Abstract
The effects of naproxen, a non-steroidal anti-inflammatory drug (NSAID), on articular cartilage degeneration in female Sprague Dawley rats was examined. OA was induced by destabilization of the medial meniscus (DMM) in each knee. Rats were treated with acetaminophen (60mg/kg), naproxen (8mg/kg), or 1% carboxymethylcellulose (placebo) by oral gavage twice daily for three weeks, beginning 2 weeks after surgery. OA severity was assessed by histological OARSI scoring and by measuring proximal tibia cartilage depth using contrast enhanced μCT (n=6 per group) in specimens collected at 2, 5, and 7 weeks after surgery as well as on pristine knees. Medial cartilage OARSI scores from the DMM knees of naproxen-treated rats were statistically lower (i.e., better) than the medial cartilage OARSI scores from the DMM knees of placebo-treated rats at 5-weeks (8.7 ± 3.6 vs. 13.2 ± 2.4, p=0.025) and 7-weeks (9.5 ± 1.2 vs. 12.5 ± 2.5, p=0.024) after surgery. At 5 weeks after DMM surgery, medial articular cartilage depth in the proximal tibia specimens was significantly greater in the naproxen (1.78 ± 0.26 mm, p=0.005) and acetaminophen (1.94 ± 0.12 mm, p<0.001) treated rats as compared to placebo-treated rats (1.34 ± 0.24 mm). However, at 7 weeks (two weeks after drug withdrawal), medial articular cartilage depth for acetaminophen-treated rats (1.36 ± 0.29 mm) was significantly reduced compared to specimens from the naproxen-treated rats (1.88 ± 0.14mm; p=0.004). The results indicate that naproxen treatment reduced articular cartilage degradation in the rat DMM model during and after naproxen treatment.
Keywords: Osteoarthritis, knee, rat, DMM, acetaminophen, naproxen, NSAIDs
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a large class of compounds commonly used to manage inflammation, swelling, and pain associated with osteoarthritis 1. NSAIDs inhibit cyclooxygenase activity to prevent synthesis of pro-inflammatory prostaglandins and thereby reduce inflammation and pain 2; 3. Two distinct cyclooxygenase enzymes, COX-1 and COX-2, are involved in prostaglandin synthesis 4-6. Each of the many NSAIDs has different pharmacological properties and inhibits COX-1 or COX-2 to different levels 7; 8.
Whether NSAID therapy provides a direct or indirect protective effect on articular cartilage preservation in a joint compromised by acute or chronic injury remains unclear. In cultured rabbit articular chondrocytes stimulated with interleukin-1, exogenous prostaglandin E2 reduced matrix metalloproteinase-9 (MMP-9) synthesis and conversely NSAIDs treatment (diclofenac or indomethacin) increased MMP-9 synthesis, suggesting that NSAID therapy can enable articular cartilage destruction 9. In another in vitro study using articular chondrocytes harvested from pigs, prednisone (a steroid) was compared to piroxicam (a non-selective COX inhibitor), and to celecoxib (a selective COX-2 inhibitor) 10. Both prednisone and celecoxib treatment decreased matrix metalloproteinase-1 (MMP-1) expression while increasing aggrecan expression. Only celecoxib treatment increased type II collagen expression. In contrast, piroxicam treatment did not affect expression of MMP-1, aggrecan, or type II collagen.
The effects of naproxen on cartilage are poorly understood, particularly in the context of injury. Naproxen inhibits COX-1 and COX-2 with near equal efficacy 7. In an in vitro study, human articular cartilage proteoglycan content increased following 7 days of naproxen treatment 11. Similarly, long-term administration of naproxen in adult beagles reduced neutral metalloprotease, gelatinase, and collagenase activity in articular cartilage extracts consistent with reduced proteoglycan release 12. Naproxen treatment also prevented cartilage loss and bone erosion in a rat model of collagen-induced arthritis 13.
We hypothesize that naproxen treatment will prevent articular cartilage loss associated with osteoarthritis (OA) progression. To test the hypothesis, rats were treated with naproxen after acute destabilization of the knee medial meniscus (DMM) to induce OA. Acetaminophen, which is recommended for managing arthritis-associated pain, was used as a treatment control in the DMM-OA model 14. In support of the hypothesis, naproxen and acetaminophen treatment prevented articular cartilage loss following the DMM procedure. However, naproxen treatment but not acetaminophen treatment had a persistent effect on preventing articular cartilage loss for two weeks following cessation of drug treatment.
Methods
Animal Model
Forty-eight female Sprague-Dawley rats that were 105-108 days old at the time of surgery were used. All procedures complied with animal welfare guidelines and were approved by the local animal care and use committee (protocol #201800021). The rats weighed on average 254g at time of surgery and no significant changes in weight were detected between groups during the experiment. No animals were lost during the study and all animals were euthanized at their designated endpoint by an overdose of inhaled isoflurane.
Prior to the DMM surgical procedure, rats were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). Sustained release buprenorphine (Sublocade, ZooPharm, Laramie, WY), 1 mg/kg) was administered by subcutaneous injection. The surgical site was then shaved and cleansed using multiple chlorhexidine washes before coating with povidone-iodine. Surgery was initiated when rats were unresponsive to tail pinch tests. Forty-two rats underwent bilateral surgeries involving a sham surgical approach on the left knee and destabilization of the medial meniscus on the right knee 15; 16. Six rats were euthanized to obtain 12 pristine knees and 6 rats were euthanized at each time point and for each treatment group to obtain 6 sham and 6 DMM knees.
