Abstract
Purpose:
To compare the degree of corneal light scatter as measured by densitometry in ultrathin Descemet Stripping Automated Endothelial Keratoplasty (UT-DSAEK) and Descemet Membrane Endothelial Keratoplasty (DMEK) in the Descemet endothelial thickness comparison trial.
Methods:
This was a prespecified secondary analysis of the Descemet endothelial thickness comparison trial, which was a prospective, randomized controlled trial. Subjects with isolated endothelial dysfunction were enrolled and were randomized to either UT-DSAEK or DMEK. Corneal opacity was quantitatively measured by Pentacam densitometry (OCULUS) at 3, 6, and 12 months.
Results:
Fifty eyes of 38 patients were enrolled at the Casey Eye Institute at Oregon Health & Science University and the Byers Eye Institute at Stanford University. Corneal densitometry for the anterior and posterior layers improved in both UT-DSAEK and DMEK after surgery. The decrease was more pronounced in the posterior layer for both groups. However, there was no difference in the degree of corneal light scatter between UT-DSAEK and DMEK at postoperative month 12, and no difference in change in densitometry was observed between the 2 arms from baseline to month 12.
Conclusions:
Both UT-DSAEK and DMEK experience an improvement in the degree of corneal light scatter after surgery. However, there was no difference in densitometry between the 2 groups at month 12. Therefore, other factors such as higher order aberrations in the posterior cornea rather than stromal–stromal interface haze mediate the superior visual outcomes in DMEK compared with UT-DSAEK.
Keywords: DMEK, UT-DSAEK, densitometry, endothelial keratoplasty
The etiology behind the superior visual acuity outcomes of Descemet Membrane Endothelial Keratoplasty (DMEK) over Descemet Stripping Automated Endothelial Keratoplasty (DSAEK) remains unclear.1 A difference in the degree of corneal haze, or corneal light scatter, has been proposed as a potential explanation. DMEK lacks the stromal–stromal interface that is present in DSAEK and therefore may produce a more consistent, clear interface with less light scatter. In addition, the overall thinner cornea in DMEK may give rise to less light scatter and, therefore, improved visual quality. One other potential explanation is that DSAEK has greater posterior corneal higher order aberrations (HOAs) compared with DMEK.2–4
With the development of an objective method to measure corneal light scatter with noninvasive Scheimpflug imaging, corneal densitometry has become a useful tool to provide information on corneal transparency. Previous studies have demonstrated significantly improved corneal light scatter as measured by densitometry after endothelial keratoplasty for Fuchs endothelial dystrophy.5,6 Corneal densitometry has also been followed up in patients undergoing corneal cross-linking, refractive surgery, and keratoplasty.7–16 A comparison between the relative corneal light scatter in DMEK versus DSAEK may help elucidate the cause of enhanced visual acuity outcomes of DMEK.
Here, we compare the degree of corneal light scatter as measured by densitometry in patients undergoing ultra-thin DSAEK (UT-DSAEK) and DMEK as part of a randomized controlled clinical trial.
MATERIALS AND METHODS
The Descemet Endothelial Thickness Comparison Trial was a two-center patient and outcome-masked randomized clinical trial comparing the outcomes of UT-DSAEK and DMEK in patients with isolated endothelial dysfunction. Patients were enrolled at the Casey Eye Institute at Oregon Health & Sciences University and the Byers Eye Institute at Stanford University. Full inclusion and exclusion criteria, along with study methodology, are described elsewhere.1 In brief, study participants were masked to their intervention, and all subjects received the same postoperative follow-up care and monitoring. The provider performing refractions was masked to the intervention as well. If both eyes of the same patient were included in the study, each eye was randomized separately. All tissue was obtained from the same eye bank, the Lions VisionGift of Portland, Oregon. Surgical techniques were standardized such that all patients underwent simultaneous cataract surgery with endothelial transplant if they were not already pseudophakic. An 8.0 mm graft was used for all cases, and the UT-DSAEK grafts were precut by the eye bank to be between 60 and 90 μm using a microkeratome.
