Abstract
Adinandra megaphylla Hu is a medicinal plant belonging to the Adinandra genus, which is well-known for its potential health benefits due to its bioactive compounds. This study aimed to assemble and annotate the chloroplast genome of A. megaphylla as well as compare it with previously published cp genomes within the Adinandra genus. The chloroplast genome was reconstructed using de novo and reference-based assembly of paired-end reads generated by long-read sequencing of total genomic DNA. The size of the chloroplast genome was 156,298 bp, comprised a large single-copy (LSC) region of 85,688 bp, a small single-copy (SSC) region of 18,424 bp, and a pair of inverted repeats (IRa and IRb) of 26,093 bp each; and a total of 51 SSRs and 48 repeat structures were detected. The chloroplast genome includes a total of 131 functional genes, containing 86 protein-coding genes, 37 transfer RNA genes, and 8 ribosomal RNA genes. The A. megaphylla chloroplast genome indicated that gene content and structure are highly conserved. The phylogenetic reconstruction using complete cp sequences, matK and trnL genes from Pentaphylacaceae species exhibited a genetic relationship. Among them, matK sequence is a better candidate for phylogenetic resolution. This study is the first report for the chloroplast genome of the A. megaphylla.
Subject terms: Biotechnology, Molecular biology, Plant sciences
Introduction
The chloroplast (cp) acts as a vital and essential organelle playing an indispensable role in several crucial biochemical processes and photosynthesis of plants1. The cp genome is uniparental inheritance and generally has a quadripartite structure including one large single-copy (LSC) region, one small single-copy (SSC) region, and two inverted repeat regions (IRs) of the same length2. In terms of gene structure and composition, the cp genome is more conserved, compared with nuclear and mitochondrial genomes3. These chloroplast DNA features were used by scientists to construct chloroplast DNA phylogenies, demonstrating to be greatly beneficial in the exploration of plant phylogenetic studies and more clarified taxonomic levels4,5. The whole chloroplast genome was reported as circular and its genes were a single evolutionary unit6,7. The chloroplast DNA genome sequencing in complex genome plants has been proven to be comparatively inexpensive and easy8,9.
Adinandra genus has been consumed as a traditional health tea beverage in China, and it has been described to have many curative effects, such as reduction of blood pressure, anti-inflammatory, antibacterial, antitumor, antitoxic, and analgesic effects10,11. Surprisingly, to date, limited reports have been published about Adinandra genus. Adinandra megaphylla Hu, belonging to the Adinandra genus, was first published in Bull. Fan Mem. Inst. Biol., Bot. 6: 172 (1935). The related information is about morphological descriptions12 and bioactivity assays13. A few species were used by molecular phylogenetics, including A. dumosa14; A. elegans, A. formosan, A. lasiostyla, A.millettii, A. yaeyamensi12; A. millettii, A. angustifolia15. To date, there have not been any studies on the genomes of A. megaphylla Hu, especially the chloroplast genome, this leads to the lack of information for estimating the phylogenetic relationships within Adinandra genus.
For the first time, we reported a new complete chloroplast genome of A. megaphylla Hu, and combined it with previously published Pentaphylacaceae complete chloroplast genomes data to visualize and evaluate the genome organization and phylogenetic relationships.
Results
Chloroplast genome assembly and annotation
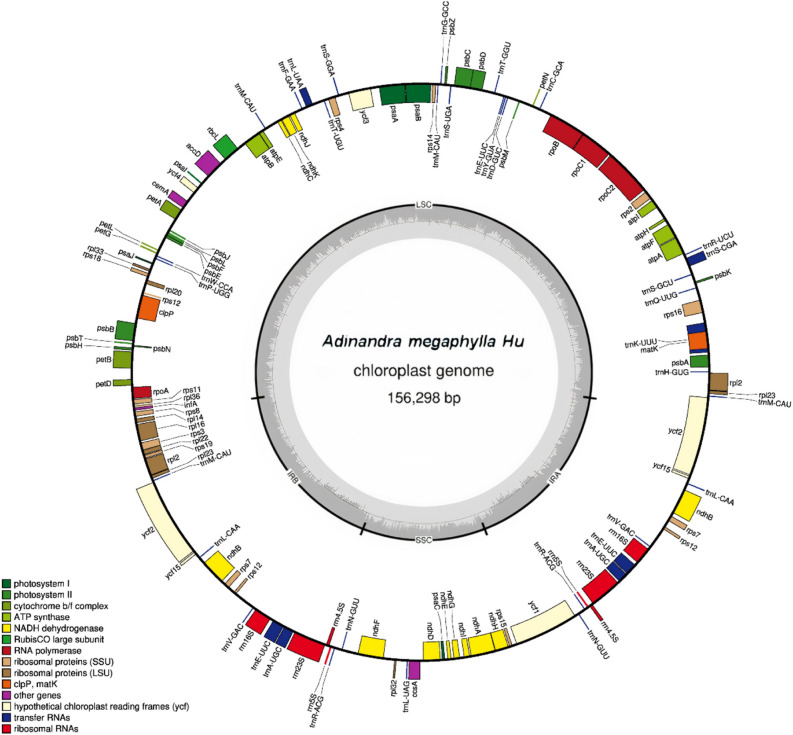
Using the PacBio SEQUEL system, 29,815,452 bp of raw sequence data of the whole genome were generated from A. megaphylla Hu (Fig. 1). The mean read length is 2938 bp, the N50 contig size is 3594 bp and approximately 5% of the genomic genome belongs to the cp genome with 188 × coverage. The cp genome size of 156,298 bp of A. megaphylla Hu was derived from the assembly. As shown in most cp genomes, the assembled A. megaphylla Hu plastome exhibited the typical quadripartite structure comprising of the four regions, a pair of inverted repeats (IRs 26,093 bp), LSC (85,688 bp), and SSC (18,424 bp). Besides, the cp genome of A. megaphylla Hu contains 131 genes, and the percent of the GC content of the cp genome was 37.4% (Table 1).