Acetaminophen and naproxen were suspended in 1% carboxymethylcellulose (CMC) before dosing. Rats were treated with placebo (1% CMC), acetaminophen (60 mg/kg), or naproxen (8 mg/kg) by oral gavage twice-a-day (morning and late afternoon) from day 15 through day 35 after DMM surgery. The naproxen and acetaminophen doses were based on prior rat studies 13; 17; 18. The dose of acetaminophen used (60 mg/kg) is the maximum, recommended daily dose of acetaminophen in humans (4,000 mg/day) and a prior study established that fracture healing was not impaired in female Sprague-Dawley rats dosed daily with either 60 or 300 mg/kg of acetaminophen 18. The dose of naproxen used (8 mg/kg) is approximately half the recommended long-term human dose of 1,000 mg/day and was effective in prior rat studies associated with arthritis 13; 17.
Six rats were euthanized 2 weeks after surgery to obtain baseline measurements of sham and DMM effects on proximal tibia articular cartilage. Six rats from each treatment group were euthanized at 5 and 7 weeks after surgery to measure drug treatment effects on proximal tibia articular cartilage and subchondral bone. The 5 and 7 week time points correspond to the end of 3 weeks of drug treatment and 2 weeks after drug treatment cessation, respectively.
Contrast-enhanced μCT analysis
Proximal tibias from the rats underwent μCT imaging. After resection, the specimens were fixed for 7 days in 10% formalin and stored in 70% ethanol at 4°C. Specimens were scanned using a Bruker Skyscan 1275 system (Bruker Corp., Billerica, MA). The μCT scan settings for unstained tibia were 70 kV, 142 μA, frame averaging of 4, rotation step of 0.4, and a 12 μm image pixel size, with a 1 mm thick aluminum filter. After the initial scan, each specimen was stained in 1% phosphotungstic acid (PTA; Sigma Aldrich Corp., St. Louis, MO) for 24 hours to visualize articular cartilage and then scanned a second time 19. For the PTA stained specimens, scan parameters were 100kV, 100 μA, frame averaging of 4, rotation step of 0.4, and a 12 μm image pixel size, with a 1 mm thick copper filter. The scan data were reconstructed using Bruker NRecon software and oriented into a standard alignment using DataViewer for later analysis.
Subchondral bone was analyzed using reconstructed images of pre-stained specimens that had been blinded for treatment. The subchondral bone volume was measured using the pre-contrast imaging data and was defined as the tissue between the articular cartilage and growth plate of the tibia. Medial subchondral bone volumes were selected from the center of each joint. Subchondral bone volumes were analyzed using CTAn (Bruker Corp., Billerica, MA) to determine the tissue volume (TV), bone volume (BV), bone volume fraction (BV/TV), and trabecular thickness (TbTh).
Reconstructed images from before (pseudo-colored red) and after 1% PTA staining (pseudo-colored green) were aligned using the Register function of AnalyzePro software (AnalyzeDirect Inc., Overland Park, KS). Co-registration of the pre- and post-staining images enabled ready visualization of the proximal tibial cartilage (Figure S-1A). Cartilage depth was measured from the apparent tidemark to the outermost edge of the PTA-stained articular cartilage at three evenly spaced locations along the medial and along the lateral aspects of the proximal tibia (Figure S-1B). Measurement locations corresponded to the zones within the medial and lateral aspects of the tibia that were evaluated for histological OARSI scoring (see below). Specimens were blinded for treatment prior to analysis.
Histology and Histomorphometry
After μCT imaging, specimens were decalcified, paraffin embedded, and sectioned in the coronal plane (5 μm thick). Histological sections were stained with safranin-o stain and fast-green 20; 21. Sections were viewed and digital images collected using an Olympus BX53 microscope and DP73 camera. Osteoarthritis severity was assessed using the Osteoarthritis Research Society International (OARSI) scoring system 15; 22. Scores ranged from 0 (normal) to 5 (vertical clefts and erosion to the calcified cartilage extending greater than 75% of the articular cartilage surface) and were summed for the three zones (0-15) of the medial and for the lateral aspects of the knee 15; 22. Cartilage area was measured using Osteomeasure software by manually tracing the remaining boundaries of the articular cartilage matrix (OsteoMetrics Inc., Decatur, GA). Osteophyte formation was also measured using the Osteomeasure software. Samples were scored from 0-4 based on osteophyte length (0 <200 μm, 1 = 200-299 μm, 2 = 300-399 μm, 3 = 400-499 μm, 4 > 500 μm) 22. The absolute lengths of osteophytes for each sample were also compared 22. Specimens were blinded for treatment prior to analysis.
Statistics
Six animals were used for each time point and treatment group based on a power analysis to detect a 50% difference in mean values at an α < 0.05 and ß > 0.8 and using mean differences in cartilage changes reported in previous rodent DMM studies 15; 16. Data were analyzed using one-way ANOVA and post-hoc Holm-Sidak corrected t-tests using Sigma Stat 4.0 (Systat Software Inc., San Jose, CA). Trabecular thickness data failed Shapiro Wilk normality test and were analyzed using a Kruskal-Wallis One Way ANOVA on RANKS with a Dunn’s multiple comparison post-hoc test. Initial statistical significance was set at p < 0.05.
Results
Thirty-six rats were necropsied to assess any drug effects on intestinal tract inflammation (12 for each treatment group, 6 for each time point). Inflamed intestine and colon were observed in 5 of 6 naproxen-treated rats at 5 weeks after surgery and within 2 hours after their final naproxen dosing. In the acetaminophen-treated rats, 1 of 6 rats had an inflamed intestinal tract at 5 weeks after surgery. In the placebo-treated rats, 1 of 6 rats had an inflamed intestinal tract at 5 weeks after surgery. At 7 weeks after DMM surgery (2 weeks after drug withdrawal), there were no signs of intestinal inflammation in any rat.
Tibias were resected, fixed, scanned by μCT, and then processed for histological examination. Representative histological images of the medial tibia plateau cartilage stained with safranin-O and fast green stained are shown in Figures 1 and S-2. Representative pre-contrast, post-contrast, and pseudo-colored combined images from the μCT analysis of 7 week post-surgery specimens are shown in Figures 3 and S-3. OARSI scores, cartilage area, cartilage depth, subchondral bone, and osteophyte measurements are summarized in Figures 2 and S-4, and Tables S-1 through S5.
