Abstract
Purpose:
To describe a case of postsurgical corneal decompensation in a patient with Fuchs endothelial dystrophy with a sectoral Descemet detachment and corneal edema that was successfully managed with a targeted Descemet stripping only (DSO) procedure.
Methods:
This is a case report and review of the literature.
Results:
A female patient with Fuchs endothelial dystrophy presented with a 6-month history of a persistent sectoral Descemet membrane detachment after cataract surgery with overlying corneal edema. Specular microscopy demonstrated moderate cell dropout with a cell density of 929 cells/mm2 in the affected eye. Intracameral air injection was attempted without improvement, and a sectoral DSO procedure was performed. Netarsudil and prednisolone were used postoperatively, and she demonstrated gradual improvement with resolution of the edema by postoperative week 10 and a central endothelial cell density of 675 cells/mm2 by postoperative month 8.
Conclusions:
DSO is a viable therapy in certain cases of postsurgical corneal decompensation.
Keywords: Descemet stripping only, DSO, Descemetorhexis without endothelial keratoplasty, DWEK, netarsudil, Fuchs endothelial dystrophy
Fuchs endothelial dystrophy is a common cause of endothelial decompensation and corneal edema in middle-aged and older patients. Dysfunctional endothelial cells have fewer sodium–potassium ATPase pumps, and they also create a thickened Descemet membrane by depositing collagen and extracellular matrix in Descemet membrane.1 Corneal guttae and edema usually start centrally and progress peripherally. Currently, corneal edema caused by decompensated Fuchs dystrophy is most commonly treated with endothelial keratoplasty.
However, Descemet stripping only (DSO) is an alternative approach gaining more interest where Descemet membrane is removed from the central visual axis, allowing healthier peripheral endothelial cells to migrate in and repopulate that area.2 Advantages of DSO include not needing donor tissue, no issues with graft rejection, and no risk of needing to rebubble a graft postoperatively. DSO has been described as a primary treatment for Fuchs endothelial dystrophy in the absence of previous surgery. Here, we describe a case of postsurgical corneal decompensation in a patient with Fuchs with a sectoral Descemet detachment extending from the periphery to the central cornea that was successfully managed with a targeted, sectoral DSO.
CASE REPORT
A 66-year-old woman with Fuchs endothelial dystrophy was referred with a persistent visually significant sectoral Descemet detachment in her right eye after cataract surgery 6 months previously. At initial evaluation, she had a visual acuity of 20/30, pinhole 20/20, intraocular pressure of 15 mm Hg, and central corneal thickness of 583 μm in the right eye and 521 μm in the left eye. Slit-lamp examination revealed a 3.2 mm (vertical) by 4.0 mm (horizontal) Descemet detachment contiguous with the inner lip of the main wound and extending to the central cornea with overlying microcystic edema (Fig. 1A). Anterior segment optical coherence tomography demonstrated a Descemet detachment from 7:00 to 9:00 from the periphery to the central cornea (Figs. 1B–C). Specular microscopy in an area of clear cornea showed cell dropout with a cell density of 929 cells/mm2 in the right eye and 961 cells/mm2 in the left eye (Fig. 1D). The patient was administered prednisolone acetate 1% 4 times daily, Muro 128 eye drops 4 times daily, and Muro 128 ointment at bedtime in the right eye.
FIGURE 1.