Figure 1.
Chloroplast map of A. megaphylla Hu in Vietnam. Genes shown inside the circle are transcribed clockwise, whereas genes outside are transcribed counterclockwise. The light gray inner circle shows the AT content, the dark gray corresponds to the GC content.
Table 1.
Summary of the chloroplast genome of A. megaphylla Hu species.
| Genome features | A. megaphylla Hu |
|---|---|
| Genome size (bp) | 156,298 |
| LSC size (bp) | 85,688 |
| SSC size (bp) | 18,424 |
| IR size (bp) | 26,093 |
| GC content (%) | 37.4 |
| No. of genes | 131 |
| No. of PCGs | 86 |
| No. of tRNA | 37 |
| No. of rRNA | 8 |
Chloroplast genome annotation
The cp genome of A. megaphylla Hu includes 37 tRNA genes, 8 rRNA genes (16S, 23S, 5S, and 4.5S), and 86 protein-coding genes (Table 1). There were 123 genes assigned into three groups based on their functions. Regarding the photosynthesis-related gene category, there are 43 genes, containing genes encoding the large subunit of Rubisco related to the photosynthetic electron transport chain and putative NADPH dehydrogenase genes. Also, 68 genes were functioning in the transcription and translation processes. The majorities are tRNA genes, and the others are rRNA genes and genes encoding subunits of RNA polymerase and ribosome proteins. The remaining twelve genes with different functions are classified in the category of other genes, including five genes with known functions in fatty acid synthesis (accD), c-type cytochrome synthesis (ccsA), carbon metabolism (cemA), (clpP) proteolysis, and RNA processing (matK). Otherwise, five genes encoding for the conserved reading frames (ycf1, ycf2, ycf3, ycf4, ycf15) with unknown functions were annotated in the plastome. Eighteen genes (all 4 rRNA genes, 7 tRNA genes, 4 ribosomal protein-coding genes, 1 NADH-dehydrogenase protein-coding gene and 2 other genes) were annotated with two copies located in IR regions (Table 2). There are 18 cp genes harbored introns, among which 13 genes (atpF, rpoC1, rpl2 (×2), ndhB (×2), ndhA, petB, rpl16, trnA-UGC (×2), trnC-ACA, trnE-UUC, trnK-UUU, trnL-UAA and trnS-CGA) contained one intron, while 2 genes (ycf3, clpP) contained 2 introns (Table 2).
Table 2.
Gene composition of A. megaphylla Hu chloroplast genome.
| Category of genes | Group of genes | Name of genes |
|---|---|---|
| Photosynthesis | Subunits of ATP synthase | atpA, atpB, atpE, atpFa, atpH, atpI |
| Subunits of NADH-dehydrogenase | ndhAa, ndhB (×2)a, ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK | |
| Subunits of cytochrome b/f complex | petL, petBa, petG, petA, petD, petN | |
| Subunits of photosystem I | psaJ, psaC, psaA, psaI, psaB | |
| Subunits of photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ | |
| Subunit of rubisco | rbcL | |
| Transcription and translation | Large subunit of ribosome | rpl14, rpl16a, rpl2 (×2)a, rpl20, rpl22, rpl23 (×2), rpl32, rpl33, rpl36 |
| DNA dependent RNA polymerase | rpoB, rpoA, rpoC1a, rpoC2 | |
| Small subunit of ribosomal proteins | rps11, rps12, rps14, rps15, rps16a, rps18, rps19 (×2), rps2, rps3, rps4, rps7 (×2), rps8 | |
| rRNA genes | rrn23S (×2), rrn16S (×2), rrn5S (×2), rrn4.5S (×2) | |
| tRNA genes | trnA-UGC (×2)a, trnC-ACAa, trnC-GCA, trnD-GUC, trnE-UUC (×2)a, trnF-GAA, trnG-GCC, trnH-GUG, trnK-UUUa, trnL-CAA (×2), trnL-UAAa, trnL-UAG, trnM-CAU (×2), trnN-GUU (×2), trnP-UGG, trnQ-UUG, trnR-ACG (×2), trnR-UCU, trnS-CGAa, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC (×2), trnW-CCA, trnY-GUA | |
| Translational initiation factor | infA | |
| Other genes | Subunit of acetyl-CoA-carboxylase (fatty acid synthesis) | accD |
| c-type cytochrome synthesis gene | ccsA | |
| Envelope membrane protein (carbon metabolism) | cemA | |
| Protease | clpPb | |
| Maturase (RNA processing) | matK | |
| Conserved open reading frames | ycf1, ycf2 (×2), ycf3b, ycf4, ycf15 (×2) |
Genes marked with the sign are the gene with a single (a) or double (b) introns and duplicated genes (×2).