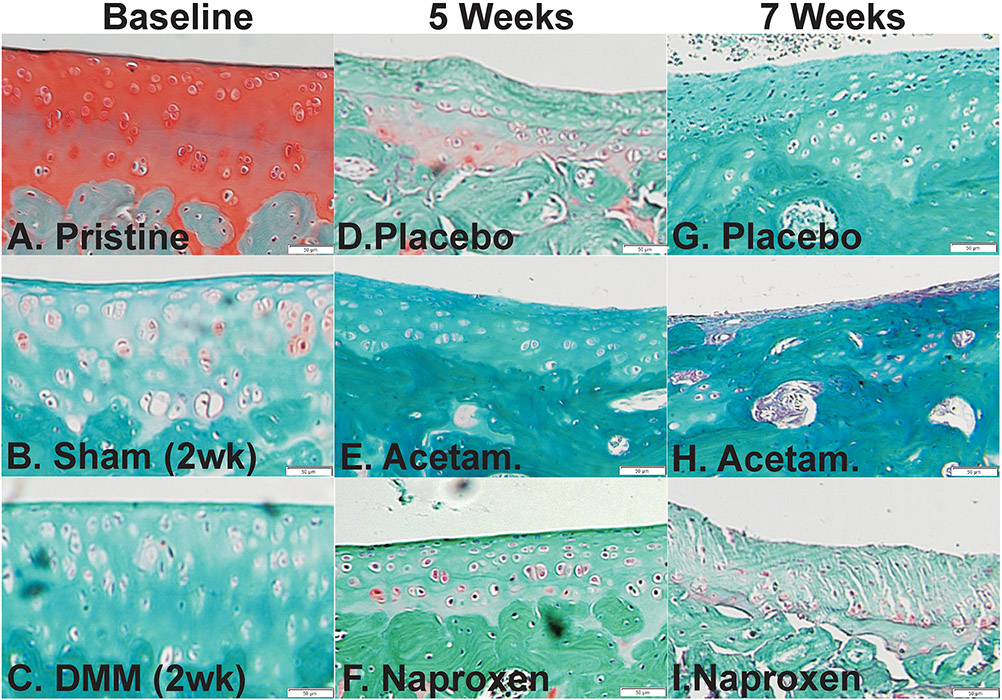
Figure 1.
Histological changes in the medial tibia cartilage following surgery and drug treatment. Shown are histology sections stained with safranin-O and fast green from pristine (A), baseline (2 weeks after surgery) sham (B), and baseline DMM (C) rat specimens. Histological sections from placebo (D, G), acetaminophen (E, H), and naproxen (F, I) treated rats are shown from specimens collected at 5 (D-F) and 7 weeks (G-I) after DMM surgery. Scale bar is 50 μm.
Figure 3.
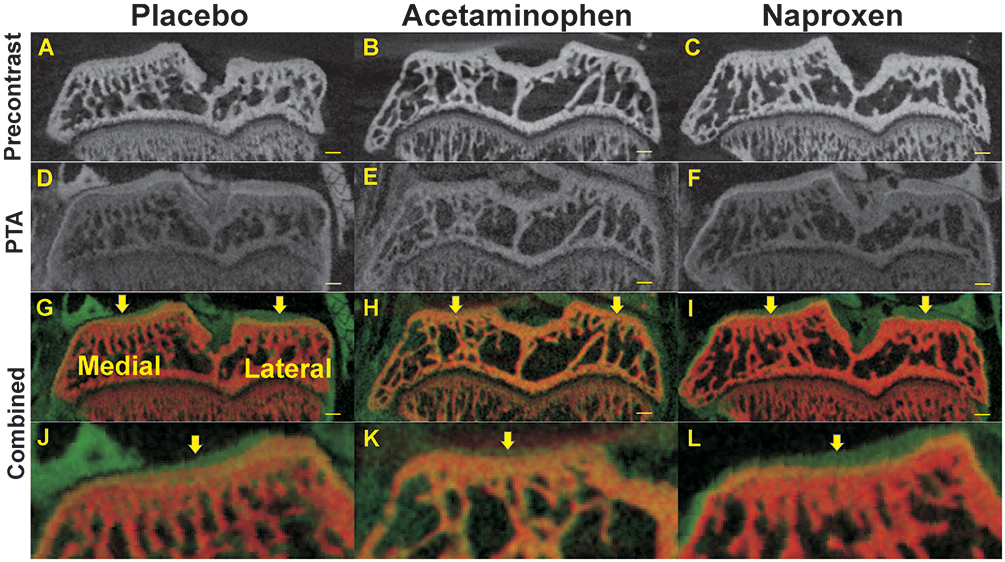
Radiographic changes in tibia plateau cartilage and subchondral bone at 7 weeks after DMM surgery. Shown are pre-contrast (A-C), PTA post-contrast (D-F), and co-registered μCT images (combined, G-I) from rats treated with placebo (A, D, G, J), acetaminophen (B, E, H, K), or naproxen (C, F, I, L). Magnified views of the medial tibia plateaus are shown (J, K, L). Arrows indicate where articular cartilage depth was measured for the medial and lateral aspects of the tibia. Scale bar is 1 mm.
Figure 2.