This prespecified secondary analysis primarily looked at corneal opacity at 12 months in UT-DSAEK and DMEK. Corneal opacity was quantitatively measured by Pentacam densitometry (OCULUS, Wetzlar, Germany). The densitometry values are expressed in grayscale units (GSU) and range from 0 (completely clear) to 100 (completely opaque). The densitometry data are broken down into the anterior 120 μm, central layer, and posterior 60 μm, as well as concentric rings composed of the central 0 to 2 mm, 2 to 6 mm, 6 to 10 mm, and 10 to 12 mm. In this study, only the central 6.0-mm zone was analyzed because it is most relevant for visual acuity and is similar to the optical zones assessed in HOA analyses.4
To calculate the corneal densitometry for the central 6-mm zone, the 0- to 2-mm zone, and 2- to 6-mm zone were combined using the following formula;
where D1 is the densitometry of the 0 to 2 mm, D2 is the densitometry of the 2 to 6 mm, and Dc is the densitometry of the 0- to 6-mm cornea. For the purpose of calculating the densitometry of the various optical zones, a volumetric formula for a cylinder was used, which is approximately the shape of a cornea when flattened.
where h is the height of cylinderThe densitometry values of the respective optical zones are weighted according to the following proportions:
These weights are applied to the original densitometry formula. This ratio is the same whether it is the anterior, central, or posterior cornea.
Statistical Analysis
The primary analysis compared corneal opacity, as measured by densitometry, in the anterior, central, and posterior cornea for each arm separately between baseline and 12-month visit using one-sided paired t tests with a significance level of 0.01 to adjust for testing 6 hypotheses. Secondary analyses compared this change in densitometry between the 2 arms using one-sided Welch t tests and also tested in the same way whether the 12-month densitometry was different between the 2 arms. As the distributions of densitometry do seem to be normal, we repeated the tests for differences at month 12 using the Mann–Whitney U test, but these analyses did not significantly change the results.
Approval from the Institutional Review Boards at the University of California, San Francisco, Oregon Health & Sciences University, and Stanford University were obtained for this study and written informed consent was obtained from all participants. This study was registered with ClinicalTrials.gov (NCT02373137) and adhered to the tenets of the Declaration of Helsinki.
RESULTS
Fifty eyes of 38 patients were enrolled in this study between January 20, 2015 and April 26, 2017. The preoperative diagnosis was Fuchs endothelial dystrophy in 48 patients (96%) and pseudophakic bullous keratopathy in 2 patients (4%). The mean graft thickness preoperatively for the UT-DSAEK group was 73 μm. Complete densitometry data were available for at least 90% of all the subjects at each time point (Table 1), and only one patient was missing values at month 12. Densitometry for both UT-DSAEK and DMEK groups decreased after surgery in the anterior and posterior layers (Fig. 1 and Table 2). The central densitometry might also decrease but not at significance level 0.01, which was chosen to compensate for testing 6 hypotheses.
TABLE 1.
Baseline Characteristics and Densitometry Data
| UT-DSAEK | DMEK | P | |
|---|---|---|---|
| Age (Years), mean (range) | 68 (51–95) | 68 (61–81) | 0.96 |
| Female sex, N (%) | 16 (64%) | 13 (52%) | 0.39 |
| Diagnosis, N (%) | |||
| Fuchs | 24 (96%) | 24 (96%) | 1.00 |
| Pseudophakic bullous keratopathy | 1 (4%) | 1 (4%) | |
| ETDRS BSCVA, mean ± SD | |||
| LogMAR | 0.27 ± 0.21 | 0.34 ± 0.29 | 0.34 |
| Approximate Snellen | 20/40 | 20/40 | |
| CCT (μM), mean ± SD | 610 ± 44 | 608 ± 52 | 0.93 |
| Completeness of densitometry data | |||
| Baseline, N (%) | 23 (92%) | 22 (88%) | |
| 3 months, N (%) | 23 (92%) | 24 (96%) | |
| 6 months, N (%) | 24 (96%) | 21 (84%) | |
| 12 months, N (%) | 25 (100%) | 24 (96%) | |
ETDRS, early treatment diabetic retinopathy study.
FIGURE 1.