Repeat sequences and codon analysis
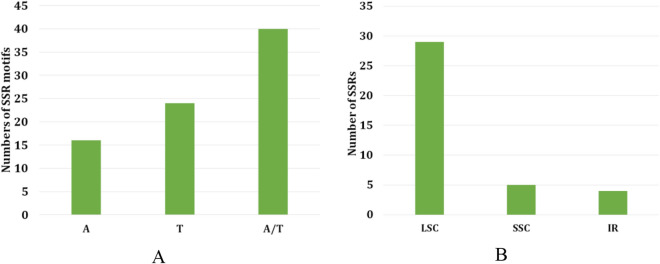
The total number of identified simple sequence repeats (SSRs) in the chloroplast genome of A. megaphylla Hu was 40. All repeats were mono repeats composed of A or T (size of 10–19) (Fig. 2A). There were no di-, tri-, tetra-, penta-, and hexa-nucleotide SSRs in the A. megaphylla Hu (Fig. 2B).
Figure 2.
Analysis of single sequence repeats of plastome in A. megaphylla Hu. (A) Number of identified SSR sequence motifs; (B) Frequency of repeat types in LSC, SSC, and IR regions.
The cp genome of A. megaphylla Hu was identified with 49 repeats consisting of 26 palindromic repeats, 19 forward and 4 reverse repeats. There were no complement repeats (Fig. 3). The smallest unit size of the repeat was 22 bp while the largest unit size was 62 bp. Most of the size of the repeats (72%) was higher than 30 bp.
Figure 3.
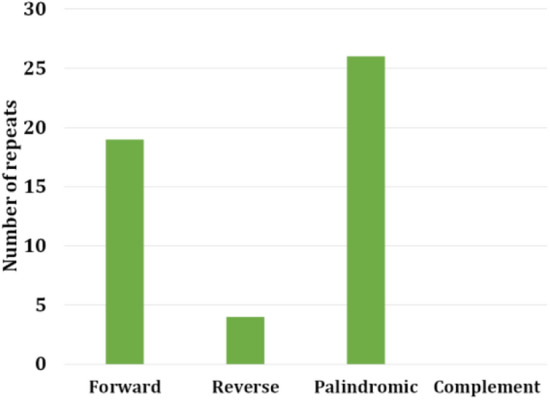
Repeat analysis on a genomic scale in A. megaphylla Hu.
The codon usage frequency of 64 protein-coding genes for three Adinandra species was evaluated. The total number of codons for protein-coding genes was 52,076 in those coding regions. G- and C-ending are found to be more frequent than their counterparts A and U (Table 3). Among the 20 amino acids, serine was the most abundant (number of codons encoding serine = 4975, 9.55%), leucine ranked second (number of codons encoding leucine = 4883, 9.37%), while the rarest one is tryptophan (677 codons, approximately 1.3%). Thirty codons were observed to be used more frequently than the expected usage at equilibrium (RSCU > 1) and thirty-one codons showed the codon usage bias: (RSCU < 1). Moreover, the frequency of use for the start codons AUG and UGG (methionine and tryptophan), as well as AUA (isoleucine) showed no bias (RSCU = 1).
Table 3.
Relative synonymous codon usage (RSCU) for protein-coding genes in A. megaphylla Hu.
| Codon | AA | ObsFreq | RCSU | Codon | AA | ObsFreq | RCSU | Codon | AA | ObsFreq | RCSU |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UAG | * | 672 | 0.73 | AUC | I | 1173 | 0.77 | CGC | R | 230 | 0.41 |
| UGA | * | 914 | 0.99 | AUU | I | 1853 | 1.22 | CGG | R | 361 | 0.65 |
| UAA | * | 1177 | 1.28 | AAA | K | 2071 | 1.33 | CGU | R | 401 | 0.72 |
| GCA | A | 382 | 1.06 | AAG | K | 1049 | 0.67 | AGC | S | 559 | 0.67 |
| GCC | A | 347 | 0.96 | CUA | L | 661 | 0.81 | AGU | S | 795 | 0.96 |
| GCG | A | 210 | 0.58 | CUC | L | 640 | 0.79 | UCA | S | 870 | 1.05 |
| GCU | A | 505 | 1.4 | CUG | L | 475 | 0.58 | UCC | S | 908 | 1.1 |
| UGC | C | 477 | 0.81 | CUU | L | 1019 | 1.25 | UCG | S | 638 | 0.77 |
| UGU | C | 697 | 1.19 | UUA | L | 934 | 1.15 | UCU | S | 1205 | 1.45 |
| GAC | D | 426 | 0.55 | UUG | L | 1154 | 1.42 | ACA | T | 680 | 1.12 |
| GAU | D | 1131 | 1.45 | AUG | M | 889 | 1 | ACC | T | 628 | 1.04 |
| GAA | E | 1336 | 1.39 | AAC | N | 798 | 0.6 | ACG | T | 412 | 0.68 |
| GAG | E | 588 | 0.61 | AAU | N | 1863 | 1.4 | ACU | T | 701 | 1.16 |
| UUC | F | 1579 | 0.83 | CCA | P | 790 | 1.28 | GUA | V | 654 | 1.18 |
| UUU | F | 2235 | 1.17 | CCC | P | 605 | 0.98 | GUC | V | 450 | 0.81 |
| GGA | G | 816 | 1.44 | CCG | P | 386 | 0.62 | GUG | V | 377 | 0.68 |
| GGC | G | 347 | 0.61 | CCU | P | 694 | 1.12 | GUU | V | 741 | 1.33 |
| GGG | G | 543 | 0.96 | CAA | Q | 1033 | 1.4 | UGG | W | 677 | 1 |
| GGU | G | 562 | 0.99 | CAG | Q | 445 | 0.6 | UAC | Y | 695 | 0.65 |
| CAC | H | 375 | 0.58 | AGA | R | 1136 | 2.04 | UAU | Y | 1452 | 1.35 |
| CAU | H | 926 | 1.42 | AGG | R | 624 | 1.12 | ||||
| AUA | I | 1523 | 1 | CGA | R | 582 | 1.05 |
*stop codon.