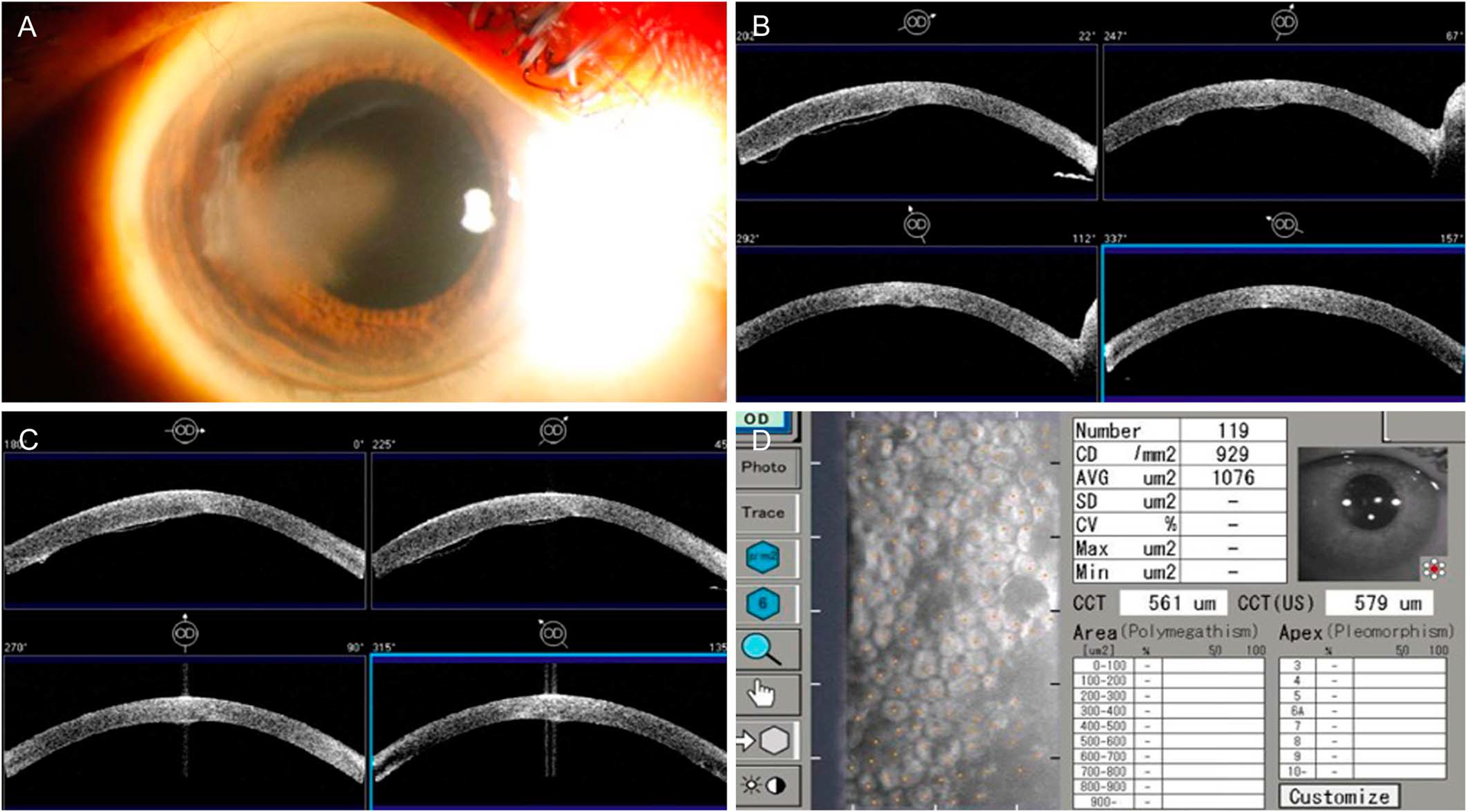
A, Slit-lamp photograph demonstrating temporal corneal edema adjacent to the main wound. B and C, Anterior segment optical coherence tomography demonstrating Descemet detachment from 7:00 to 9:00 from the periphery to central cornea. D, Specular microscopy demonstrating cell dropout with a cell density of 929 cells/mm2 in an area of clear cornea in the right eye.
There was no clinical improvement after 1 week, so she underwent intracameral air injection with full-time postoperative supine positioning for 2 days. Three weeks later, she was seen at her referring doctor’s office and was noted to have improved attachment of the Descemet membrane with 20/20 visual acuity in the right eye. However, over the next few weeks, her vision declined to 20/70 with worsening corneal edema and progressive redetachment of the Descemet membrane. The patient wanted to avoid a corneal transplant if possible, so DSO was suggested and she elected to proceed.
Intraoperatively, trypan blue and intraoperative OCT were used to help visualize the edges of the Descemet membrane. A reverse Sinskey hook was used to lift off the edge of the Descemet membrane peripherally, and then, bent internal limiting membrane forceps were used to perform the targeted sectoral Descemetorhexis, encompassing the entirety of the detached area.