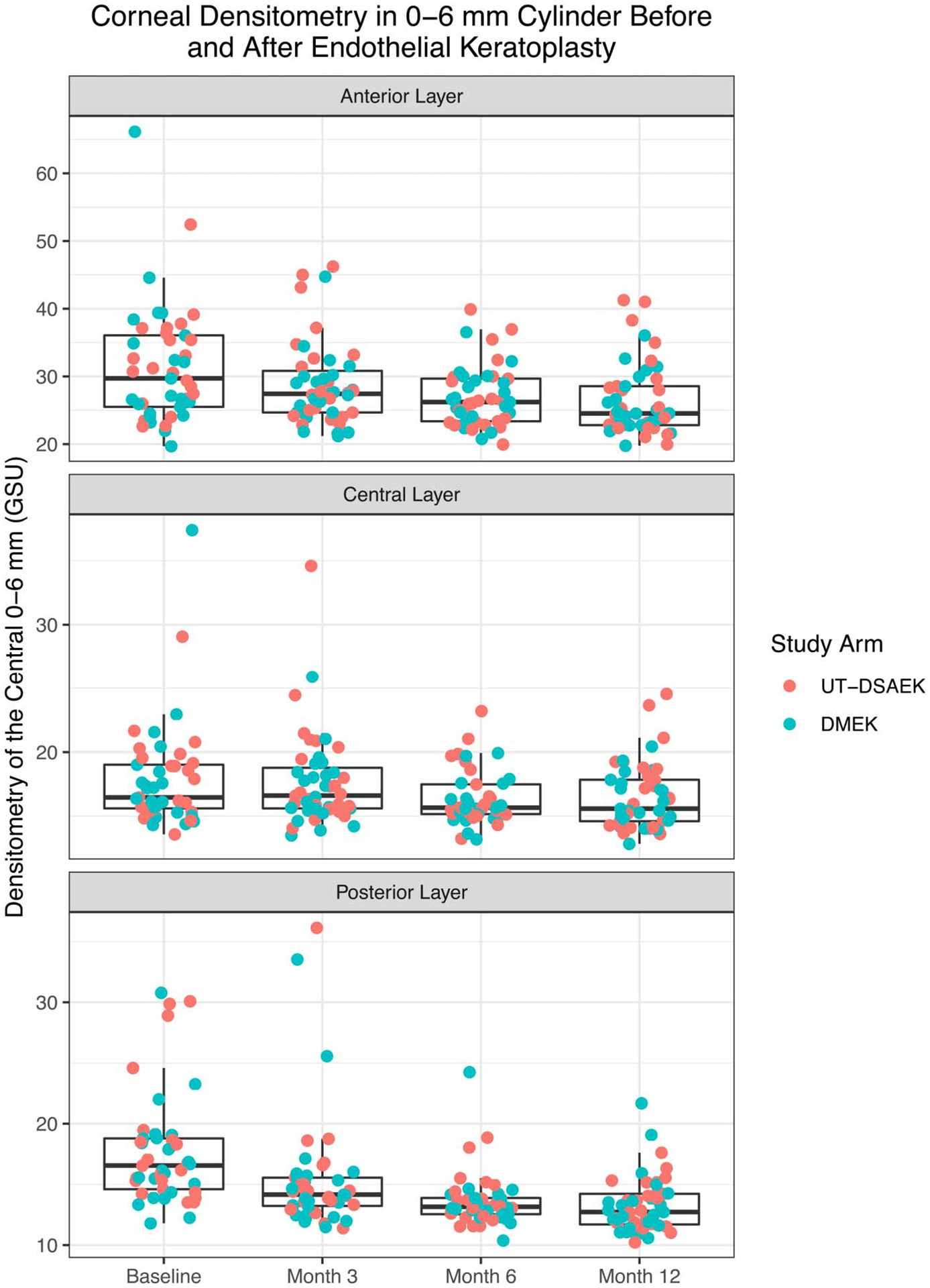
Corneal densitometry of the 0- to 6-mm cylinder preoperatively and at postoperative months 3, 6, and 12 for UT-DSAEK and DMEK.
TABLE 2.
Change in Densitometry for Each Layer of the Cornea in UT-DSAEK and DMEK at 12 Months
| Surgery Type | Layer of Cornea | Average Change in Densitometry at 12 Months | Standard Error | Confidence Interval Upper Bound | P |
|---|---|---|---|---|---|
| UT-DSAEK N = 23 | |||||
| Anterior | −4.41 | 1.12 | −2.49 | 0.0004 | |
| Central | −0.97 | 0.49 | −0.13 | 0.0302 | |
| Posterior | −5.05 | 1.09 | −3.19 | 0.0001 | |
| DMEK N = 21 | |||||
| Anterior | −5.99 | 2.11 | −2.35 | 0.0051 | |
| Central | −2.02 | 1.12 | −0.08 | 0.0435 | |
| Posterior | −4.09 | 1.02 | −2.34 | 0.0003 | |
Secondary analysis found no statistically significant difference in the improvement in densitometry between arms (Fig. 2 and Table 3).
