholar]
- Butelman ER, France CP, Woods JH (1995) Agonist and antagonist effects of dynorphin A-(1–13) in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther 275:374–380 [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiro K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH (1998) kappa-Opioid receptor binding populations in rhesus monkey brain: relationship to an assay of thermal antinociception. J Pharmacol Exp Ther 285:595–601 [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek M (1999a) Apparent efficacy of kappa-opioid receptor ligands on serum prolactin levels in rhesus monkeys. Eur J Pharmacol 383:305–309 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ (1999b) Effects of E-2078, a stable dynorphin A(1–8) analog, on sedation and serum prolactin levels in rhesus monkeys. Psychopharmacology 147:73–80 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Perez A, Kreek MJ (1999c) Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther 290:678–686 [PubMed] [Google Scholar]
- Butelman ER, Vivian JA, Yu J, Kreek MJ, Woods JH (1999d) Systemic effects of E-2078, a stabilized dynorphin A(1–8) analog, in rhesus monkeys. Psychopharmacology 143:190–196 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ, Woods JH (2001) GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys. J Pharmacol Exp Ther 298:1049–1059 [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Kreek MJ (2002) Comparison of the discriminative and neuroendocrine effects of centrally penetrating kappa-opioid agonists in rhesus monkeys. Psychopharmacology 164:115–120 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Harris TJ, Kreek MJ (2003) Topical capsaicin-induced allodynia in unanesthetized primates: pharmacological modulation. J Pharmacol Exp Ther 306:1106–1114 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ball JW, Kreek MJ (2004) Peripheral selectivity and apparent efficacy of dynorphins: comparison to non-peptidic kappa-opioid agonists in rhesus monkeys. Psychoneuroendocrinology 29:307–326 [DOI] [PubMed] [Google Scholar]
- Butelman ER, Rus S, Prisinzano TE, Kreek MJ (2010) The discriminative effects of the kappa-opioid hallucinogen salvinorin A in nonhuman primates: dissociation from classic hallucinogen effects. Psychopharmacology 210:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Carlezon WA Jr (2013) Development of κ opioid receptor antagonists. J Med Chem 56:2178–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Koob GF (2016) Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology 41:373–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D (2013) Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 4:2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SD, Abi-Dargham A (2019) The role of dynorphin and the kappa opioid receptor in the symptomatology of schizophrenia: a review of the evidence. Biol Psychiatry 86:502–511 [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Carroll ME (2002) Effects of bremazocine on self-administration of smoked cocaine base and orally delivered ethanol, phencyclidine, saccharin, and food in rhesus monkeys: a behavioral economic analysis. J Pharmacol Exp Ther 301:993–1002 [DOI] [PubMed] [Google Scholar]
- Cowan A, Gmerek DE (1986) In-vivo studies on kappa opioid receptors. Trends Pharmacol Sci 7:69–72 [Google Scholar]
- Cowan A, Ko MC (2020) Opioid receptors in itch and pain processing. In: Yosipovitch G, Andersen HH, Arendt-Nielsen L (eds) Itch and pain: similarities, interactions, and differences. Wolters Kluwer N.V., Philadelphia, pp 203–213 [Google Scholar]
- Cowan A, Kehner GB, Inan S (2015) Targeting itch with ligands selective for κ opioid receptors. Handb Exp Pharmacol 226:291–314 [DOI] [PubMed] [Google Scholar]
- Cox BM, Opheim KE, Teschemacher H, Goldstein A (1975) A peptide-like substance from pituitary that acts like morphine. 2. Purification and properties. Life Sci 16:1777–1782 [DOI] [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR (2015) Challenges for opioid receptor nomenclature: IUPHAR review 9. Br J Pharmacol 172:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CW, Rothman RB, Prisinzano TE (2011) Neuropharmacology of the naturally occurring kappa-opioid hallucinogen salvinorin A. Pharmacol Rev 63:316–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Kieffer BL (2018) Opioid receptors: drivers to addiction? Nat Rev Neurosci 19:499–514 [DOI] [PubMed] [Google Scholar]