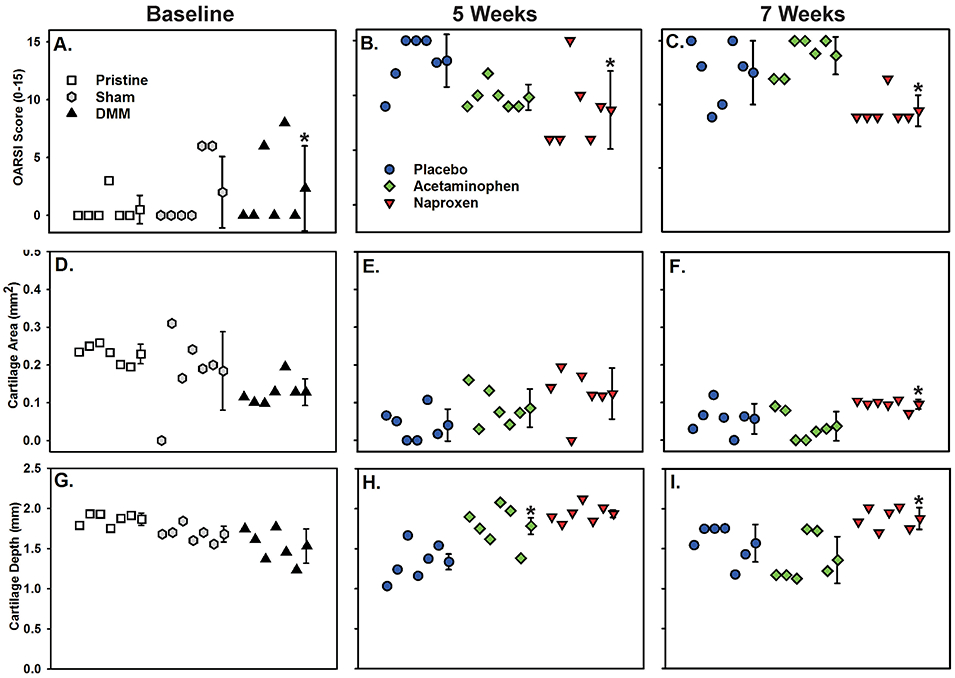
Quantitative analysis of tibia articular cartilage. Plots detail OARSI scoring, cartilage area, and cartilage depth measurements taken from the medial aspect of tibias following DMM surgery at baseline (A, D, G), 5 weeks (B, E, H), and 7 weeks (C, F, I) after surgery (n=6 per group). Mean and standard deviation are shown after individual samples for each group. The Symbol legends are shown in panels A and B. Values significantly different than the pristine (panels A) or placebo control (panels B, C, F, and H) are indicated (*). Cartilage depth was also significantly greater in the 7 week naproxen group as compared to the acetaminophen group (panel I, *). See Tables S-2 and S-3 for numerical values.
As expected, histological observation of the pristine specimens showed a low, mean OARSI score of 1.9 and the articular cartilage appeared well defined with abundant glycosaminoglycans based on the intense safranin-O staining (Figure 1A). In contrast, 2 weeks after surgeries, both the sham (Figure 1B) and DMM (Figure 1C) treated rat specimens exhibited early indications of OA including decreased safranin-O staining intensity and changes in chondrocyte morphology. At 5 weeks after surgery (3 weeks of drug treatment), placebo treated rat specimens (Figure 1D) appeared to have minimal articular cartilage consistent with development of OA. Comparatively, acetaminophen (Figure 1E) and naproxen (Figure 1F) treated rat specimens still had evident articular cartilage. At 7 weeks after surgery (2 weeks after drug withdrawal), little or no articular cartilage was evident in the placebo treated rats though some islands of apparent fibrocartilage were evident (Figure 1G). The acetaminophen (Figure 1H) treated rat specimens also exhibited indications of severe OA with the near or complete absence of articular cartilage 2 weeks after drug withdrawal. Unexpectedly, 2 weeks after drug withdrawal, articular cartilage was still evident in the naproxen (Figure 1I) treated rat specimens, though cartilage fissures were visible. Lower magnification of these images is shown in Figure S-2.
At 2 weeks after DMM or sham surgery, proximal tibia medial and lateral cartilage was compared to that from pristine knees (Figure 2, and Table S-1). The OARSI score 2 weeks after DMM surgery for the proximal tibia medial cartilage was, as expected, significantly higher than the OARSI score from pristine tibia (p<0.001). No additional significant differences were detected at 2 weeks after the DMM or sham surgery for the OARSI scores, cartilage area or cartilage depth between the medial or lateral values from the pristine, DMM, or sham specimens. Cartilage depth was also measured by comparing pre- and post-contrast μCT images of each knee (Figure S-3).
The OARSI scores, cartilage area, and cartilage depth values at 5 weeks after DMM surgery were compared (Figure 2 and Table S-2). Naproxen DMM specimens had a statistically lower medial OARSI score than the placebo DMM specimens (p=0.025). There was no statistical difference in medial OARSI scores between acetaminophen and naproxen (p=0.079) or placebo and acetaminophen (p=0.444) specimens. Medial cartilage area at 5 weeks was not significantly different between groups (p=0.056). However, medial cartilage depth was significantly greater in the naproxen and acetaminophen DMM groups as compared to the placebo DMM group (p=0.005 and p<0.001, respectively).
At 7 weeks after the DMM surgical procedures, naproxen DMM specimens had a statistically lower medial OARSI score than the placebo DMM (p=0.024 vs. placebo) and acetaminophen DMM groups (p=0.003 vs. acetaminophen, (Figure 2 and Table S-3). There was no statistical difference in medial OARSI scores between placebo DMM and acetaminophen DMM (p=0.224) samples. Medial cartilage area was significantly greater for naproxen DMM samples (p=0.024), compared to acetaminophen DMM samples (Figure 2 and Table S-3). There was no statistical difference in medial cartilage area between placebo DMM and naproxen DMM (p=0.116) or placebo DMM and acetaminophen DMM (p=0.323) samples. Cartilage depth was also measured by comparing pre- and post-contrast μCT images of each knee (Figure 3). Medial cartilage depth was significantly greater in the naproxen DMM group as compared to the acetaminophen group (p=0.004) but only approached being significantly greater than the placebo DMM group (p=0.066).