FIGURE 2.
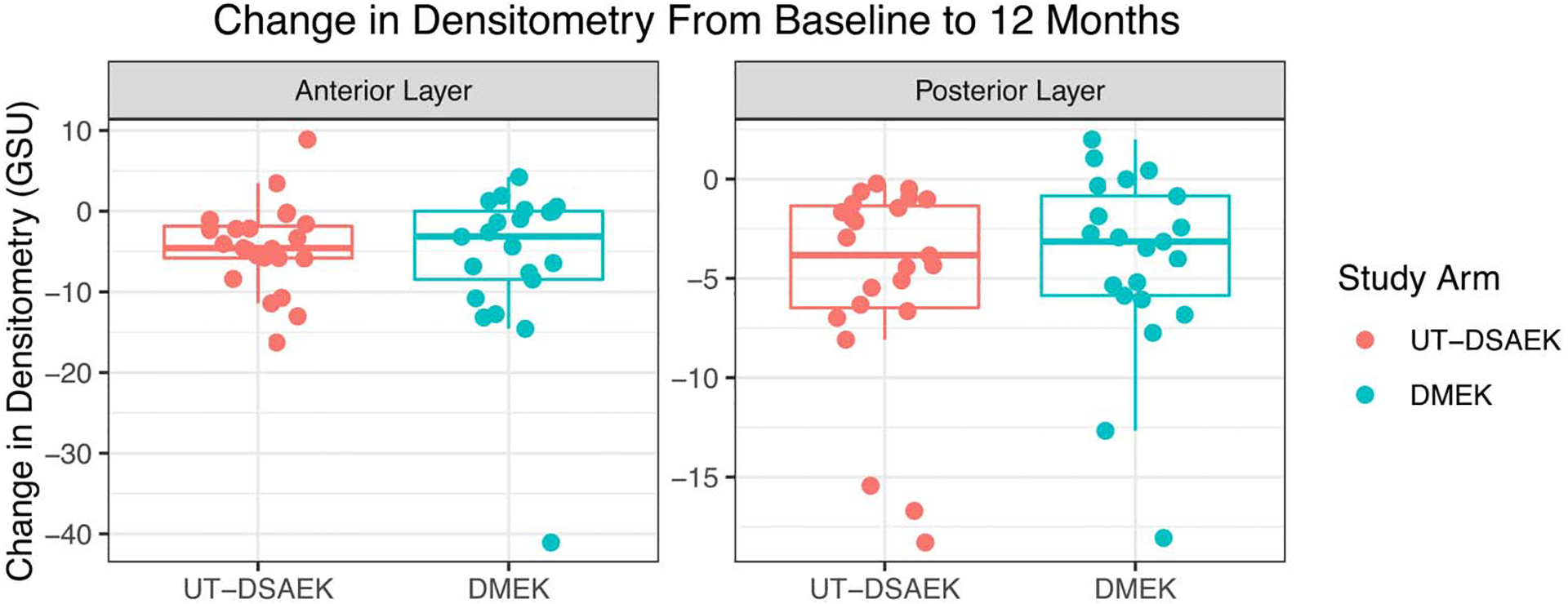
Change in anterior and posterior corneal densitometry from 0 to 6 mm in UT-DSAEK and DMEK from baseline to 12 months.
TABLE 3.