- de Laat B, Nabulsi N, Huang Y, O’Malley SS, Froehlich JC, Morris ED, Krishnan-Sarin S (2020) Occupancy of the kappa opioid receptor by naltrexone predicts reduction in drinking and craving. Mol Psychiatry. 10.1038/s41380-41020-40811-41388 [DOI] [PubMed] [Google Scholar]
- Ding H, Hayashida K, Suto T, Sukhtankar DD, Kimura M, Mendenhall V, Ko MC (2015) Supraspinal actions of nociceptin/orphanin FQ, morphine and substance P in regulating pain and itch in non-human primates. Br J Pharmacol 172:3302–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Czoty PW, Kiguchi N, Cami-Kobeci G, Sukhtankar DD, Nader MA, Husbands SM, Ko MC (2016) A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc Natl Acad Sci U S A 113:E5511–E5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Yasuda D, Daga PR, Polgar WE, Lu JJ, Czoty PW, Kishioka S, Zaveri NT, Ko MC (2018) A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci Transl Med 10:eaar3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Kiguchi N, Perrey DA, Nguyen T, Czoty PW, Hsu FC, Zhang Y, Ko MC (2020) Antinociceptive, reinforcing, and pruritic effects of the G-protein signalling-biased mu opioid receptor agonist PZM21 in non-human primates. Br J Anaesth 125:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham RA, Johnson JD, Moore KE, Lookingland KJ (1996) Evidence that D2 receptor-mediated activation of hypothalamic tuberoinfundibular dopaminergic neurons in the male rat occurs via inhibition of tonically active afferent dynorphinergic neurons. Brain Res 732:113–120 [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, Woods JH (1987) Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther 242:413–420 [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M (2001) Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol 57:141–152 [DOI] [PubMed] [Google Scholar]
- Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F (2020) A phase 3 trial of difelikefalin in hemodialysis patients with pruritus. N Engl J Med 382:222–232 [DOI] [PubMed] [Google Scholar]
- Flynn SM, Epperly PM, Davenport AT, Cami-Kobeci G, Husbands SM, Ko MC, Czoty PW (2019) Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys. Neuropsychopharmacology 44:1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL (2014) Assessment of the kappa opioid agonist, salvinorin A, as a punisher of drug self-administration in monkeys. Psychopharmacology 231:2751–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, France CP (2016) Effects of lorcaserin on cocaine and methamphetamine self-administration and reinstatement of responding previously maintained by cocaine in rhesus monkeys. J Pharmacol Exp Ther 359:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, Manandhar P, Santiago M, Fritzwanker S, Schmiedel F, Katte TA, Reekie T, Grimsey NL, Kassiou M, Kellam B, Krasel C, Halls ML, Connor M, Lane JR, Schulz S, Christie MJ, Canals M (2020) Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal 13:eaaz3140. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A (1984) In vivo evidence for benzomorphan-selective receptors in rats. J Pharmacol Exp Ther 230:110–115 [PubMed] [Google Scholar]
- Gmerek DE, Dykstra LA, Woods JH (1987) Kappa opioids in rhesus monkeys. III. Dependence associated with chronic administration. J Pharmacol Exp Ther 242:428–436 [PubMed] [Google Scholar]
- Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L (1981) Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci U S A 78:7219–7223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greedy BM, Bradbury F, Thomas MP, Grivas K, Cami-Kobeci G, Archambeau A, Bosse K, Clark MJ, Aceto M, Lewis JW, Traynor JR, Husbands SM (2013) Orvinols with mixed kappa/mu opioid receptor agonist activity. J Med Chem 56:3207–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero M, Urbano M, Kim EK, Gamo AM, Riley S, Abgaryan L, Leaf N, Van Orden LJ, Brown SJ, Xie JY, Porreca F, Cameron MD, Rosen H, Roberts E (2019) Design and synthesis of a novel and selective kappa opioid receptor (KOR) antagonist (BTRX-335140). J Med Chem 62:1761–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herling S, Woods JH (1981) Discriminative stimulus effects of narcotics: evidence for multiple receptor-mediated actions. Life Sci 28:1571–1584 [DOI] [PubMed] [Google Scholar]
- Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, Henderson G (2018) The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175:2653–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Platt DM, Brasfield M, Follett ME, Prisinzano TE, Blough BE, Freeman KB (2020) Quantification of observable behaviors induced by typical and atypical kappa-opioid receptor agonists in male rhesus monkeys. Psychopharmacology 237:2075–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML (2016) Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict Biol 21:360–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan S, Cowan A (2005) Reduced kappa-opioid activity in a rat model of cholestasis. Eur J Pharmacol 518:182–186 [DOI] [PubMed] [Google Scholar]
- Jacobson ML, Browne CA, Lucki I (2020) Kappa opioid receptor antagonists as potential therapeutics for stress-related disorders. Annu Rev Pharmacol Toxicol 60:615–636 [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hökfelt T (1995) Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci 15:8156–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Babalonis S, Marcus R, Vince B, Kelsh D, Lofwall MR, Fraser H, Paterson B, Martinez S, Martinez DM, Nunes EV, Walsh SL, Comer SD (2020) A randomized, double-blind, placebo-controlled study of the kappa opioid receptor antagonist, CERC-501, in a human laboratory model of smoking behavior. Addict Biol 25:e12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon AP, Polgar E, Hachisuka J, Snyder LM, Cameron D, Savage S, Cai X, Karnup S, Fan CR, Hemenway GM, Bernard CS, Schwartz ES, Nagase H, Schwarzer C, Watanabe M, Furuta T, Kaneko T, Koerber HR, Todd AJ, Ross SE (2014) Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 82:573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis A, Holleran KM, Jones SR (2017) Dynorphin/kappa opioid receptor signaling in preclinical models of alcohol, drug, and food addiction. Int Rev Neurobiol 136:53–88 [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Cami-Kobeci G, Sukhtankar DD, Czoty PW, DeLoid HB, Hsu FC, Toll L, Husbands SM, Ko MC (2019) BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br J Anaesth 122:e146–e156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Ko MC (2020) Therapeutic potentials of NOP and MOP receptor coactivation for the treatment of pain and opioid abuse. J Neurosci Res. 10.1002/jnr.24624 (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Zheng MQ, Nabulsi N, Labaree D, Ropchan J, Najafzadeh S, Carson RE, Huang Y, Morris ED (2013) Determination of the in vivo selectivity of a new kappa-opioid receptor antagonist PET tracer 11C-LY2795050 in the rhesus monkey. J Nucl Med 54:1668–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC (2015) Neuraxial opioid-induced itch and its pharmacological antagonism. Handb Exp Pharmacol 226:315–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Husbands SM (2009) Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther 328:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH (1998) Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther 285:518–526 [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH (1999a) Activation of peripheral kappa opioid receptors inhibits capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther 289:378–385 [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH (1999b) Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther 291:1113–1120 [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Willmont KJ, Burritt A, Hruby VJ, Woods JH (2000) Local inhibitory effects of dynorphin A-(1–17) on capsaicin-induced thermal allodynia in rhesus monkeys. Eur J Pharmacol 402:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Lee H, Harrison C, Clark MJ, Song HF, Naughton NN, Woods JH, Traynor JR (2003a) Studies of mu-, kappa-, and delta-opioid receptor density and G protein activation in the cortex and thalamus of monkeys. J Pharmacol Exp Ther 306:179–186 [DOI] [PubMed] [Google Scholar]
- Ko MC, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, Naughton NN (2003b) Activation of kappa-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther 305:173–179 [DOI] [PubMed] [Google Scholar]
- Ko MC, Willmont KJ, Lee H, Flory GS, Woods JH (2003c) Ultra-long antagonism of kappa opioid agonist-induced diuresis by intracisternal nor-binaltorphimine in monkeys. Brain Res 982:38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Divin MF, Lee H, Woods JH, Traynor JR (2006) Differential in vivo potencies of naltrexone and 6beta-naltrexol in the monkey. J Pharmacol Exp Ther 316:772–779 [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H (2010) Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 25:1251–1257 [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T, Suzuki H (2012) Efficacy and safety of a novel k-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol 36:175–183 [DOI] [PubMed] [Google Scholar]