No significant differences were detected when comparing data from the proximal tibia lateral cartilage of the DMM operated knees at 5 or 7 weeks (Figure 2 and Tables S-2 and S-3). No significant differences were detected when comparing OARSI scores or cartilage area from the proximal tibia medial or lateral cartilage specimens of the sham operated knees at 5 or 7 weeks (Tables S-4 and S-5). However, medial cartilage depth in the sham surgery knees of the placebo treated rat specimens was significantly less than that from the acetaminophen or naproxen treated rat specimens at 5 weeks after surgery.
Medial subchondral bone structure was analyzed to determine whether subchondral bone structure was affected by acetaminophen or naproxen treatment. As shown in Table S-6, both acetaminophen and naproxen appeared to prevent bone loss at 5 weeks after the DMM surgery. The medial subchondral bone volume (BV) and relative bone volume (BV/TV) were significantly less in the placebo treated rat specimens than in the acetaminophen and naproxen treated rats at 5 weeks, while trabecular thickness (TbTh) was reduced in the placebo treated rat specimens as compared to the naproxen treated rat specimens. At 7 weeks after DMM and 2 weeks after cessation of acetaminophen and naproxen treatment, BV and BV/TV were significantly less in the placebo and acetaminophen treated rat specimens as compared to the naproxen treated rat specimens, though TbTh was only reduced in the placebo treated rat specimens when compared to the naproxen treated rat specimens.
Osteophyte analyses found a significant increases in scores and osteophyte length for the DMM baseline group, compared to pristine (p = 0.005) and sham controls (p = 0.005; Figure S-4).
Discussion
The results of this study indicate that naproxen treatment can slow OA progression the rat DMM OA model. Specifically, specimens from naproxen treated rats maintained medial articular cartilage depth and subchondral bone and had lower OARSI scores at 5 and 7 weeks after DMM surgery and more cartilage area at 7 weeks after DMM surgery as compared to placebo treated rat specimens (Figure 2 and Tables S-2, S-3, and S-6). Acetaminophen treatment also appeared to prevent articular cartilage loss in the rats at 5 weeks after DMM surgery (Figure 2 and Table S-2). Interestingly, the positive effects of naproxen treatment on preventing articular cartilage loss after DMM surgery persisted after naproxen treatment was withdrawn (Figure 2 and Table S-3). In sharp contrast, withdrawal of acetaminophen treatment led to rapid loss of any remaining medial articular cartilage (Figure 2 and Table S-3). This observation suggests that unlike acetaminophen, naproxen may have some disease modifying effects that persist after drug withdrawal. The pharmacokinetics of naproxen and acetaminophen may be consistent with the persistent effects on naproxen on preventing articular cartilage loss after drug withdrawal. The naproxen dose used in the present study (8 mg/kg, twice a day) should produce serum naproxen concentrations greater than 40 μg/ml, well above the 7 μg/ml IC50 needed to inhibit cyclooxygenase 7; 23. In humans, naproxen is rapidly transported into the synovial compartment from which the elimination T1/2 is over 24 hours 24. In contrast, acetaminophen is rapidly eliminated from rat plasma and human synovial fluid with a T1/2 of approximately 1 hour 25; 26.
Similar to the findings here, other studies using animal models of OA found that naproxen treatment generally restricted OA progression. In early studies, naproxen treatment reduced hind paw bone and cartilage erosion in the rat model of Freund’s adjuvant-induced arthritis 17; 27. In a rabbit model of Staphylococcus aureus induced arthritis, antibiotic treatment with naproxen reduced GAG loss by 50% as compared to antibiotic treatment alone 28. Similarly, in a destabilized canine knee model of OA, naproxen treatment reduced loss of cartilage proteoglycan and reduced MMP activity 29. In contrast, when inactivated Mycobacterium tuberculosis was directly injected in rat knees to induce arthritis, naproxen inhibited bone loss at the inflamed knee joint, but naproxen treatment caused greater GAG loss in the patellar tendon 30.
Though the primary pharmacological effect of NSAID administration is inhibition of COX-1 and COX-2 4, NSAID effects on cartilage biology appear to extend beyond inhibition of cyclooxygenase and vary from one drug to another 31. For instance, Palmonski and Brandt found that the non-selective NSAIDs indomethacin and sulindac sulfoxide had no effect on glycosaminoglycan (GAG) synthesis, while ibuprofen, fenoprofen, and salicylate inhibited (GAG) synthesis, and conversely, that benoxaprofen increased GAG synthesis in organ cultures of canine articular cartilage 32; 33. In a rat in vivo model of OA, celecoxib treatment appeared to reduce OA progression while indomethacin and ibuprofen appeared to enhance OA progression 34. Conversely, genetic ablation of COX-1 or COX-2 had no effect on OA progression in articular cartilage in mice 35. These varied findings indicate that ability of an NSAID to affect OA progression is drug specific and may be independent of the ability of each NSAID to inhibit COX-1 or COX-2.
Whether naproxen would have protective effects against OA induced cartilage loss would be difficult to predict based on cell culture studies. In micromass cultures, naproxen inhibited IL-1ß induction of MMP1, MMP13, and ADAMTS5, consistent with the protective effects of naproxen on OA related cartilage degradation noted in the present study 36. In human MSCs cultured in chondrogenic media containing insulin and TGFß3, naproxen treatment inhibited expression of the matrix degradation enzymes MMP13 and ADAMTS5 but also inhibited the expression of multiple cartilage matrix genes 37. Naproxen induced type X collagen expression in cultures of human MSCs from normal and OA donors, which if operable within the context of articular cartilage would promote articular cartilage degradation 38. Based on the results presented here and the studies noted above, the protective effects of naproxen may involve targeting processes in other tissues, such as the synovium, rather than having a direct protective effect in chondrocytes 39. A limitation of our study was that we did not investigate the effects of naproxen and other anti-inflammatory drugs on DMM-induced synovitis. Our PTA-enhanced cartilage imaging protocol precluded us from leaving the joint intact, and thus we did not address the role of naproxen on the synovium.