Comparative chloroplast genomic analysis
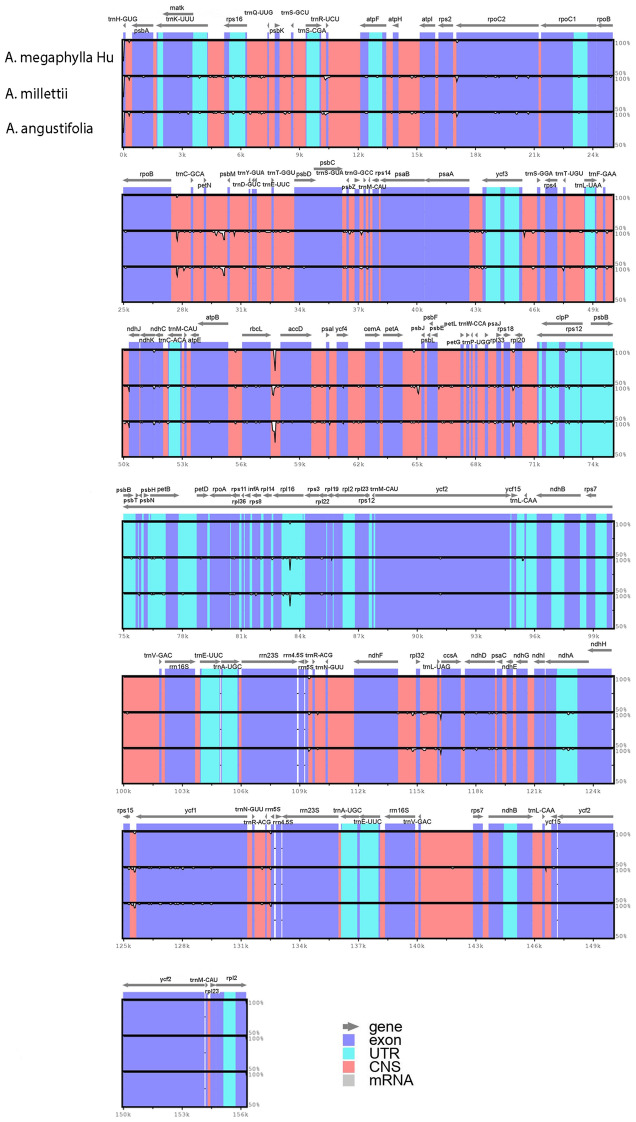
To characterize genome divergence, the annotation of A. megaphylla Hu was taken as references. The comparison revealed that three chloroplast genomes were highly similar (Fig. 4). The plastome sequences were fairly conserved across the three data with a few regions with a variation. The results exhibited the divergence in LSC and SSC regions were higher than in IR regions. Besides, the sequences in the coding regions tended to be more conserved whereas most of the variations detected were found in conserved non-coding sequences (CNS). The sequences of exons were nearly identical throughout the three taxa. Among the coding genes, the highly disparate regions included matK, rpoC2, ndhK, ndhD, ycf1.
Figure 4.
Identity plot comparing the chloroplast genomes of three Adinandra species.
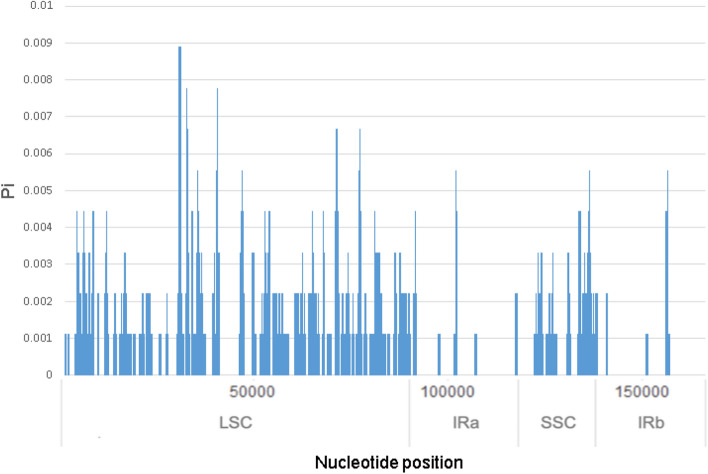
The sliding window analysis showed that the average pi value of the LSC (Pi = 0.001569) and SSC (Pi = 0.001339) regions was much higher than that in the IR (Pi = 0.000219) regions, which showed that LSC and SSC regions contained the most of the variation (Fig. 5). Among the 3 Adinandra species, the average value of nucleotide diversity (Pi) was 0.00119.