- Lee H, Ko MC (2015) Distinct functions of opioid-related peptides and gastrin-releasing peptide in regulating itch and pain in the spinal cord of primates. Sci Rep 5:11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MC (2007) Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology 107:478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zheng MQ, Naganawa M, Kim S, Gao H, Kapinos M, Labaree D, Huang Y (2019) Development and in vivo evaluation of a κ-opioid receptor agonist as a PET radiotracer with superior imaging characteristics. J Nucl Med 60:1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Ko MC (2013) The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci 4:214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ (2013) Peripherally restricted opioid analgesics. In: Ko MC, Husbands SM (eds) Research and development of opioid-related ligands. American Chemical Society, Washington, pp 201–222 [Google Scholar]
- Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, Xue L, Cook C, Hakimian JK, Severino AL, Lueptow L, Komarek K, Taylor AMW, Olmstead MC, Carroll FI, Bass CE, Andrews AM, Walwyn W, Trang T, Evans CJ, Leslie FM, Cahill CM (2019) Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci 39:4162–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malver LP, Brokjaer A, Staahl C, Graversen C, Andresen T, Drewes AM (2014) Electroencephalography and analgesics. Br J Clin Pharmacol 77:72–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Eades CG (1964) A comparison between acute and chronic physical dependence in the chronic spinal dog. J Pharmacol Exp Ther 146:385–394 [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipolito L, Markovic T, Yoon HJ, Liu S, Walicki MC, Bhatti DL, Sirohi S, Klaas A, Walker BM, Neve R, Cahill CM, Shoghi KI, Gereau RWt, McCall JG, Al-Hasani R, Bruchas MR, Moron JA(2019) Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102:564–573.e566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS (1998) Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther 286:812–824 [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H (1993) Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci U S A 90:9954–9958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniaga CS, Iwamoto S, Kitamura T, Fujishiro M, Takahashi N, Kina K, Ogawa H, Tominaga M, Takamori K (2019) Plasma dynorphin a concentration reflects the degree of pruritus in chronic liver disease: a preliminary report. Acta Derm Venereol 99:442–443 [DOI] [PubMed] [Google Scholar]
- Moy JK, Hartung JE, Duque MG, Friedman R, Nagarajan V, Loeza-Alcocer E, Koerber HR, Christoph T, Schröder W, Gold MS (2020) Distribution of functional opioid receptors in human dorsal root ganglion neurons. Pain 161:1636–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE (1997) Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 282:44–55 [PubMed] [Google Scholar]
- Negus SS, Schrode K, Stevenson GW (2008) Mu/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol 16:386–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe JE, Williams KL, Mukhopadhyay P, Rice KC, Woods JH, Ko MC (2008) Effects of mu, kappa, and delta opioid receptor agonists on the function of hypothalamic-pituitary-adrenal axis in monkeys. Psychoneuroendocrinology 33:478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB (1999) Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88:1093–1135 [DOI] [PubMed] [Google Scholar]
- Peters GR, Ward NJ, Antal EG, Lai PY, deMaar EW (1987) Diuretic actions in man of a selective kappa opioid agonist: U-62,066E. J Pharmacol Exp Ther 240:128–131 [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776 [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, t Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML (2014) Why primate models matter. Am J Primatol 76:801–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek MS, Schroeder FA, Che T, Wey HY, Neelamegam R, Wang C, Roth BL, Hooker JM (2019) Discrepancies in kappa opioid agonist binding revealed through PET imaging. ACS Chem Neurosci 10:384–395 [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP, Woods JH (2011) The effects of nociceptin/orphanin FQ receptor agonist Ro 64–6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology 213:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece PA, Sedman AJ, Rose S, Wright DS, Dawkins R, Rajagopalan R (1994) Diuretic effects, pharmacokinetics, and safety of a new centrally acting kappa-opioid agonist (CI-977) in humans. J Clin Pharmacol 34:1126–1132 [DOI] [PubMed] [Google Scholar]