Acetaminophen is also the recommended therapeutic for treating OA pain and in previous clinical studies, naproxen and acetaminophen had similar efficacy in reducing pain and improving function in OA patients 40-43. The acetaminophen dose used in this study (60 mg/kg) can produce analgesia in rats since a previous study found that the same acetaminophen dose reduced bone fracture pain in a rat model 44. However, acetaminophen does not target COX-1 or COX-2 45; 46. Van der Kraan et al. found that 200 mg/kg daily acetaminophen administration reduced serum sulfate levels which decreased patellar GAG content in male Wistar rats 47. We did not quantify GAG content in our study. However, we noted near complete loss of safranin-O staining of GAG in the articular cartilage by 2 weeks after DMM surgery (Figures 1 and S-2). Neither acetaminophen nor naproxen treatment restored safranin-O staining in this study. Brandt and Albrecht noted that naproxen at the therapeutic dose (30 μg/ml) had no effect on GAG synthesis in organ cultures of canine articular cartilage, whereas Dingle et al. noted that naproxen failed to prevent IL-1α induced loss of GAG in organ cultures of porcine articular cartilage 48; 49.
The sustained effects of naproxen after treatment cessation on better preservation of articular cartilage is intriguing but the mechanism is unknown. At 5 weeks after DMM surgery, articular cartilage and subchondral bone values were similar between the naproxen and acetaminophen treatment groups (Tables S-2 and S-6). By 7 weeks after DMM surgery, articular cartilage values and subchondral bone volume for the acetaminophen treated rats were equivalent to placebo values, while the naproxen values remained significantly better (Tables S-3 and S-6). This suggests that simply preserving cartilage and bone tissues at 5 weeks was not sufficient to account for better values at 7 weeks. As noted above, chondrocyte and cartilage in vitro studies suggest that inhibited synthesis of matrix degradation enzymes could account for the sustained preservation of articular cartilage in the naproxen treated rats. Additional experiments to measure reductions in the levels of MMP and other catabolic enzymes in the naproxen treated rat knee joints would be needed to confirm this potential mechanism.
Another possibility is that preservation of subchondral bone during naproxen treatment helps delay articular cartilage erosion following cessation of naproxen treatment. The DMM model is associated with greater osteoclast activity and bone loss in mouse and rat subchondral bone, as would occur during OA pathogenesis 16; 50. Previous studies found that naproxen treatment can reduce bone loss in rabbits and ovariectomized rats 51; 52, in support of a potential mechanism for delaying articular cartilage erosion. Conversely, abnormally rapid loss of articular cartilage and subchondral bone after cessation of acetaminophen treatment may underlie the observed differences between acetaminophen and naproxen treatment withdrawal. Additional research is needed to understand the effects of naproxen, acetaminophen, and other analgesics on cartilage and bone biology.
Supplementary Material
Figure S-1. Reconstructed images from before (pseudo-colored red) and after 1% PTA staining (pseudo-colored green) were aligned using the Register function of AnalyzePro software (AnalyzeDirect Inc., Overland Park, KS). Panel A shows pre-stained (left), post-PTA stained (right), and co-registered (middle) images from the proximal tibia of a pristine control sample. Cartilage depth was measured from the apparent tidemark to the outermost edge of the PTA-stained articular cartilage at three evenly spaced locations along the medial and along the lateral aspects of the proximal tibia (B). One measurement is shown as a yellow line for the medial and lateral articular cartilage.
Figure S-3. Radiographic changes in tibia plateau cartilage and subchondral bone at baseline. Shown are pre-contrast (A-C), PTA post-contrast (D-F), and co-registered μCT images (combined, G-I) from pristine rats (A, D, G, J) and rats 2 weeks after sham (B, E, H, K) or DMM surgery (C, F, I, L). Magnified views of the medial tibia plateaus are shown (J, K, L). Arrows indicate where articular cartilage depth was measured for the medial and lateral aspects of the tibia. Scale bar is 1 mm.
Figure S-4. Plots detail osteophyte scoring and osteophyte length measurements taken from tibias following sham and DMM surgery at baseline (A, D), 5 weeks (B, E), and 7 weeks (C, F) after surgery, as well as pristine controls (n=6 per group). Mean and standard deviation are shown after individual samples for each group. Symbol legends are shown in panels A and B. The osteophyte score and mean osteophyte length for the baseline DMM group were significantly greater than the pristine and baseline sham values (*).
Figure S-2. Histological changes in the medial tibia cartilage following surgery and drug treatment. Shown are histology sections stained with safranin-O and fast green from pristine (A), baseline (2 weeks after surgery) sham (B), and baseline DMM (C) rat specimens. Histological sections from placebo (D, G), acetaminophen (E, H), and naproxen (F, I) treated rats are shown from specimens collected at 5 (D-F) and 7 weeks (G-I) after DMM surgery. Scale bar (bottom right) is 500 μm.
Acknowledgments
Funding
This study was supported by a grant from Bayer Healthcare LLC and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR069044 to JPOC.
