Abstract
Purpose:
To assess the association of severity of ocular discomfort with measures of quality of life among patients with moderate to severe dry eye disease (DED).
Methods:
A prospective, observational, cohort study within a randomized clinical trial. Patients (N=535) in the DREAM Study with moderate to severe DED completed the Ocular Surface Disease Index (OSDI) on DED symptoms, the SF-36 on quality of life, the Brief Ocular Discomfort (BODI) questionnaire, and had a comprehensive ophthalmic assessment by a study-certified clinician. The ocular discomfort on average over the past week was scored on an 11-point scale (0 for no discomfort, 10 for discomfort as bad as you can imagine)
Results:
Average ocular discomfort scores for patients ranged from 0 to 10 with a mean of 4.28. Discomfort scores did not vary with demographic characteristics, signs of DED, self-reported depression, or self-reported non-ocular pain conditions. Ocular discomfort scores did correlate moderately to strongly with total OSDI scores (Spearman correlation coefficient, rs, 0.47 to 0.67) and with measures of interference with activities of daily living (general activity level, mood, walking ability, ability for normal work, relations with other people, sleep, and enjoyment of life; [rs=0.39 to 0.65]).
Conclusion:
Among DREAM patients, worse ocular discomfort was associated with worse overall dry eye symptoms and interfered to a greater degree with activities of daily living. Ocular discomfort is an important part of the assessment of dry eye patients.
Keywords: Dry Eye Disease, Ocular Discomfort, Social Function, Activities of Daily Living
INTRODUCTION
Dry eye disease (DED) is one of the most common eye conditions that patients seek care for.1 A combination of symptoms and signs are used to make the clinical diagnosis. Symptoms of the disease include various types of ocular discomfort and visual disturbance, as well as conjunctival bulbar redness, heavy lid sensation, and, seemingly paradoxically to patients, tearing.2 The ocular discomfort of DED has been characterized as dryness, burning, irritation, foreign body sensation, itching, or grittiness.3
The fact that DED is the most common reason for seeking medical eye care and that expenditures in the United States for management and remedies to relieve the symptoms of DED exceed $3.84 billion per year are indirect, but compelling, evidence of the negative impact of DED on quality of life.3,4 Reports from several previous studies have established that people with DED have worse scores on a variety of health-related and vision-related quality of life measures than people with no ocular disease.3,5 Many of these studies included only patients with Sjögren’s Syndrome who may have other systemic symptoms that affect quality of life. Most studies did not explore the severity or specific aspect of DED symptomatology associated with the lower quality of life scores among DED patients.
The DREAM Study is a multicenter, double-masked, randomized clinical trial of omega-3 fatty acid supplementation versus placebo (refined olive oil) for treatment of dry eye disease. DREAM patients received a comprehensive clinical evaluation for DED and completed questionnaires addressing severity of symptoms and quality of life, including a questionnaire specifically targeting the symptom of ocular discomfort and its interference with activities of daily living.6 In this report, we use the responses to the questionnaires and data from the examinations to describe the association of ocular discomfort with severity of other symptoms of DED, severity of signs of DED, and quality of life measures.
MATERIALS AND METHODS
The design and methods of the DREAM Study have been published previously.6,7 Salient features of the study relevant to this report are included here.
Patients
Patients were recruited from 27 sites across the United States between October 2014 and July 2016. The institutional review board associated with each study center approved the protocol and consent statement prior to initiation of the study. Written informed consent was obtained from each study patient. The study was conducted in accordance with regulations of the Health Insurance Portability and Accountability Act. The study was registered with ClinicalTrials.gov (NCT02128763) and operated under an Investigational New Drug IND (106,387) application to the Food and Drug Administration.
To qualify for the study, candidates needed to have both symptoms and signs of moderate to severe dry eye disease at each of 2 clinic visits separated by approximately 2 weeks. Symptoms were measured with the Ocular Surface Disease Index (OSDI); eligibility criteria required a score between 25 and 80 on a scale of 0 to 100 on the first visit and between 21 and 80 on the second visit.8,9 Note that ocular discomfort is one of many symptoms and was not required for entry into the study. At each of the 2 visits, candidates needed to have at least 1 eye with at least 2 of the following 4 signs: conjunctival lissamine green staining score ≥1 on a scale of 0–6; corneal fluorescein staining score ≥4 on a scale of 0–15; tear film break up time ≤ 7 seconds; and Schirmer test with anesthesia measurement ≥1 to ≤ 7 mm/5min. The same qualifying signs had to be present in the same eye at both visits. Candidates were excluded if they had worn contact lenses within 30 days, had a history of laser-assisted in situ keratomileusis, ocular infection, or recent ocular surgery. Patients were assigned randomly in a 2:1 ratio to either 12 months of daily omega-3 fatty acid supplements (2 g of eicosapentaenoic acid (EPA) and 1 g of docosahexaenoic acid (DHA)) or placebo supplements (5 g of refined olive oil).
Assessments of clinical signs of dry eye disease
Clinicians who had completed a DREAM certification program examined patients at each of the two visits for determination of eligibility for the study; however, only the evaluations from the second visit are analyzed in this report. Measurement of tear beak-up time (TBUT) involved instillation of 5μl of fluorescein 2% in the inferior cul de sac of the eye, waiting 30 seconds, and viewing the cornea through a slit lamp using broad beam cobalt blue illumination and a yellow barrier filter. The examiner instructed the patient to blink and used a stopwatch to measure the time between the blink and the appearance of the first discontinuity in the tear film. TBUT was measured 3 time and averaged for each eye. Schirmer’s test involved instillation of a topical anesthetic, waiting approximately 5 minutes, hanging test strips onto the lower conjunctival sac in the temporal one-third of the lid of each eye , and instructing the patient to close both eyes. After 5 minutes, the strips were removed and the length of wetting of the strip recorded in millimeters.