Figure 5.
Comparative analysis of nucleotide diversity (Pi) values among the three Adinandra species cp genome sequences.
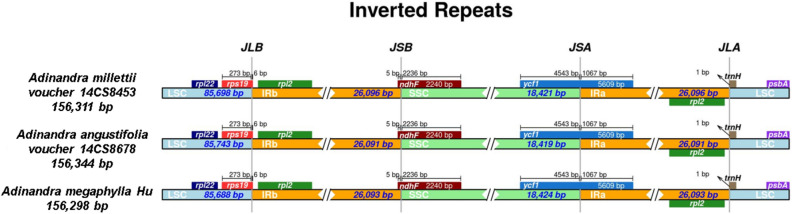
IR contraction and expansion in the chloroplast genome
The IR and SC boundaries of the three Adinandra were compared. Overall, the results indicated that the size, organization and gene content of the chloroplast genomes were highly similar among the three species. The size of IR ranges from 26,089 bp (A. megaphylla Hu) to 26,095 bp (A. millettii). And the size of IR of A. angustifolia was 26,092 bp. The ndhF gene was situated within the LSC region with a 5 bp overlap with the IRa for all three Adinandra species. Similarly, the rps19 gene was positioned within the LSC region with a 6 bp overlap with the IRb. The border across IRa and SSC was located in the region of the ycf1 gene with 1067 bp tail section of the gene placed in the IRa (Fig. 6). Results of the IR analysis witnessed neither expansion nor contraction of IR regions in the three species.
Figure 6.
Comparison of LSC, IR and SSC junction positions among the three chloroplast genomes. JLB (junction IRb/LSC), JSB (junction IRb/SSC), JSA (junction IRa/SSC), JLA (junction IRa/LSC).
Phylogenetic inference
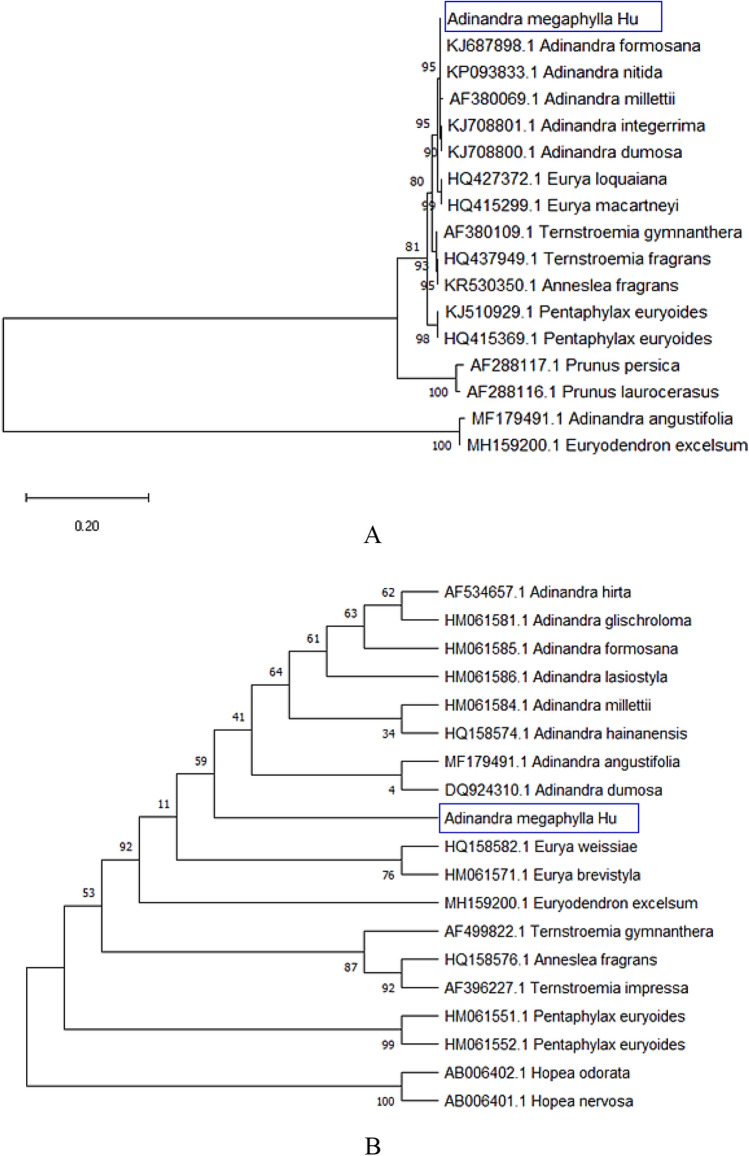
As shown in Fig. 7A, the phylogenetic analysis was based on matK sequences recovered good resolution among genera. In the Pentaphylacaceae, Euryodendron and Adinandra angustifolia separates outside other genera. The clade of genus Ternstroemia and Anneslea were sisters to the clade of Adinandra and Eurya genera. Indeed, all six Adinandra species are grouped in one clade, which is divided into three subclades with 95% support; A. millettii stood alone in one subclade, A. integerrima and A. dumosa formed the second subclade, three other species separated into the third one. These results were different from the previous study15, in which phylogenetic analysis of A. angustifolia, A. millettii, Anneslea fragan and Ternstroemia gymnanthera inferred from the LSC dataset indicated that they belong to one clade (bootstrap values = 100%). This difference might be due to the shortcoming of indicates in phylogenetic analysis when only these four species were representatives of the Pentaphylacaceae appearing in Zhang et al.’s study. In contrast with the matK sequence, the trnL region dataset yielded less phylogenetic resolution than the bootstrap value was 59% at the clade of the genus Adinandra (Fig. 7B). Additionally, the Adinandra was separated into six subclades; one constructed by the studied A. megaphylla Hu, A. formosana and A. lasiostyla constructed two distinct subclades. A. hirta and A. glischroloma; A. millettii and A. hainanensis; A. angustifolia and A. dumosa formed three separated subclades, respectively (Fig. 7B). In the case of barcoding among the Pentaphylacaceae family, the matK sequence is suggested for better phylogenetic resolution.