References
- 1.Felson DT. 2006. Clinical practice. Osteoarthritis of the knee. N Engl J Med 354:841–848. [DOI] [PubMed] [Google Scholar]
- 2.Seibert K, Zhang Y, Leahy K, et al. 1994. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proceedings of the National Academy of Sciences USA 91:12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vane JR. 1971. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 231:232–235. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR, Bakhle YS, Botting RM. 1998. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120. [DOI] [PubMed] [Google Scholar]
- 5.Kujubu DA, Fletcher BS, Varnum BC, et al. 1991. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem 266:12866–12872. [PubMed] [Google Scholar]
- 6.Lysz TW, Needleman P. 1982. Evidence for two distinct forms of fatty acid cyclooxygenase in brain. J Neurochem 38:1111–1117. [DOI] [PubMed] [Google Scholar]
- 7.Warner TD, Giuliano F, Vojnovic I, et al. 1999. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proceedings of the National Academy of Sciences of the United States of America 96:7563–7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Theken KN. 2018. Variability in analgesic response to non-steroidal anti-inflammatory drugs. Prostaglandins Other Lipid Mediat 139:63–70. [DOI] [PubMed] [Google Scholar]
- 9.Ito A, Nose T, Takahashi S, et al. 1995. Cyclooxygenase inhibitors augment the production of pro-matrix metalloproteinase 9 (progelatinase B) in rabbit articular chondrocytes. FEBS Lett 360:75–79. [DOI] [PubMed] [Google Scholar]
- 10.Cho H, Walker A, Williams J, et al. 2015. Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. Biomed Res Int 2015:595273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastbergen SC, Jansen NW, Bijlsma JW, et al. 2006. Differential direct effects of cyclo-oxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study. Arthritis Res Ther 8:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratcliffe A, Azzo W, Saed-Nejad F, et al. 1993. In vivo effects of naproxen on composition, proteoglycan metabolism, and matrix metalloproteinase activities in canine articular cartilage. J Orthop Res 11:163–171. [DOI] [PubMed] [Google Scholar]
- 13.Katri A, Dabrowska A, Lofvall H, et al. 2019. Combining naproxen and a dual amylin and calcitonin receptor agonist improves pain and structural outcomes in the collagen-induced arthritis rat model. Arthritis Res Ther 21:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conaghan PG, Arden N, Avouac B, et al. 2019. Safety of Paracetamol in Osteoarthritis: What Does the Literature Say? Drugs Aging 36:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasson SS, Blanchet TJ, Morris EA. 2007. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 15:1061–1069. [DOI] [PubMed] [Google Scholar]
- 16.Iijima H, Aoyama T, Ito A, et al. 2014. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage 22:1036–1043. [DOI] [PubMed] [Google Scholar]
- 17.Martel RR, Klicius J, Metcalf G, et al. 1984. Comparative effects of long term treatment with etodolac, naproxen and ibuprofen on articular and bone changes associated with adjuvant arthritis in rats. Agents Actions 15:403–412. [DOI] [PubMed] [Google Scholar]
- 18.Bergenstock M, Min W, Simon AM, et al. 2005. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma 19:717–723. [DOI] [PubMed] [Google Scholar]
- 19.Das Neves Borges P, Forte AE, Vincent TL, et al. 2014. Rapid, automated imaging of mouse articular cartilage by microCT for early detection of osteoarthritis and finite element modelling of joint mechanics. Osteoarthritis Cartilage 22:1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg L 1971. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am 53:69–82. [PubMed] [Google Scholar]
- 21.Paglia DN, Wey A, Hreha J, et al. 2014. Local vanadium release from a calcium sulfate carrier accelerates fracture healing. J Orthop Res 32:727–734. [DOI] [PubMed] [Google Scholar]
- 22.Gerwin N, Bendele AM, Glasson S, et al. 2010. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage 18Suppl 3:S24–34. [DOI] [PubMed] [Google Scholar]
- 23.Doherty NS, Anttila M, Dean PB. 1977. Penetration of naproxen and salicylate into inflammatory exudates in the rat. Ann Rheum Dis 36:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruno R, Iliadis A, Jullien I, et al. 1988. Naproxen kinetics in synovial fluid of patients with osteoarthritis. Br J Clin Pharmacol 26:41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegers CP, Strubelt O, Schutt A. 1978. Relations between hepatotoxicity and pharmacokinetics of paracetamol in rats and mice. Pharmacology 16:273–278. [DOI] [PubMed] [Google Scholar]
- 26.Owen SG, Francis HW, Roberts MS. 1994. Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br J Clin Pharmacol 38:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackerman NR, Rooks WH 2nd, Shott L, et al. 1979. Effects of naproxen on connective tissue changes in the adjuvant arthritic rat. Arthritis Rheum 22:1365–1374. [DOI] [PubMed] [Google Scholar]
- 28.Smith RL, Kajiyama G, Schurman DJ. 1997. Staphylococcal septic arthritis: antibiotic and nonsteroidal anti-inflammatory drug treatment in a rabbit model. J Orthop Res 15:919–926. [DOI] [PubMed] [Google Scholar]
- 29.Ratcliffe A, Rosenwasser MP, Mahmud F, et al. 1993. The in vivo effects of naproxen on canine experimental osteoarthritic articular cartilage: composition, metalloproteinase activities and metabolism. Agents Actions Suppl 39:207–211. [DOI] [PubMed] [Google Scholar]
- 30.Seed MP, Gardner CR. 2005. The modulation of intra-articular inflammation, cartilage matrix and bone loss in mono-articular arthritis induced by heat-killed Mycobacterium tuberculosis. Inflammopharmacology 12:551–567. [DOI] [PubMed] [Google Scholar]
- 31.Nakata K, Hanai T, Take Y, et al. 2018. Disease-modifying effects of COX-2 selective inhibitors and non-selective NSAIDs in osteoarthritis: a systematic review. Osteoarthritis Cartilage 26:1263–1273. [DOI] [PubMed] [Google Scholar]
- 32.Palmoski MJ, Brandt KD. 1980. Effects of some nonsteroidal antiinflammatory drugs on proteoglycan metabolism and organization in canine articular cartilage. Arthritis Rheum 23:1010–1020. [DOI] [PubMed] [Google Scholar]