Measurement of symptoms of dry eye disease and quality of life
Each patient completed the OSDI questionnaire for assessment of symptoms related to chronic dry eye during the last week, their severity, and their impact on the patient’s ability to function.8 The OSDI is a 12-item questionnaire with scores based on the frequency of experiencing symptoms, limitations on specific tasks, and experiencing problems with their eyes under specific conditions. The OSDI has 3 corresponding subscales (ocular symptoms [sensitivity to light, feeling gritty, painful or sore], vision-related function [blurred vision, poor vision, reading, driving at night, working with a computer or bank machine, watching TV], and environmental triggers [windy conditions, areas with low humidity, air conditioned areas) each with scores that range from 0 to 100, with higher scores indicating greater disability.10
The measure of the ocular discomfort score used in this report was the response the question “Please rate your ocular discomfort by circling the one number that best describes your ocular discomfort on the average in the last week.” The circled number (0 to 10) was multiplied by 10 to provide a score ranging from 0 (no discomfort) to 100 (discomfort as bad as you can imagine). This was asked as item 3 of the Brief Ocular Discomfort Inventory (BODI). The BODI is a modification of the Brief Pain Inventory (BPI) that is used widely to assess pain.11 The BPI was modified by replacing each mention of “pain” with “ocular discomfort”. Four BODI items address the intensity of ocular discomfort during the previous week (worst, least, average, and current) scored from 0 (no discomfort) to 10 (discomfort as bad as you can imagine), 7 items address interference in the past week by ocular discomfort (general activity, mood, walking ability, work, relations with other people, sleep, and enjoyment of life) scored from 0 (does not interfere) to 10 (completely interferes) The last item addresses relief from ocular treatments or medications, scored from 0% (no relief) to 100% (complete relief).
Patients also completed the SF-36 (v2) Health Survey, a 36-item on health-related quality of life. The SF-36 has two component scores (physical and mental health) and 8 subscales (physical functioning, role limitations due to physical health, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health).12 All SF-36 scores range from 0 to 100 with higher scores indicating better quality of life.
Statistical Analysis
Patients from both treatment groups were combined into 1 group because, despite the fact that symptom scores improved substantially between baseline and follow-up, there were no differences between treatment groups on the primary outcome measure (total OSDI score change from baseline) or secondary outcome measures (change in total BODI score, summary SF-36 scores, conjunctival and corneal staining, TBUT, Schirmer test).6 The mean of measurements from both eyes was used as a person-specific score for the ocular signs of conjunctival and corneal staining, TBUT, and Schirmer test.
Spearman correlation coefficients were used to determine the association between the level of ocular discomfort scores and other continuous measures of patient and ocular characteristics; t-tests or analysis of variance (ANOVA) were used for categorical measures of characteristics. The average ocular discomfort score was divided into 4 ordered categories (minimal 0–20, mild 30–40, moderate 50–60, severe 70–100) that were used for display of data; however, all tests of statistical significance used the continuous scores. Correlation coefficients are described using the terminology of Evans: 0–0.19 = very weak, 0.20–0.39 = weak, 0.40–0.59 = moderate, 0.60 – 0.79 = strong, 0.80–0.99 = very strong.13
Statistical analyses were performed using the Statistical Analysis Software (SAS version 9.4, North Carolina, USA). P values less than .05 were considered as statistically significant.
RESULTS
Patient characteristics
A detailed description of the characteristics of the 535 patients enrolled in the DREAM study has been published previously.6 In brief, the mean (standard deviation [SD]) age was 58 (13.2), 434 (81%) of patients were female, 398 (74%) were white, 64 (12%) black, and 68 (13%) Hispanic. The mean (SD) score for the OSDI was 42.1 (15.5) and for signs of DED was 2.9 (1.4) for conjunctival staining, 3.8 (2.8) for corneal staining, 3.1 (1.6) seconds for tear break-up time, and 9.6 (6.7) mm in 5 minutes for Schirmer’s test.
Distribution of Average Ocular Discomfort Score and Its Association with Systemic Factors
The distribution at baseline of the self-reported average ocular discomfort score over the last week spanned the full range of possible scores, from 0 to 100, although 502 (93.8%) of the 535 patients reported a score between 10 and 70 (Table 1). The mean (SD) of the average discomfort score at baseline was 42.8 (19.1) and decreased to 35.3 (21.3) at 6 months, and 32.8(22.3) at 12 months.
Table 1.
Distribution of self-reported average discomfort in the last week from the Brief Ocular Discomfort Index (BODI), over time
| Time | ||||
|---|---|---|---|---|
| Average Discomfort Score Category |
Score | Baseline (N=535) | 6 Months (N=481) | 12 Months (N=488) |
| Minimal | 0 | 11 (2.1%) | 39 (8.1%) | 48 (9.8%) |
| 10 | 35 (6.6%) | 48 (10.0%) | 78 (16.0%) | |
| 20 | 64 (12.0%) | 84 (17.5%) | 78 (16.0%) | |
| Mild | 30 | 65 (12.2%) | 74 (15.4%) | 70 (14.3%) |
| 40 | 91 (17.0%) | 67 (13.9%) | 66 (13.5%) | |
| Moderate | 50 | 129 (24.2%) | 87 (18.1%) | 68 (13.9%) |
| 60 | 79 (14.8%) | 40 (8.3%) | 40 (8.2%) | |
| Severe | 70 | 39 (7.3%) | 24 (5.0%) | 20 (4.1%) |
| 80 | 18 (3.4%) | 12 (2.5%) | 12 (2.5%) | |
| 90 | 1 (0.2%) | 5 (1.0%) | 5 (1.0%) | |
| 100 | 2 (0.4%) | 1 (0.2%) | 3 (0.6%) | |
| Missing | Missing | 1 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Mean (SD)a | 42.8 (19.1) | 35.3 (21.3) | 32.8 (22.3) | |
| Median (IQR)b | 50 (30,60) | 30 (20,50) | 30 (10, 50) | |
SD is standard deviation
IQR is interquartile range (25th percentile, 75th percentile)
The correlation between the average ocular discomfort experienced score and age was very weak (rs = −0.04; p=0.36) at baseline and remained very weak at 6 months (rs = 0.03; p=0.57) and 12 months (rs = 0.02; p=0.66). The mean value of the ocular discomfort score at each of the 3 time points did not differ by gender, ethnicity, race, cigarette smoking, or patient-reported history of rheumatoid arthritis, osteoarthritis, diabetes, depression, use of antidepressant medications, fibromyalgia, or Sjögren syndrome (Table 2).