Figure 7.
Phylogenetic relationship was inferred using the Maximum Likelihood method based on matK (A) and trnL (B) genes.
Discussion
Pentaphylacaceae is a family of flowering plants and contains 12 genera including approximately 345 species over the world16. A total of 8 cp genomes in the Pentaphylacaceae family have been published currently, 2 of which belong to Adinandra. The genus Adinandra consists of about 85 species mainly distributed in Bangladesh, Cambodia, China, India, Indonesia, Southern Japan, Laos, Malaysia, Myanmar, New Guinea, Philippines, Sri Lanka, Thailand, and the African tropical forest17. Because of bioactive compounds, many species in the genus Adinandra are of interest18–23.
In the present study, we recently sequenced whole cp genomes for one Vietnamese.
Adinandra megaphylla Hu and implemented comparative analyses on three Adinandra cp genomes to explore the structure of cp genomes in the taxa. Gene organization together with codon usage patterns was characterized and results indicated the high conservation, which can be helpful for phylogenetic and population genetics studies.
Angiosperm chloroplast genomes have a highly conserved structure and gene content24,25. Roughly 129 genes are usually found across the angiosperm chloroplast genomes, among which 18 genes include introns. The analyzed Adinandra chloroplast genomes specified the typical quadripartite structure and showed the expected size range (~ 15.6 kb) for angiosperm plants and the conserved gene contents25,26. Our gene annotation results were similar to the genetic properties of angiosperm chloroplast genomes. The number of genes present in the cp genome from A. megaphylla Hu was 131 and there 18 genes related to introns.
Apart from the two copies of inverted repeats, 48 small repeats were spread out within coding and non-coding regions of the three Adinandra taxa. The repeat numbers are not remarkably higher but comparable to other counterparts (the number of dispersed repeats: 49 in Papaver spp.; 21 in Paris spp.; 36 in Passiflora; 37 in Aconitum)27–29. Repeats are highly associated with the plastome reconstruction in several angiosperm taxa and can be considered as an indication of recombination30. Due to the potential to generate secondary structures, repeated sequences can act as recognition signals during the recombination process31. It is supposed that recombination rarely occurs in angiosperms because of the predominance of uniparental inheritance. Nevertheless, evidence of intermolecular homologous recombination in flowering plants has been reported32,33. To date, studies screening plastome recombination in the taxa are entirely lacking. There was no research demonstrating the presence of plastome recombination in Pentaphylacaceae. In this study, the higher number of repeats in comparison with previous estimates might not be substantiation for inter- and intra-specific plastome recombination.
In terms of constructing phylogenetic relationships of plants, complete chloroplast genomes contribute adequate information and have proven their effectiveness in the capability of classification in lower taxonomic levels34,35. matK is one of the common DNA barcodes used in plants36. However, the phylogeny results indicated that using only a single gene for species classification may generate different results from different genes. The combination of these barcodes can lead to better species identification.
Conclusion
In this study, three complete chloroplast genomes of Adinandra were investigated, including one firstly sequenced chloroplast genomes (A. megaphylla Hu) comparatively analyzed with other published genus in the family of Pentaphylacaceae for the first time. We assemble the complete chloroplast genome of A. megaphylla Hu with 156,298 bp. The structure and gene content of the chloroplast genome of three Adinandra were similar and appeared highly conserved. Finally, the phylogenetic relationships built for species of Pentaphylacaceae, in terms of comparison public date with our novel sequence of Adinandra species. This study provides the potential of chloroplast genome sequences for enhancing species classification and phylogenetic research for in-depth study within Pentaphylacaceae.