Change in Densitometry and Visual Acuity in UT-DSAEK and DMEK at 12 Months
| Measurement | Average Change in UT-DSAEK | Average Change in DMEK | Difference Between Arms | Standard Error | Confidence Interval Lower Bound | P |
|---|---|---|---|---|---|---|
| Densitometry anterior layer | −4.41 | −5.99 | 1.58 | 2.39 | −2.47 | 0.2565 |
| Densitometry posterior layer | −5.05 | −4.09 | −0.96 | 1.49 | −3.47 | 0.7383 |
| LogMAR visual acuity | −0.11 | −0.32 | 0.21 | 0.07 | 0.09 | 0.0025 |
DISCUSSION
Corneal haze as measured by densitometry did improve in both arms after surgery, which is consistent with previous studies.6 However, there was no significant difference in the improvement in corneal densitometry between UT-DSAEK and DMEK groups. The same subset of eyes had significantly greater improvement in visual acuity in the DMEK arm than the UT-DSAEK arm (P = 0.0025), so the study should be adequately powered to detect a difference in densitometry between arms if it explained the difference in acuity outcomes. In fact, for the observed standard deviation in the posterior layer of 1.5, we would have 80% power to detect a difference of 1.5 in improvement; instead, the average improvement in the DMEK arm is actually less than that in the UT-DSAEK arm. Similarly, there was no statistically significant difference between arms in the 12-month densitometry measurements, but there was a difference between arms in 12-month visual acuity (P = 0.0034) (Table 4). Thus, corneal haze may not be the etiology of the visual acuity difference.
TABLE 4.
Densitometry and Visual Acuity at 12 Months in UT-DSAEK and DMEK
| Measurement | Average 12-Month Value in UT-DSAEK | Average 12-Month Value in DMEK | Difference Between Arms | Standard Error | Confidence Interval Lower Bound | P |
|---|---|---|---|---|---|---|
| Densitometry anterior layer | 26.98 | 25.77 | 1.22 | 1.47 | −1.27 | 0.2074 |
| Densitometry central layer | 16.85 | 16.10 | 0.75 | 0.72 | −0.46 | 0.1518 |
| Densitometry posterior layer | 13.28 | 13.23 | 0.05 | 0.65 | −1.04 | 0.4696 |
| LogMAR visual acuity | 0.16 | 0.04 | 0.12 | 0.04 | 0.05 | 0.0034 |
Previous studies have reported greater light scatter in edematous corneas than normal corneas. For example, total corneal densitometry in normal corneas range from 15.2 to 17.4 GSU for the central 0- to 2-mm zone and 14.95 to 17.6 GSU for the 2- to 6-mm zone, whereas in patients with Fuchs endothelial dystrophy or bullous keratopathy, it ranges from 28.0 to 34.4 for the central 0- to 2-mm zone and 24.4 to 30.2 for the 2- to 6-mm zone.17–20 Our study’s baseline total densitometry values range from 16.1 to 42.3 with median 21.2, so our values might be slightly lower overall than those previously reported. One potential explanation for this may be a difference in disease severity, as indicated by preoperative central corneal thickness (CCT). The baseline mean CCT in this study was 610 μm in the UT-DSAEK arm and 608 μm in the DMEK arm. By contrast, Droutsas et al20 reported a baseline mean CCT of 702 μm in their DMEK arm and 634 μm in their DSAEK group.
The anterior aspect of the cornea is responsible for most of the light scattering in both healthy corneas and those with Fuchs endothelial dystrophy.5,19,21 Our study found that corneal light scatter was most pronounced in the anterior 120 μm both before and after endothelial keratoplasty. Similarly, Schaub et al5 found that in patients with Fuchs endothelial dystrophy undergoing DMEK, the largest improvement in densitometry occurred in the anterior and total layers, with most of the improvement occurring in the first 6 months after surgery but also continuing through the second year. This may be explained by microcystic edema and subepithelial haze, which tend to resolve or improve after surgery.
A compelling argument in favor of DMEK is the pure anatomic replacement of Descemet membrane against the host corneal stroma, thereby avoiding the stromal–stromal interface in DSAEK where haze or contour irregularity can compromise best spectacle-corrected visual acuity (BSCVA). Previous studies comparing the 2 surgeries have used thicker DSAEK grafts and primarily analyzed total corneal densitometry inclusive of all layers. Alnawaiseh et al6 used DSAEK grafts less than 120 μm thick and found that at 6 and 12 months, the total densitometry improved in the 0- to 2-mm zone for DSAEK and in the 0- to 2-mm and 2- to 6-mm zones for DMEK. Droutsas et al20 used conventionally thick DSAEK grafts with an average thickness of 140 μm and found that for the 0- to 2-mm and 2- to 6-mm zones, at 3 and 6 months, the DSAEK group had higher total densitometry than the DMEK group, but there were no differences at 12 and 24 months. The longer time required for thicker DSAEK grafts to deturgesce may underlie its delayed improvement in densitometry compared with the DMEK arm. This is in contrast to the findings in our study, which did not find a significant difference in densitometry between UT-DSAEK and DMEK at any of the postoperative time points.