Table 2.
Association of baseline characteristics with average ocular discomfort in the past week on the Brief Ocular Discomfort Index, over time
| Baseline (N=535) | 6 Months (N=481) | 12 Months (N=488) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Mean(SD) | P-value* | N | Mean(SD) | P-valuea | N | Mean(SD) | P-value* |
| Gender | |||||||||
| Male | 101 | 39.6 (20.7) | 0.06 | 90 | 35.4 (21.1) | 0.96 | 88 | 31.0 (22.6) | 0.42 |
| Female | 433 | 43.6 (18.7) | 391 | 35.3 (21.4) | 400 | 33.2 (22.3) | |||
| Ethnicity | |||||||||
| Hispanic or Latino | 68 | 41.5 (21.9) | 0.53 | 51 | 34.7 (24.6) | 0.82 | 55 | 33.5 (25.4) | 0.81 |
| Other | 466 | 43.0 (18.7) | 430 | 35.4 (20.9) | 433 | 32.7 (21.9) | |||
| Race | |||||||||
| White | 397 | 42.9 (18.7) | 0.93 | 363 | 34.6 (20.5) | 0.34 | 370 | 32.6 (21.5) | 0.86 |
| Black | 64 | 43.1 (22.1) | 58 | 38.8 (25.2) | 57 | 34.2 (24.4) | |||
| Other | 73 | 42.1 (19.1) | 60 | 36.5 (22.2) | 61 | 32.1 (25.4) | |||
| Cigarette smoking | |||||||||
| Never | 366 | 42.2 (19.2) | 0.93 | 329 | 33.7 (21.2) | 0.34 | 337 | 31.3 (21.7) | 0.86 |
| Former | 142 | 43.1 (18.5) | 133 | 37.6 (20.4) | 134 | 35.4 (23.1) | |||
| Current | 26 | 49.6 (20.9) | 19 | 47.9 (24.9) | 17 | 41.8 (26.3) | |||
| Rheumatoid arthritis | |||||||||
| No | 485 | 42.7 (19.3) | 0.75 | 439 | 35.3 (21.3) | 0.79 | 446 | 32.6 (22.5) | 0.55 |
| Yes | 49 | 43.7 (18.0) | 42 | 36.2 (21.9) | 42 | 34.8 (21.0) | |||
| Osteoarthritis | |||||||||
| No | 393 | 42.9 (19.3) | 0.84 | 349 | 35.1 (22.0) | 0.65 | 356 | 32.4 (22.7) | 0.60 |
| Yes | 141 | 42.6 (18.6) | 132 | 36.1 (19.5) | 132 | 33.6 (21.3) | |||
| Diabetes | |||||||||
| No | 472 | 43.1 (18.8) | 0.34 | 427 | 35.1 (21.1) | 0.45 | 433 | 32.5 (22.2) | 0.41 |
| Yes | 62 | 40.6 (21.8) | 54 | 37.4 (22.8) | 55 | 35.1 (23.2) | |||
| Depression | |||||||||
| No | 412 | 42.9 (19.4) | 0.94 | 369 | 34.6 (21.6) | 0.19 | 376 | 31.7 (22.7) | 0.06 |
| Yes | 122 | 42.7 (18.1) | 112 | 37.7 (20.4) | 112 | 36.3 (20.9) | |||
| Taking anti-depressants | |||||||||
| No | 405 | 42.7 (19.4) | 0.73 | 353 | 35.0 (21.9) | 0.51 | 355 | 32.2 (22.6) | 0.36 |
| Yes | 129 | 43.3 (18.4) | 128 | 36.4 (19.7) | 133 | 34.3 (21.8) | |||
| Fibromyalgia | |||||||||
| No | 522 | 42.9 (19.2) | 0.72 | 469 | 35.3 (21.4) | 0.72 | 477 | 32.7 (22.4) | 0.69 |
| Yes | 12 | 40.8 (17.3) | 12 | 37.5 (16.6) | 11 | 35.5 (19.7) | |||
| Sjögren syndrome | |||||||||
| No | 455 | 42.4 (19.3) | 0.29 | 408 | 34.9 (21.0) | 0.51 | 416 | 32.1 (22.3) | 0.37 |
| Yes | 52 | 45.4 (18.8) | 47 | 37.0 (23.6) | 46 | 35.2 (22.4) | |||
T-test except that ANOVA was used for Race and Cigarette smoking
Association of the Average Ocular Discomfort Scores with Signs and Symptoms of Dry Eye Disease
The correlation coefficients between the average ocular discomfort experienced over the last week and signs of dry eye disease are displayed in Table 3. Although some of the correlation coefficients were significantly different from 0 at a nominal level of 0.05, the magnitude of all of the coefficients indicated a very weak correlation (rs ≤0.12). This was also true when the average ocular discomfort was compared to the signs at 6 and 12 months.
Table 3.