Postoperative day 1 visual acuity was 20/60, and there was significant sectoral corneal edema in the area of the Descemetorhexis. Gatifloxacin and prednisolone acetate 1% were administered 4 times daily along with netarsudil twice daily. At postoperative week 1, visual acuity was 20/30–2 with improving corneal edema. By postoperative week 10, the corneal edema had fully resolved with a central corneal thickness of 548 μm. Visual acuity was 20/30+3, and on slit-lamp examination, guttae were apparent in the area of the Descemetorhexis. However, no cells were yet seen on specular microscopy. Prednisolone was decreased to once a day, and netarsudil and Muro 128 were continued. By postoperative month 4, central corneal thickness had improved to 492 μm and specular microscopy was able to detect a cell density of 318 cells/mm2. All eye drops were discontinued at this time. By postoperative month 8, there was continued improvement in the posterior corneal scar with guttae centrally in the area devoid of Descemet membrane (Fig. 2). The cell density had improved to 675 cells/mm2.
FIGURE 2.

A and B, Slit-lamp photographs at postoperative month 8 demonstrating resolution of corneal edema. C, The faint Descemetorhexis outline is most apparent at the central leading edge (red arrows).
DISCUSSION
This case report expands the indications for DSO to include postsurgical cornea edema. To this point, DSO has been described as a primary alternative to endothelial keratoplasty for Fuchs endothelial dystrophy.3,4 The success of DSO relies on a sufficient reserve of healthy peripheral endothelial cells and thus is not typically indicated for aphakic or pseudophakic bullous keratopathy where endothelial cells are diffusely damaged. However, when the endothelial cell loss is more localized, such as with clear corneal wound edema, an extreme form of which is epitomized in this case with a large Descemet detachment, a targeted DSO can be curative. Not only does the surgery avoid the risk of immunogenic rejection, but DSO also more precisely targets the area of pathology compared with endothelial keratoplasty. If traditional Descemet membrane endothelial keratoplasty (DMEK) or Descemet stripping automated endothelial keratoplasty (DSAEK) was performed for this particular case, recovery would not necessarily be any shorter than that with DSO because peripheral corneal edema and bullae would be expected until endothelial cell migration covered this area.
Although this present case carries an underlying diagnosis of Fuchs dystrophy, we categorize it as postsurgical corneal edema, more similar to a bullous keratopathy phenotype because the postoperative course after the initial cataract surgery is typical of non-Fuchs patients with a Descemet detachment complication. In fact, the endothelial cell reserve before cataract surgery was likely fairly robust because the cornea outside of the Descemet detachment was clear and compact. That the contralateral eye had a typically successful, rapid recovery after cataract surgery suggests that this eye’s corneal edema resulted from surgical factors rather than from inherent Fuchs decompensation. It is possible that the location of the Descemet detachment adjacent to the main wound, where there might have been more endothelial cell loss from surgery, prevented healthy peripheral endothelial cells from migrating in to cover the detached area. Perhaps, if the Descemet detachment was located elsewhere, peripheral endothelial cells could have migrated in successfully.
No standard protocol for DSO exists, but there is consensus that only a limited Descemetorhexis can be removed for subsequent successful corneal clearing. Koenig reported persistent corneal edema after a central 6 mm Descemetorhexis in eyes with Fuchs endothelial dystrophy, yet Borkar et al reported favorable results when only the central 4 mm of Descemet membrane was removed.5,6 In the present case, a targeted sectoral Descemetorhexis was performed with approximate dimensions of 6 mm (horizontal) by 4 mm (vertical). This case illustrates that in DSO, a Descemetorhexis does not have to be exclusively central to be successful.