- 33.Brandt KD. 1987. Effects of nonsteroidal anti-inflammatory drugs on chondrocyte metabolism in vitro and in vivo. American Journal of Medicine 83:29–34. [DOI] [PubMed] [Google Scholar]
- 34.Ou Y, Tan C, An H, et al. 2012. Selective COX-2 inhibitor ameliorates osteoarthritis by repressing apoptosis of chondrocyte. Med Sci Monit 18:Br247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukai A, Kamekura S, Chikazu D, et al. 2012. Lack of a chondroprotective effect of cyclooxygenase 2 inhibition in a surgically induced model of osteoarthritis in mice. Arthritis Rheum 64:198–203. [DOI] [PubMed] [Google Scholar]
- 36.Greco KV, Iqbal AJ, Rattazzi L, et al. 2011. High density micromass cultures of a human chondrocyte cell line: a reliable assay system to reveal the modulatory functions of pharmacological agents. Biochem Pharmacol 82:1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antoniou J, Wang HT, Hadjab I, et al. 2015. The Effects of Naproxen on Chondrogenesis of Human Mesenchymal Stem Cells. Tissue Eng Part A 21:2136–2146. [DOI] [PubMed] [Google Scholar]
- 38.Almaawi A, Wang HT, Ciobanu O, et al. 2013. Effect of acetaminophen and nonsteroidal anti-inflammatory drugs on gene expression of mesenchymal stem cells. Tissue Eng Part A 19:1039–1046. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai Y, Fujita M, Kawasaki S, et al. 2019. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 160:895–907. [DOI] [PubMed] [Google Scholar]
- 40.Williams HJ, Ward JR, Egger MJ, et al. 1993. Comparison of naproxen and acetaminophen in a two-year study of treatment of osteoarthritis of the knee. Arthritis Rheum 36:1196–1206. [DOI] [PubMed] [Google Scholar]
- 41.Hochberg MC, Altman RD, April KT, et al. 2012. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 64:465–474. [DOI] [PubMed] [Google Scholar]
- 42.Golden HE, Moskowitz RW, Minic M. 2004. Analgesic efficacy and safety of nonprescription doses of naproxen sodium compared with acetaminophen in the treatment of osteoarthritis of the knee. Am J Ther 11:85–94. [DOI] [PubMed] [Google Scholar]
- 43.Temple AR, Benson GD, Zinsenheim JR, et al. 2006. Multicenter, randomized, double-blind, active-controlled, parallel-group trial of the long-term (6-12 months) safety of acetaminophen in adult patients with osteoarthritis. Clin Ther 28:222–235. [DOI] [PubMed] [Google Scholar]
- 44.Cottrell JA, Meyenhofer M, Medicherla S, et al. 2009. Analgesic effects of p38 kinase inhibitor treatment on bone fracture healing. Pain 142:116–126. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell JA, Akarasereenont P, Thiemermann C, et al. 1993. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A 90:11693–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Botting RM. 2000. Mechanism of action of acetaminophen: is there a cyclooxygenase 3? Clin Infect Dis 31Suppl 5:S202–210. [DOI] [PubMed] [Google Scholar]
- 47.van der Kraan PM, Vitters EL, de Vries BJ, et al. 1990. The effect of chronic paracetamol administration to rats on the glycosaminoglycan content of patellar cartilage. Agents Actions 29:218–223. [DOI] [PubMed] [Google Scholar]
- 48.Brandt KD, Albrecht M. 1990. Effect of naproxen sodium on the net synthesis of glycosaminoglycans and protein by normal canine articular cartilage in-vitro. J Pharm Pharmacol 42:738–740. [DOI] [PubMed] [Google Scholar]
- 49.Dingle JT, Leeming MR, Martindale JJ. 1993. Effect of tenidap on cartilage integrity in vitro. Ann Rheum Dis 52:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang H, Huang L, Welch I, et al. 2018. Early Changes of Articular Cartilage and Subchondral Bone in The DMM Mouse Model of Osteoarthritis. Sci Rep 8:2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodman S, Ma T, Trindade M, et al. 2002. COX-2 selective NSAID decreases bone ingrowth in vivo. Journal of Orthopaedic Research 20:1164–1169. [DOI] [PubMed] [Google Scholar]
- 52.Lane N, Coble T, Kimmel DB. 1990. Effect of naproxen on cancellous bone in ovariectomized rats. J Bone Miner Res 5:1029–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S-1. Reconstructed images from before (pseudo-colored red) and after 1% PTA staining (pseudo-colored green) were aligned using the Register function of AnalyzePro software (AnalyzeDirect Inc., Overland Park, KS). Panel A shows pre-stained (left), post-PTA stained (right), and co-registered (middle) images from the proximal tibia of a pristine control sample. Cartilage depth was measured from the apparent tidemark to the outermost edge of the PTA-stained articular cartilage at three evenly spaced locations along the medial and along the lateral aspects of the proximal tibia (B). One measurement is shown as a yellow line for the medial and lateral articular cartilage.
Figure S-3. Radiographic changes in tibia plateau cartilage and subchondral bone at baseline. Shown are pre-contrast (A-C), PTA post-contrast (D-F), and co-registered μCT images (combined, G-I) from pristine rats (A, D, G, J) and rats 2 weeks after sham (B, E, H, K) or DMM surgery (C, F, I, L). Magnified views of the medial tibia plateaus are shown (J, K, L). Arrows indicate where articular cartilage depth was measured for the medial and lateral aspects of the tibia. Scale bar is 1 mm.
Figure S-4. Plots detail osteophyte scoring and osteophyte length measurements taken from tibias following sham and DMM surgery at baseline (A, D), 5 weeks (B, E), and 7 weeks (C, F) after surgery, as well as pristine controls (n=6 per group). Mean and standard deviation are shown after individual samples for each group. Symbol legends are shown in panels A and B. The osteophyte score and mean osteophyte length for the baseline DMM group were significantly greater than the pristine and baseline sham values (*).
Figure S-2. Histological changes in the medial tibia cartilage following surgery and drug treatment. Shown are histology sections stained with safranin-O and fast green from pristine (A), baseline (2 weeks after surgery) sham (B), and baseline DMM (C) rat specimens. Histological sections from placebo (D, G), acetaminophen (E, H), and naproxen (F, I) treated rats are shown from specimens collected at 5 (D-F) and 7 weeks (G-I) after DMM surgery. Scale bar (bottom right) is 500 μm.