Correlation between the average severity of ocular discomfort over the last week and signs of dry eye disease, the Ocular Surface Disease Index (OSDI) scores, SF-36 scores, interference scores on the Brief Ocular Discomfort Index , over time
| Baseline (N=535) | 6 Months (N=481) | 12 Months (N=488) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Mean(SD)a | rs | P | N | Mean(SD)a | rs | P | N | Mean(SD)a | rs | P |
| Signs | ||||||||||||
| Conjunctival staining score | 535 | 2.9 (1.4) | 0.04 | 0.36 | 481 | 2.6 (1.5) | 0.04 | 0.41 | 488 | 2.5 (1.5) | 0.10 | 0.02 |
| Corneal staining score | 535 | 3.8 (2.8) | 0.12 | 0.006 | 481 | 3.3 (2.7) | 0.11 | 0.02 | 488 | 3.2 (2.7) | 0.06 | 0.17 |
| Tear break-up time (seconds) | 535 | 3.1 (1.6) | −0.05 | 0.25 | 481 | 3.7 (2.5) | −0.08 | 0.08 | 488 | 3.8 (2.3) | −0.07 | 0.13 |
| Schirmer test (mm) | 535 | 9.6 (6.7) | −0.10 | 0.02 | 477 | 10.0 (6.6) | −0.01 | 0.84 | 484 | 9.9 (6.4) | −0.04 | 0.39 |
| OSDI (lower is better) | ||||||||||||
| Total score | 535 | 42.1 (15.5) | 0.47 | <0.001 | 481 | 32.2 (19.1) | 0.61 | <0.001 | 488 | 30.5 (18.6) | 0.67 | <0.001 |
| Vision-related function | 535 | 35.2 (19.2) | 0.23 | <0.001 | 481 | 26.5 (21.1) | 0.47 | <0.001 | 488 | 24.7 (20.7) | 0.53 | <0.001 |
| Ocular symptoms | 535 | 45.1 (19.1) | 0.44 | <0.001 | 481 | 34.7 (21.8) | 0.56 | <0.001 | 488 | 32.9 (22.1) | 0.57 | <0.001 |
| Environmental triggers | 529 | 52.6 (26.8) | 0.38 | <0.001 | 475 | 41.1 (29.6) | 0.50 | <0.001 | 481 | 39.6 (29.0) | 0.51 | <0.001 |
| SF-36 (higher is better) | ||||||||||||
| Summary: Physical Health | 535 | 47.5 (9.7) | −0.10 | 0.02 | 478 | 47.7 (10.3) | −0.19 | <0.001 | 486 | 47.7 (10.2) | −0.18 | <0.001 |
| Physical Functioning | 535 | 48.3 (9.6) | −0.06 | 0.13 | 478 | 48.4 (9.7) | −0.12 | 0.01 | 487 | 48.4 (9.7) | −0.17 | <0.001 |
| Role Limitations-Physical Health | 535 | 47.5 (9.6) | −0.14 | 0.001 | 479 | 47.4 (10.0) | −0.22 | <0.001 | 487 | 48.0 (10.0) | −0.23 | <0.001 |
| Bodily Pain | 535 | 47.3 (9.7) | −0.10 | 0.02 | 479 | 47.3 (10.1) | −0.28 | <0.001 | 487 | 47.5 (9.9) | −0.21 | <0.001 |
| General Health Perceptions | 535 | 52.0 (10.2) | −0.13 | 0.003 | 479 | 51.5 (10.7) | −0.16 | <0.001 | 487 | 51.5 (10.4) | −0.13 | 0.003 |
| Summary: Mental Health | 535 | 52.3 (9.4) | −0.11 | 0.01 | 479 | 51.7 (9.8) | −0.19 | <0.001 | 486 | 52.2 (9.0) | −0.18 | <0.001 |
| Vitality | 535 | 51.3 (9.8) | −0.12 | 0.005 | 479 | 51.1 (10.3) | −0.21 | <0.001 | 486 | 51.3 (9.7) | −0.21 | <0.001 |
| Social Functioning | 535 | 49.6 (9.6) | −0.12 | 0.006 | 479 | 49.6 (9.9) | −0.24 | <0.001 | 487 | 49.6 (9.4) | −0.18 | <0.001 |
| Role Limitations-Emotional Problems | 535 | 50.1 (9.3) | −0.12 | 0.007 | 479 | 49.6 (9.6) | −0.16 | <0.001 | 487 | 50.3 (9.3) | −0.20 | <0.001 |
| Mental Health | 535 | 52.4 (8.6) | −0.13 | 0.004 | 479 | 51.6 (9.2) | −0.22 | <0.001 | 486 | 52.2 (8.6) | −0.20 | <0.001 |
| Brief Ocular Discomfort Index | ||||||||||||
| Interference with activities of daily living (lower is better) | ||||||||||||
| General activity | 535 | 33.1 (23.1) | 0.60 | <0.001 | 481 | 25.2 (24.7) | 0.63 | <0.001 | 488 | 22.2 (24.2) | 0.65 | <0.001 |
| Mood | 535 | 28.6 (24.1) | 0.52 | <0.001 | 481 | 21.9 (23.7) | 0.55 | <0.001 | 488 | 18.4 (22.1) | 0.56 | <0.001 |
| Walking ability | 534 | 18.2 (22.2) | 0.37 | <0.001 | 481 | 13.7 (21.4) | 0.39 | <0.001 | 488 | 12.2 (19.6) | 0.44 | <0.001 |
| Normal work | 535 | 29.9 (23.7) | 0.51 | <0.001 | 481 | 21.5 (23.3) | 0.56 | <0.001 | 488 | 19.3 (22.5) | 0.57 | <0.001 |
| Relations with other people | 535 | 17.1 (21.0) | 0.40 | <0.001 | 481 | 12.6 (19.9) | 0.44 | <0.001 | 488 | 11.7 (19.4) | 0.48 | <0.001 |
| Sleep | 535 | 20.0 (23.8) | 0.40 | <0.001 | 481 | 15.3 (20.9) | 0.44 | <0.001 | 488 | 13.3 (20.4) | 0.40 | <0.001 |
| Enjoyment of life | 535 | 25.7 (23.5) | 0.51 | <0.001 | 481 | 19.3 (22.4) | 0.52 | <0.001 | 488 | 17.4 (22.1) | 0.56 | <0.001 |
| Relief from treatment (higher is better) | 534 | 38.5 (27.5) | −0.02 | 0.60 | 479 | 44.2 (31.3) | −0.08 | 0.07 | 483 | 45.8 (32.0) | −0.02 | 0.61 |
SD is standard deviation
At baseline, the correlation of the average ocular discomfort score with the total OSDI score was moderate (rs =0.47), indicating worse symptom scores with greater average discomfort (Table 3). To more fully describe the association of average ocular discomfort scores with the total OSDI scores at baseline and 12 months, the mean OSDI score for each of the ocular discomfort categories, as given in Table 1, is displayed in Figure 1. There was a weaker correlation (rs =0.23) with the vision-related function subscale than with the ocular symptoms (rs =0.44) and environmental triggers (rs =0.38) subscales. At 6 and 12 months, the OSDI total and subscales scores were approximately 10 points lower (better). The correlation coefficients indicated stronger associations (rs =0.47 to 0.67) at follow-up visits than at the baseline visit. The association of average ocular discomfort with the OSDI total score was strong at both 6 (rs=0.61) and 12 months (rs =0.67).