The case supports the theory as to why DSO succeeds: removing a cellular or mechanical barrier that prevents endothelial cell migration. Small Descemet detachments after cataract surgery usually self-seal, but in this case, the waxing and waning nature of the Descemet detachment reflects an insufficient endothelial cell reserve. One month after the intracameral air injection, the Descemet detachment did improve, typically a sign of progression in the recovery process. A previous case report noted a similar phenomenon with a late Descemet detachment after cataract surgery that spontaneously reattached.7 The authors suggested that the temporal location may have allowed gravitational forces to help reappose Descemet membrane, which may have also played a role in our patient’s case. However, our patient’s Descemet detachment subsequently worsened, a reflection of severely impaired endothelial cells acting as a barrier to the migration of functional endothelial cells into the afflicted area. It is also possible that the detached Descemet had become taut because of the chronicity of the detachment, which prevented it from reattaching. Davies et al suggested a mechanical barrier phenomenon by describing differential success with DSO depending on the surgical method used. They reported greater success with removing Descemet membrane using a smooth tear rather than scoring and stripping, which may create a mechanical barrier to cell migration.8
Increasing evidence supporting the clinical efficacy of rho kinase inhibitors in treating corneal edema has emerged, mostly with ripasudil as monotherapy or adjunctive treatment to DSO in the postoperative period.9,10 Ripasudil has been shown to enhance visual recovery and endothelial cell counts after DSO,9,10 but because it is not commercially available in the United States, netarsudil, another rho kinase inhibitor approved for glaucoma, was used off-label in this case. The benefit of netarsudil in this case cannot be known for certain, and it is possible that it aids in cell migration more than cell function. Future research investigating DSO, netarsudil, and ripasudil would be beneficial.
Although endothelial keratoplasty is still the gold standard for the surgical management of endothelial dysfunction, DSO is a valuable alternative not only for Fuchs dystrophy but also for postsurgical corneal edema: if tissue is not readily available, the patient wants to avoid any risk of graft rejection, or if there is a strong motivation to minimize or eliminate the need for postoperative steroid use. This case report supports the theory that dysfunctional endothelial cells act as a barrier for the migration of healthy endothelial cells into a damaged area, necessitating the removal of that area of Descemet membrane and endothelial cells for corneal edema to resolve. In addition, this case demonstrates that the Descemetorhexis does not have to be centrally located and describes for the first time the adjunctive use of topical netarsudil in the postoperative period after DSO.
Acknowledgments
This work was supported by National Eye Institute core grant P30-026877 (Stanford) and Research to Prevent Blindness. The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Nanda GG, Alone DP. Review: current understanding of the pathogenesis of Fuchs’ endothelial corneal dystrophy. Mol Vis. 2019;25:295–310. [PMC free article] [PubMed] [Google Scholar]
- 2.Arbelaez JG, Price MO, Price FW. Long-term follow-up and complications of stripping descemet membrane without placement of graft in eyes with Fuchs endothelial dystrophy. Cornea. 2014;33:1295–1299. [DOI] [PubMed] [Google Scholar]
- 3.Iovieno A, Neri A, Soldani AM, et al. Descemetorhexis without graft placement for the treatment of Fuchs endothelial dystrophy: preliminary results and review of the literature. Cornea. 2017;36:637–641. [DOI] [PubMed] [Google Scholar]
- 4.Huang MJ, Kane S, Dhaliwal DK. Descemetorhexis without endothelial keratoplasty versus DMEK for treatment of Fuchs endothelial corneal dystrophy. Cornea. 2018;37:1479–1483. [DOI] [PubMed] [Google Scholar]
- 5.Koenig SB. Planned Descemetorhexis without endothelial keratoplasty in eyes with Fuchs corneal endothelial dystrophy. Cornea. 2015;34: 1149–1151. [DOI] [PubMed] [Google Scholar]
- 6.Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by descemet stripping without endothelial keratoplasty. Cornea. 2016;35:1267–1273. [DOI] [PubMed] [Google Scholar]
- 7.Ball JL, Stewart O, Taylor R. Spontaneous reattachment of extensive Descemet’s membrane detachment following uneventful phacoemulsification surgery. Eye (Lond). 2004;18:962. [DOI] [PubMed] [Google Scholar]
- 8.Davies E, Jurkunas U, Pineda R. Predictive factors for corneal clearance after Descemetorhexis without endothelial keratoplasty. Cornea. 2018; 37:137–140. [DOI] [PubMed] [Google Scholar]
- 9.Moloney G, Petsoglou C, Ball M, et al. Descemetorhexis without grafting for Fuchs endothelial dystrophy-supplementation with topical ripasudil. Cornea. 2017;36:642–648. [DOI] [PubMed] [Google Scholar]
- 10.Macsai MS, Shiloach M. Use of topical rho kinase inhibitors in the treatment of Fuchs dystrophy after Descemet stripping only. Cornea. 2019;38:529–534. [DOI] [PubMed] [Google Scholar]