Densitometry of the posterior layer of the cornea is interesting to analyze because it may capture the donor–recipient interface in UT-DSAEK and DMEK grafts. The UT-DSAEK grafts used in our study were less than 100 μm with an average thickness of 73 μm. Considering that donor DSAEK grafts deturgesce with time, it is possible that the densitometry of the posterior layer (60 μm) captured the stromal–stromal interface and thus provides insight into the potential difference in graft–host interface clarity between DMEK and UT-DSAEK surgeries. Our study did not find a significant difference in the densitometry for any layer at month 12 for UT-DSAEK versus DMEK, which suggests that interface haze does not explain the poorer BSCVA observed in patients who underwent UT-DSAEK. However, the improvement in posterior densitometry after surgery in both groups may be explained by the removal of guttae, especially given the fact that all but one of the patients in each group had a preoperative diagnosis of Fuchs endothelial dystrophy.22 Although there can be changes in forward and back scatter after DSAEK, our study’s Pentacam measurements specifically measure backscatter.
Because the degree of corneal light scatter did not differ significantly between UT-DSAEK and DMEK groups, there may be other factors at play to explain the superior visual acuity outcomes of DMEK over UT-DSAEK. Perhaps, the additional stroma and increased CCT influence the BSCVA in a way not captured by densitometry. In a separate secondary analysis of the Descemet endothelial thickness comparison trial, our group found that at 3, 6, and 12 months, the DMEK group had significantly less HOAs of the posterior corneal surface compared with UT-DSAEK and that these correlate with BSCVA.4 Rudolph et al3 similarly found that DMEK had significantly lower mean HOA than DSAEK for the central 4- and 6-mm zones of the posterior corneal surface. Dirisamer et al23 also found that coma and trefoil at the posterior surface were lower in DMEK than in DSAEK.
It is found that 12 months were not long enough to detect significant differences in densitometry between the 2 groups. Schaub et al5 found that densitometry measurements continued to improve into the second postoperative year after DMEK despite the visual acuity plateauing after 1 year. Our study was also not powered to assess the difference in densitometry between the 2 arms. Rather, it was powered to detect a difference in visual acuity of 0.12 logarithm of the minimum angle of resolution (LogMAR).1 An additional limitation relates to the approximations we used to calculate corneal densitometry because the thickness of the graft and recipient cornea can vary from the center to the periphery, which was not taken into account in our formula.
In conclusion, there was no statistically significant difference in densitometry between UT-DSAEK and DMEK arms to explain the superior visual outcomes with DMEK. Therefore, it seems that other factors such as HOAs in the posterior cornea mediate the superior visual outcomes in DMEK compared with UT-DSAEK.
Acknowledgments
Supported by grants K23 EY025025 (Rose-Nussbaumer), core grants P30-026877 (Stanford) and P30 EY010572 (Casey Eye Institute) from the National Eye Institute and Research to Prevent Blindness. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Chamberlain W, Lin CC, Austin A, et al. Descemet endothelial thickness comparison trial: a randomized trial comparing ultrathin Descemet stripping automated endothelial keratoplasty with Descemet membrane endothelial keratoplasty. Ophthalmology. 2019;126:19–26. [DOI] [PubMed] [Google Scholar]
- 2.Maier AK, Gundlach E, Gonnermann J, et al. Retrospective contralateral study comparing Descemet membrane endothelial keratoplasty with Descemet stripping automated endothelial keratoplasty. Eye (Lond) 2015;29:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudolph M, Laaser K, Bachmann BO, et al. Corneal higher-order aberrations after Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119:528–535. [DOI] [PubMed] [Google Scholar]