Figure 1.
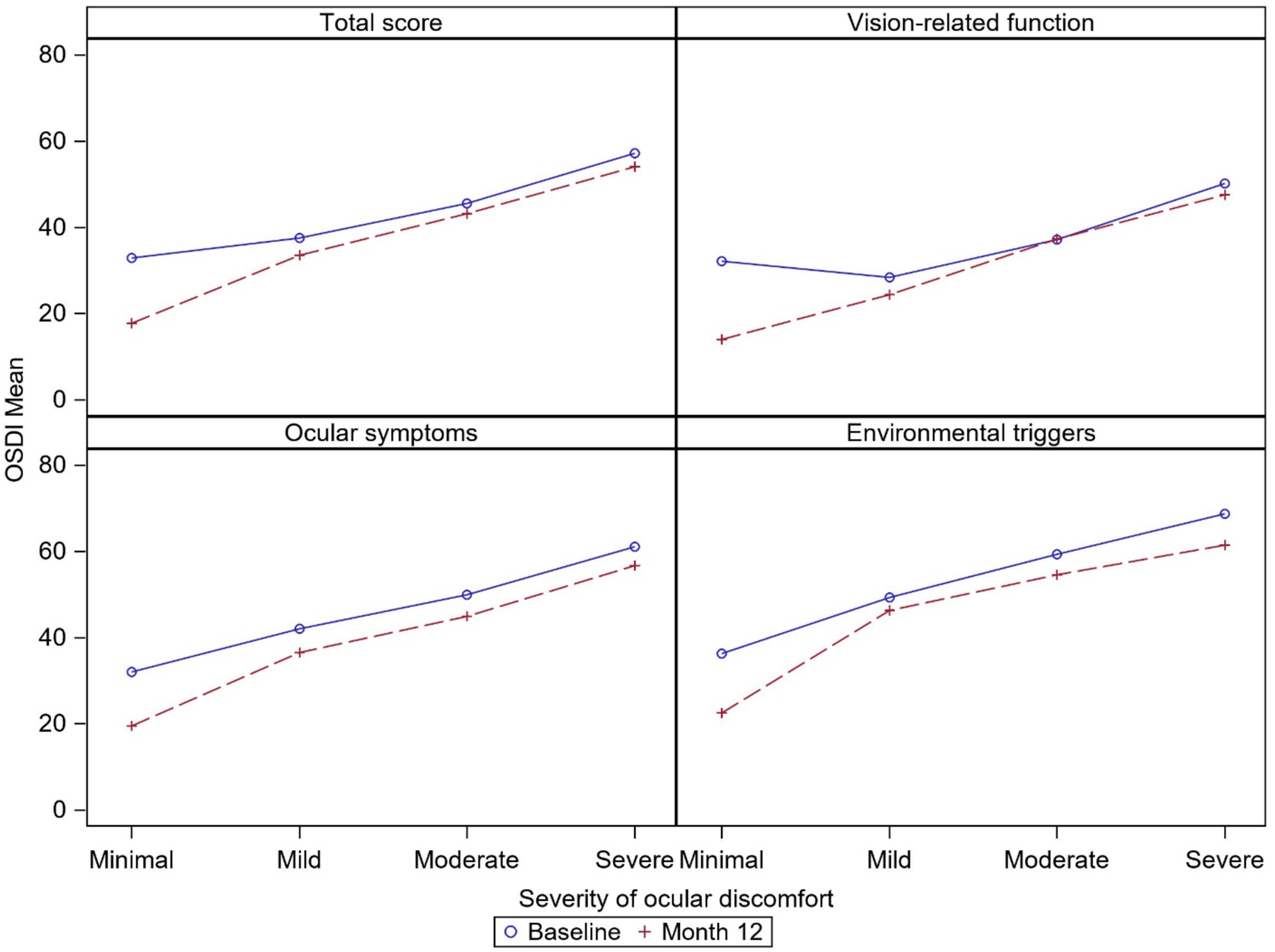
Mean Ocular Surface Disease Index (OSDI) scores by severity of average ocular discomfort in the last week at baseline and 12 months.
Association of the Average Ocular Discomfort Scores with Measures of Quality of Life
The correlation coefficients for the association of average discomfort and SF-36 scores were all negative, indicating worse health-related quality of life scores associated with greater average discomfort (Table 3; Figure 2). At baseline, the correlation of average discomfort with the summary scores for physical health and mental health were very weak (rs= −0.10 and −0.11, respectively). The correlation coefficients for the subscale scores within these two domains were of similar magnitude. At 6 and 12 months, the mean scores for all the SF-36 subscales were nearly unchanged from the baseline levels. The correlation coefficients at 6 and 12 months for all subscales were greater than at baseline; however, they were all approximately −0.2 (weak correlation).
Figure 2.