Material and methods
Sample collection
Samples were collected in Hoang Lien—Van Ban Nature Reserve that belongs to Liem Phu Commune, Van Ban District, Lao Cai Province, Vietnam in August 2019 (code number: Nguyen Huu Quan 01), 1200 m, 21°59′15″N; 104°19′28″E. The taxonomic identification is authenticated by Associate Professor Danh Thuong SY, the head of Botany Department in Faculty of Biology, Thai Nguyen University of Education; the voucher specimens were placed in the Herbarium of the Institute of Ecology and Biological Resources (HN), Hanoi, Vietnam. Fresh leaves with the same code number were used to extract genomic DNA (Fig. 8).
Figure 8.
Morphological characteristic of A. megaphylla Hu. (A) Habit; (B) flowering twig; (C) bud and flower; Photos by Huu Quan Nguyen.
The collection of plant materials has complied with relevant institutional of Hoang Lien—Van Ban Nature Reserve, Vietnamese and international guidelines and legislation.
DNA extraction and chloroplast genome sequencing
Genomic DNA was extracted from young plant leaves using a modified CTAB method37. A260/280 and A260/A230 ratios were measured with the Shimadzu Biospec Nano to assess DNA sample purity. The accurate concentration of double-stranded DNA was determined with Qubit 3 Fluorometer and Qubit HS DNA reagents. Genomic DNA integrity was assessed by agarose gel electrophoresis with 0.8% agarose. Also, DNA libraries were prepared from total genomic DNA using SMRTbell Express Template Prep Kit 2.0 (Pacific Biosciences, Menlo Park, CA), and adapter ligation was subsequently performed, following the manufacturer’s protocol for genomic DNA above 20 kb (Pacific Biosciences). SMRTbell libraries were loaded on one chip and sequenced on a Pacbio SEQUEL system at the Key Laboratory for Gene Technology, Institution of Biotechnology (Hanoi, Vietnam).
Genome assembly and annotation
The total gDNA was sequenced in the PacBio platform by the resequencing method. The sequences derived from the cp genome were identified via the local Blast program38 using Adinandra angustifolia (MF179491) cp genomes as the reference15. Subsequently, the software HGAP439 was used to assemble the cp genome. The protein-coding, rRNA, and tRNA genes were annotated by the CpGAVAS pipeline40. The tRNAscan-SE ver. 1.21 software41 was applied to verify the tRNA genes with default parameters. The OrganellarGenomeDRAW tool (OGDRAW) ver. 1.3.142 was selected to create the circular gene map. Repeat elements were found using two approaches. Web-based simple sequence repeats finder MISA-web43 was used to detect microsatellites, including 10 repeat units for mono-, 5 repeat units for di-, 4 repeat units for tri-, and 3 repeat units for tetra-, penta-, and hexa-nucleotide SSRs. Among the SSRs of each type, comparing the size of SSRs was employed to count the polymorphic SSRs among the three species. The size and type of repeats in the three Adinandra plastomes were investigated using REPuter44 with the set parameters as follows: a minimal repeat size of 20 bp, hamming distance of 3 kb, and 90% or greater sequence identity.
Genome comparison
For comparative purposes, we collected two available cp genomes of A. angustifolia (#MF179491) and A. millettii (#MF179492) from GenBank (https://www.ncbi.nlm.nih.gov/genbank/). The overall genome structure, genome size, gene content and repeats across all three Adinandra species were compared15. The whole plastome sequences of the three Adinandra plants were aligned with the MAFFT server45 and visualized using LAGAN mode in mVISTA46. For the mVISTA plot, we used the annotated cp genome of A. megaphylla Hu as a reference. The Irscope47 was employed to visually display and compare the borders of large single-copy (LSC), small single-copy (SSC), and inverted repeat (IR) regions among the three Adinandra species. We also determined the codon usage bias and the sequence divergence among the three Adinandra species through a sliding window analysis computing pi among the chloroplast genomes in DnaSP ver. 6.12.0348. For the sequence divergence analysis, we applied the window size of 600 bp with a 200 bp step size.
Phylogenetic identification
The sequences of matK and trnL from all Adinandra species and other members of the family Pentaphylacaceae from Genbank (https://www.ncbi.nlm.nih.gov/genbank/) were used to identify the taxonomic position of the studied A. megaphylla Hu. These sequences were aligned with ClustalW mode in Unipro UGENE software v36.049 before a maximum likelihood (ML)50 phylogenetic tree was constructed using Mega-X software51 with 1000 bootstraps. The chosen methods followed the previous study of this genus15.
Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.02-2018.338.
Author contributions
H.Q.N., T.N.L.N., D.T.S., H.H.C. and H.M.C. conceived and designed the experiments; H.Q.N., T.N.L.N., T.N.D., T.L.L. and M.H.P. performed the experiments; D.T.S., H.Q.N., M.H.P, T.L.L. and T.T.N.N. performed data analyses; H.Q.N., T.N.D., T.N.L.N., D.T.S. and H.M.C. prepared the manuscript; T.N.L.N, H.H.C and H.M.C. made the Proof-reading. All authors approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/3/2022
The original online version of this Article was revised: In the original version of this Article, there was an error in the name of the species Adinandra megaphylla, which has now been corrected in the original Article.
Contributor Information
Thi Ngoc Lan Nguyen, Email: ntnlan.dhsptn@tnu.edu.vn.