- Roach JJ, Shenvi RA (2018) A review of salvinorin analogs and their kappa-opioid receptor activity. Bioorg Med Chem Lett 28:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A 99:11934–11939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Gavériaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattéi MG, Charron G, Bloch B, Kieffer B (1995) kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci U S A 92:7006–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR (1999) Mu and kappa1 opioid-stimulated [35S] guanylyl-50-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience 94:651–662 [DOI] [PubMed] [Google Scholar]
- Snyder LM, Chiang MC, Loeza-Alcocer E, Omori Y, Hachisuka J, Sheahan TD, Gale JR, Adelman PC, Sypek EI, Fulton SA, Friedman RL, Wright MC, Duque MG, Lee YS, Hu Z, Huang H, Cai X, Meerschaert KA, Nagarajan V, Hirai T, Scherrer G, Kaplan DH, Porreca F, Davis BM, Gold MS, Koerber HR, Ross SE (2018) Kappa opioid receptor distribution and function in primary afferents. Neuron 99(1274–1288):e1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89:2046–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MA, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34:468–483 [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Wentland MP, Bidlack JM, Mello NK, Negus SS (2004) Effects of the mixed-action kappa/mu opioid agonist 8-carboxamidocyclazocine on cocaine- and food-maintained responding in rhesus monkeys. Eur J Pharmacol 506:133–141 [DOI] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC (2014) Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology 231:1377–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Bonci A (2019) Dynorphin/kappa-opioid receptor control of dopamine dynamics: implications for negative affective states and psychiatric disorders. Brain Res 1713:91–101 [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM, Chefer V, O’Donnell P, Shippenberg TS (2013) Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschemacher H, Opheim KE, Cox BM, Goldstein A (1975) A peptide-like substance from pituitary that acts like morphine. I. Isolation. Life Sci 16:1771–1775 [DOI] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A (1997) The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol 120:781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW (2010) Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain 26(Suppl 10):S10–S15 [DOI] [PubMed] [Google Scholar]
- Veening JG, Gerrits PO, Barendregt HP (2012) Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasa Y, Fujiwara A, Umeuchi H, Endoh T, Okano K, Tanaka T, Nagase H (2004) Inhibitory effects of TRK-820 on systemic skin scratching induced by morphine in rhesus monkeys. Life Sci 75:2947–2957 [DOI] [PubMed] [Google Scholar]
- Wikstrom B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y (2005) Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol 16:3742–3747 [DOI] [PubMed] [Google Scholar]
- Williams KL, Woods JH (1998) Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the mu-, kappa-, or delta-receptor. Alcohol Clin Exp Res 22:1634–1639 [DOI] [PubMed] [Google Scholar]
- Williams KL, Ko MC, Rice KC, Woods JH (2003) Effect of opioid receptor antagonists on hypothalamic-pituitary-adrenal activity in rhesus monkeys. Psychoneuroendocrinology 28:513–528 [DOI] [PubMed] [Google Scholar]
- Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI (1993) Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci U S A 90:6736–6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Butelman ER, Woods JH, Chait BT, Kreek MJ (1997) Dynorphin A (1–8) analog, E-2078, is stable in human and rhesus monkey blood. J Pharmacol Exp Ther 280:1147–1151 [PubMed] [Google Scholar]
- Zamarripa C, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB (2018) The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend 192:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Shi YG, Woods JH, Watson SJ, Ko MC (2007) Central kappa-opioid receptor-mediated antidepressant-like effects of nor-binaltorphimine: behavioral and BDNF mRNA expression studies. Eur J Pharmacol 570:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen C, Xue JC, Kunapuli S, DeRiel JK and Liu-Chen LY (1995) Cloning of a human kappa opioid receptor from the brain. Life Sci 56:Pl201–Pl207 [DOI] [PubMed] [Google Scholar]
- Zöllner C, Stein C (2007) Opioids. Handb Exp Pharmacol 177:31–63 [DOI] [PubMed] [Google Scholar]