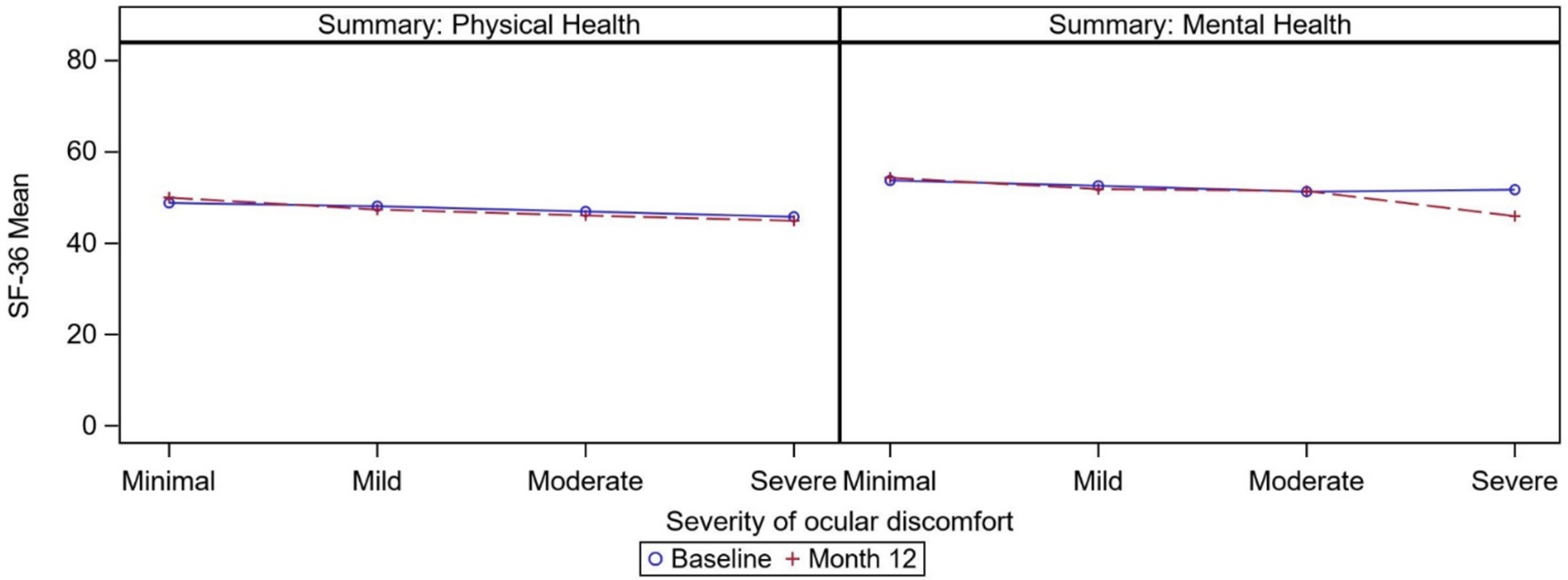
Mean scores for SF-36 summary scores by severity of average ocular discomfort in the last week at baseline and 12 months
The associations of average ocular discomfort with ratings of interference of ocular discomfort with activities of daily living are summarized in Table 3 (Figure 3). At baseline, the correlation coefficients ranged from weak (rs= 0.37 (walking ability)) to strong (rs= 0.60 (general activity)). The correlations observed at 6 and 12 months were similar to those at baseline. The mean score for interference with each type of activity increased steadily with increases in intensity of discomfort. Relief from treatment was not correlated with average ocular discomfort (rs= −0.02; p=0.60) at baseline. At 6 and 12 months the mean percentage relief from treatment increased from baseline, but the lack of correlation with average ocular discomfort remained at both 6 months (rs= −0.08; p=0.07) and 12 months (rs= −0.02; p=0.61).
Figure 3.
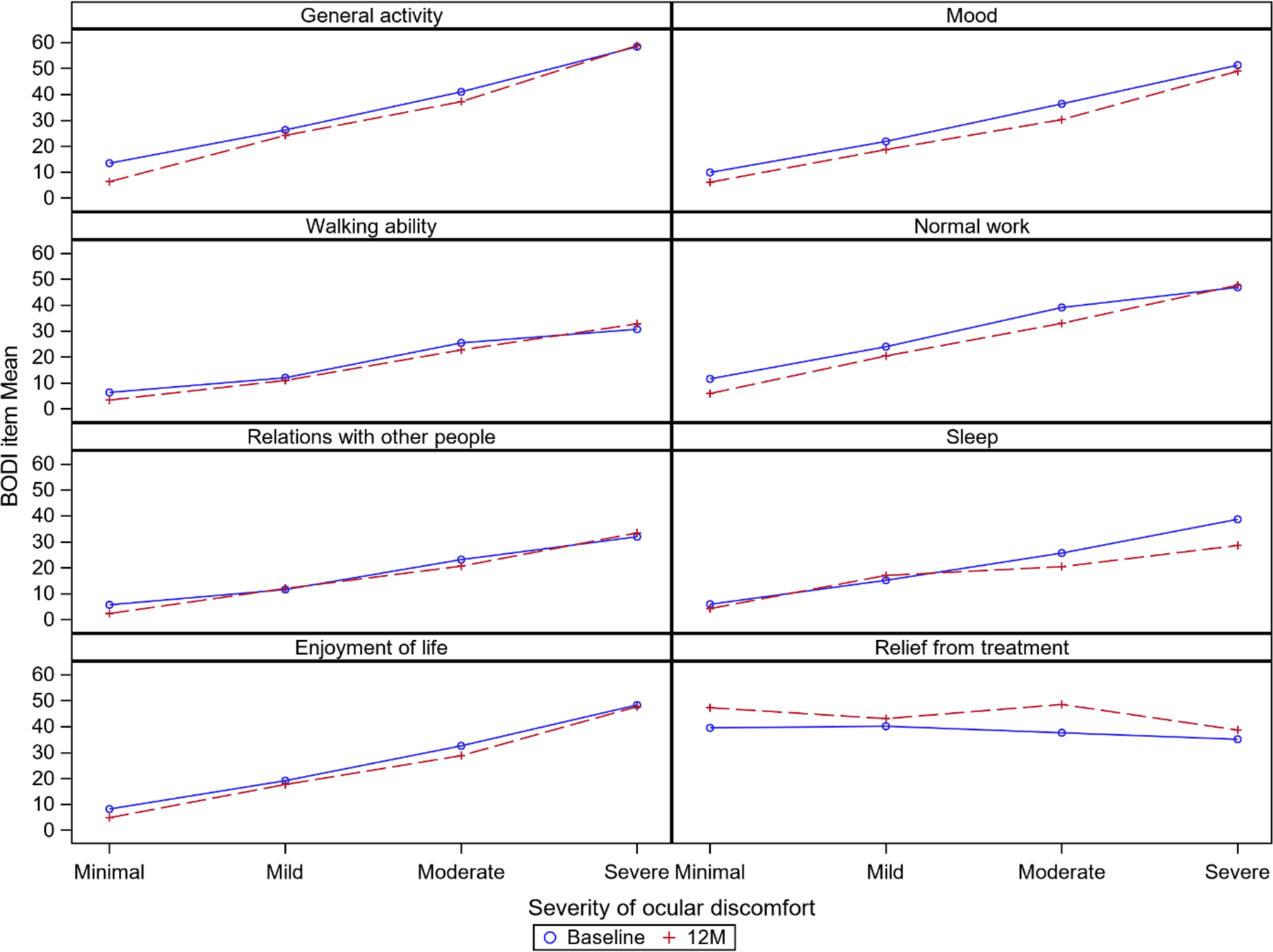
Mean scores for interference with areas on the Brief Ocular Discomfort Index (BODI) by severity of average ocular discomfort in the last week at baseline and 12 months.
DISCUSSION
We found a high prevalence of ocular discomfort in our large, demographically diverse cohort of patients with moderate to severe DED. Greater ocular discomfort was associated with greater interference with several activities of daily living. Although ocular discomfort scores decreased after patients were placed on either active omega-3 or placebo supplements, the amount of interference associated with each category of ocular discomfort changed little between baseline and 12 months (Figure 3). This highlights the constancy of the relationship between ocular discomfort and its impact on the various activities, as the interference on these activities is dependent on the specific category of discomfort level the subject has at the time the questionnaires are administered. Higher ocular discomfort scores were also moderately associated with higher (worse) scores on the OSDI, a patient reported outcome measure that includes a broader range of DED symptoms including decreased visual function and responses to environmental conditions known to exacerbate symptoms of DED.10 The associations of more severe ocular discomfort with worse scores on the summary and subscale measures from the generic SF-36 health survey of health-related quality of life, were very weak (≤ 0.19) or weak (0.20 to 0.39) at baseline and follow-up, although significantly different from 0. Thus, patients who report worse average ocular discomfort, are likely to report higher interference with daily activities and more overall symptoms of DED, but not necessarily worse overall physical or mental health.