- 4.Duggan MJ, Rose-Nussbaumer J, Lin CC, et al. Corneal higher-order aberrations in Descemet membrane endothelial keratoplasty versus ultrathin DSAEK in the Descemet endothelial thickness comparison trial: a randomized clinical trial. Ophthalmology. 2019;126:946–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaub F, Enders P, Bluhm C, et al. Two-year course of corneal densitometry after Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2017;175:60–67. [DOI] [PubMed] [Google Scholar]
- 6.Alnawaiseh M, Rosentreter A, Prokosch V, et al. Changes in corneal densitometry in patients with fuchs endothelial dystrophy after endothelial keratoplasty. Curr Eye Res. 2017;42:163–167. [DOI] [PubMed] [Google Scholar]
- 7.Otri AM, Fares U, Al-Aqaba MA, et al. Corneal densitometry as an indicator of corneal health. Ophthalmology. 2012;119:501–508. [DOI] [PubMed] [Google Scholar]
- 8.Chan TCY, Chan JCK, Wang YM, et al. Survival analysis of corneal densitometry after collagen cross-linking for progressive keratoconus. Cornea. 2018;37:1449–1456. [DOI] [PubMed] [Google Scholar]
- 9.Fares U, Otri AM, Al-Aqaba MA, et al. Wavefront-optimized excimer laser in situ keratomileusis for myopia and myopic astigmatism: refractive outcomes and corneal densitometry. J Cataract Refract Surg. 2012;38:2131–2138. [DOI] [PubMed] [Google Scholar]
- 10.Poyales F, Garzón N, Mendicute J, et al. Corneal densitometry after photorefractive keratectomy, laser-assisted in situ keratomileusis, and small-incision lenticule extraction. Eye (Lond). 2017;31:1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shajari M, Wanner E, Rusev V, et al. Corneal Densitometry after Femtosecond Laser-Assisted In Situ Keratomileusis (Fs-LASIK) and Small Incision Lenticule Extraction (SMILE). Curr Eye Res. 2018;43: 605–610. [DOI] [PubMed] [Google Scholar]
- 12.Pircher N, Pachala M, Prager F, et al. Changes in straylight and densitometry values after corneal collagen crosslinking. J Cataract Refract Surg. 2015;41:1038–1043. [DOI] [PubMed] [Google Scholar]
- 13.Kim BZ, Jordan CA, McGhee CN, et al. Natural history of corneal haze after corneal collagen crosslinking in keratoconus using Scheimpflug analysis. J Cataract Refract Surg. 2016;42:1053–1059. [DOI] [PubMed] [Google Scholar]
- 14.Alzahrani K, Dardin SF, Carley F, et al. Corneal clarity measurements in patients with keratoconus undergoing either penetrating or deep anterior lamellar keratoplasty. Clin Ophthalmol. 2018;12: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt UK, Fares U, Rahman I, et al. Outcomes of deep anterior lamellar keratoplasty following successful and failed “big bubble”. Br J Ophthalmol. 2012;96:564–569. [DOI] [PubMed] [Google Scholar]
- 16.Koh S, Maeda N, Nakagawa T, et al. Quality of vision in eyes after selective lamellar keratoplasty. Cornea. 2012;31 (suppl 1):S45–S49. [DOI] [PubMed] [Google Scholar]
- 17.Dong J, Zhang Y, Zhang H, et al. Corneal densitometry in high myopia. BMC Ophthalmol. 2018;18:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garzón N, Poyales F, Illarramendi I, et al. Corneal densitometry and its correlation with age, pachymetry, corneal curvature, and refraction. Int Ophthalmol. 2017;37:1263–1268. [DOI] [PubMed] [Google Scholar]
- 19.Alnawaiseh M, Zumhagen L, Wirths G, et al. Corneal densitometry, central corneal thickness, and corneal central-to-peripheral thickness ratio in patients with Fuchs endothelial dystrophy. Cornea. 2016;35:358–362. [DOI] [PubMed] [Google Scholar]
- 20.Droutsas K, Lazaridis A, Giallouros E, et al. Scheimpflug densitometry after DMEK versus DSAEK-two-year outcomes. Cornea. 2018;37:455–461. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen E, Ivarsen A, Kristensen S, et al. Fuchs’ endothelial corneal dystrophy: a controlled prospective study on visual recovery after endothelial keratoplasty. Acta Ophthalmol. 2016;94:780–787. [DOI] [PubMed] [Google Scholar]
- 22.Patel SV, Baratz KH, Hodge DO, et al. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127:153–160. [DOI] [PubMed] [Google Scholar]
- 23.Dirisamer M, Parker J, Naveiras M, et al. Identifying causes for poor visual outcome after DSEK/DSAEK following secondary DMEK in the same eye. Acta Ophthalmol. 2013;91:131–139. [DOI] [PubMed] [Google Scholar]