As in most previous studies of the association of symptoms and signs of DED, the association between the symptoms of dry eye, as well as the severity of ocular discomfort and the severity of four key signs evaluated in DREAM was either not statistically significant or very weak (≤ 0.19 ; Table 2).3 Others have found greater discordance between symptoms and signs or greater ocular pain among patients with conditions associated with non-ocular pain; however, mean ocular discomfort scores were similar in the DREAM patients for those with or without self-reported rheumatoid arthritis, osteoarthritis, and fibromyalgia (Table 2).14–17 Because the eligibility criteria for the DREAM clinical trial specified thresholds for signs and a restricted range for the baseline OSDI symptom score (23–80), the DREAM study population was not well suited for exploration of patients with highly discordant symptoms and signs.
Greater ocular discomfort was associated with worse OSDI total scores both at baseline and during follow-up (Table 3), consistent with previous studies smaller or less diverse populations. Satitpitakul and colleagues studied 84 patients with DED who had a mean OSDI score (45.6) similar to the DREAM study patients at baseline (42.8) and used a question on ocular pain and scoring system similar to the BODI question on ocular discomfort and found a moderate correlation (rs =0.49) nearly equal to the correlation among DREAM patients (rs =0.47).18 Kalangara and colleagues studied 154 patients at a Veterans Administration medical center (91% male) who had a mean OSDI score of 39 and used a question on ocular pain and the same scoring system as used in the BODI. They found a strong correlation (rs =0.61) similar to the correlation among DREAM patients during follow up (rs =0.61 at 6 months, rs =0.67 at 12 months).19
Depression and use of anti-depressant medications have been associated in many studies with having DED; however, less is known about whether the association differs with severity of DED symptoms and pain.3 Satitpitakul et al found that ocular pain severity was associated with use of anti-depressant medications and both Ong et al and Vehof et al found greater discordance between symptoms and signs among those with depression or using of anti-depressant medications.15,16,18 However, among DREAM patients the mean ocular discomfort score did not differ by presence of self-reported depression or use of anti-depressant medications (Table 2).
DED has a significant impact on visual function, daily activities, social and physical functioning, workplace productivity, and quality of life.3 We found a similar impact ocular discomfort on the BODI interference with activities of daily living questions, with a strong correlation between the increasing levels of ocular discomfort and interference with general activity, mood, walking ability, normal work (includes both work outside the home and housework), relations with other people, sleep, and enjoyment of life. These findings highlight the importance of assessing the level of ocular discomfort in DED suffers as it strongly reflects the impact of DED on their daily lives.
While this study provides valuable insight into the impact of dry eye-related ocular discomfort on patients’ quality of life, there are limitations in the study’s inclusion criteria that can influence our results. One limitation is the absence of a control group with no dry eye so that only associations with severity could be assessed. Another is the exclusion of subjects with dry eye symptoms but no signs, an important group which includes patients with what has increasingly been recognized as having ocular neuropathic pain.20 Finally, the exclusion of patients with prior refractive surgery and current contact lens wearers limits the generalizability of these results.21
Similar to other pain syndromes, the dry eye experience likely is based on a combination of the peripheral signs (e.g., changes in the ocular surface) and a central adaptation/sensitization to the peripheral process. Currently, we are not able to accurately predict the degree to which the two processes are involved within the individual patient. Thus, asking about the level of ocular discomfort not only enhances the information we gain from the patient but is a key component to our understanding of the patient experience which will facilitate our ability to further explore and address other features of the disease and improve treatment of our patients. Further studies examining the impact of improving patients’ level of discomfort with treatment on their wellbeing and social functioning are important to ascertain its value in routine clinical practice.
Supplementary Material
Funding/Support:
This study was supported by cooperative agreements U10EY022879 and U10EY022881 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services.
Footnotes
Conflict of Interest
Rony R. Sayegh, MD has received personal fees from Allergan and Novartis. Penny A. Asbell, MD has received research grants from the National Eye Institute, MC2 Therapeutics, Miotech, and Regeneron; personal fees from Shire, Medscape, MC2 Therapeutics, Miotech, Bausch& Lomb, ECLA, Astra Zeneca,Topovert, Regeneron, Blephex, Senju, Axero, Eyepoint, Novaliq, Kala, Dompe, Alcon, Allados, Motive, Sun; and non-financial support from ECLA and Regeneron. John T. Farrar, MD, PhD has received research grants and contracts from US Food and Drug Administration, and National Institutes of Health and consulting fees from Analgesic Solutions, Aptinyx, Biogen, Opioid Post-Marketing Consortium, Daiichi Sankyo, DepoMed, Evadera, Jansen, Lilly, Novartis, Vertex, and Pfizer; payment for or data and safety monitoring committee services from NIH-NIA and Cara Therapeutics. Maureen G. Maguire, PhD has received grants from the National Eye Institute and Foundation Fighting Blindness; personal fees from Genentech and Regenera for data and safety monitoring committee services. For the remaining authors, none were declared.
Supplemental Digital Content
Supplemental Digital Content (SDC) Table 1: Credit Roster for the DRy Eye Assessment And Management (DREAM) Study
REFERENCES
- 1.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop. Ocul Surf 2007;5(2):93–107. [DOI] [PubMed] [Google Scholar]
- 2.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua AH, Joo C-K, Liu A, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II definition and classification report. Ocul Surf 2017;15(3):276–283. [DOI] [PubMed] [Google Scholar]
- 3.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na K-S, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L. TFOS DEWS II epidemiology report. Ocul Surf 2017;15(3):334–365. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 2011;30(4):379–387. [DOI] [PubMed] [Google Scholar]
- 5.Uchino M, Schaumberg D. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep 2014;1(2)51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Dry Eye Assessment and Management Study Research Group. n-3 fatty acid supplementation for treatment of dry eye disease. N Engl J Med 2018(18);378:1681–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asbell PA, Maguire MG, Peskin E, Kuklinski E, Bunya VT, the DREAM Study Research Group. The Dry Eye Assessment and Management (DREAM©) Study: Study design and baseline characteristics. Contemporary Clinical Trials 2018; 71:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.12-Item Ocular Surface Disease Index (OSDI) administration and scoring manual. Version 1.0. Allergan I, CA. November 2004. [Google Scholar]
- 9.Miller KL, Walt JG, Mink DR, Satram-Hoang S, Wilson SE, Perry HD, Asbell PA, Pflugfelder SC. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol 2010;128(1):94–101. [DOI] [PubMed] [Google Scholar]
- 10.Walt JG, Rowe MM, Stern KL. Evaluating the Functional Impact of Dry-eye: The Ocular Surface Disease Index. Drug Information Journal. 1997;31(4):1436. [Google Scholar]
- 11.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von ST, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–121. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE. SF-36 health survey update. Spine 2000;25(24):3130–3139. [DOI] [PubMed] [Google Scholar]
- 13.Evans JD. Straightforward Statistics for the Behavioral Sciences. Pacific Grove: Brooks/Cole Publishing; 1996. [Google Scholar]
- 14.Galor A, Felix ER, Feuer W, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol 2015;99(8):1126–1129. [DOI] [PubMed] [Google Scholar]
- 15.Ong ES, Felix ER, Levitt RC, Feuer WJ, Sarantopoulos CD, Galor A. Epidemiology of discordance between symptoms and signs of dry eye. Br J Ophthalmol 2018;102(5):674–679. [DOI] [PubMed] [Google Scholar]
- 16.Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, et al. Predictors of discordance between symptoms and signs in dry eye disease. Ophthalmology 2017;124(3):280–6. [DOI] [PubMed] [Google Scholar]
- 17.Yamanishi R, Uchino M, Kawashima M, Dogru M, Matsuguma S, Tsubota K. Analysis of the association between the severity of ocular and systemic pain. Ocul Surf 2019;17(3):434–439. [DOI] [PubMed] [Google Scholar]
- 18.Satitpitakul V, Kheirkhah A, Crnej A, Hamrah P, Dana R. Determinants of ocular pain severity in patients with dry eye disease. Am J Ophthalmol 2017;170:198–204. [DOI] [PubMed] [Google Scholar]
- 19.Kalangara JP, Galor A, Levitt RC, Covington DB, McManus KT, Sarantopouos CD, Felx ER. Characteristics of ocular pain complaints in patients with idiopathic dry eye symptoms. Eye & Conact Lens 2017;43(3):192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain. Is it real? Ocul Surf 2009;7(1)28–40. [DOI] [PubMed] [Google Scholar]
- 21.Toda I. Dry eye after LASIK. Invest Ophthalmol Vis Sci 2018;59(14):DES109–DES115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