Hoang Mau Chu, Email: chuhoangmau@tnu.edu.vn.
References
- 1.Neuhaus HE, Emes MJ. Nonphotosynthetic metabolism in plastids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- 2.Bendich AJ. Circular chloroplast chromosomes: The grand illusion. Plant Cell. 2004;16:1661–1666. doi: 10.1105/tpc.160771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asaf S, et al. Comparative analysis of complete plastid genomes from wild soybean (Glycine soja) and nine other Glycine species. PLoS ONE. 2017;12:e0182281. doi: 10.1371/journal.pone.0182281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alwadani KG, Janes JK, Andrew RL. Chloroplast genome analysis of box-ironbark Eucalyptus. Mol. Phylogenet. Evol. 2019;136:76–86. doi: 10.1016/j.ympev.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves DJP, Simpson BB, Ortiz EM, Shimizu GH, Jansen RK. Incongruence between gene trees and species trees and phylogenetic signal variation in plastid genes. Mol. Phylogenet. Evol. 2019;138:219–232. doi: 10.1016/j.ympev.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Gitzendanner MA, Soltis PS, Wong GKS, Ruhfel BR, Soltis DE. Plastid phylogenomic analysis of green plants: A billion years of evolutionary history. Am. J. Bot. 2018;105:291–301. doi: 10.1002/ajb2.1048. [DOI] [PubMed] [Google Scholar]
- 7.Gu C, Ma L, Wu Z, Chen K, Wang Y. Comparative analyses of chloroplast genomes from 22 Lythraceae species: Inferences for phylogenetic relationships and genome evolution within Myrtales. BMC Plant Biol. 2019;19:281. doi: 10.1186/s12870-019-1870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo X, et al. Plastome phylogeny and early diversification of Brassicaceae. BMC Genomics. 2017;18:176. doi: 10.1186/s12864-017-3555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saarela JM, et al. A 250 plastome phylogeny of the grass family (Poaceae): Topological support under different data partitions. PeerJ. 2018;6:e4299. doi: 10.7717/peerj.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, She G, Chen H. Study on antitumor activities of substances extracted from Adinandra nitida Merr. ex H. L. Li. Nat. Sci. 1997;12:43–45. [Google Scholar]
- 11.Yu J, Chen M. Studies on flavonoids extraction from Adinandra nitida Merr. ex H. L. Li. and on their antioxidative and bacteriostatic bioactivities. J. Shantou Univ. 1997;12:52–58. [Google Scholar]
- 12.Wu CC, Hsu ZF, Tsou CH. Phylogeny and taxonomy of Eurya (Ternstroemiaceae) from Taiwan, as inferred from ITS sequence data. Bot. Stud. 2007;48:97–116. [Google Scholar]
- 13.Nguyen TNL, et al. Antibacterial, antioxidant and anti—Cancerous activities of Adiandra megaphylla Hu leaf extracts. Biosci. Biotechnol. Res. Commun. 2020;13:1015–1020. doi: 10.21786/bbrc/13.3/5. [DOI] [Google Scholar]
- 14.Rosea JP, et al. Phylogeny, historical biogeography, and diversification of angiosperm order Ericales suggest ancient Neotropical and East Asian connections. Mol. Phylogenet. Evol. 2018;122:59–79. doi: 10.1016/j.ympev.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Yu XQ, et al. Insights into the historical assembly of East Asian subtropical evergreen broadleaved forests revealed by the temporal history of the tea family. New Phytol. 2017;215:1235–1248. doi: 10.1111/nph.14683. [DOI] [PubMed] [Google Scholar]
- 16.Steven, P. F. Angiosperm Phylogeny Website. Version 14, July 2017 [and More or Less Continuously Updated Since]. http://www.mobot.org/MOBOT/research/APweb/ (2001 onwards).
- 17.Min, T. L. & Bruce, B. B. Theaceae. In Flora of China, Vol. 12 (eds Wu Z. Y. et al.) 435–443 (Science Press, Beijing and Missouri Botanical Garden Press, 2007).
- 18.Yuan C, Huang L, Suh JH, Wang Y. Bioactivity-guided isolation and identification of antiadipogenic compounds in Shiya tea (leaves of Adinandra nitida) J. Agric. Food Chem. 2019;67:6785–6791. doi: 10.1021/acs.jafc.9b01326. [DOI] [PubMed] [Google Scholar]
- 19.Brad K, Zhang Y. Study on extraction and purification of apigenin and the physical and chemical properties of its complex with lecithin. Pharmacogn. Mag. 2018;14:203–206. doi: 10.4103/pm.pm_159_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Chen G, Fu X, Liu RH. Phytochemical profiles and antioxidant activity of different varieties of Adinandra tea (Adinandra Jack) J. Agric. Food Chem. 2015;63:169–176. doi: 10.1021/jf503700v. [DOI] [PubMed] [Google Scholar]
- 21.Gao H, Liu B, Liu F, Chen Y. Anti-proliferative effect of camellianin A in Adinandra nitida leaves and its apoptotic induction in human Hep G2 and MCF-7 cells. Molecules. 2010;15:3878–3886. doi: 10.3390/molecules15063878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Yang J, Ma Y, Yuan E, Chen C. Antioxidant and angiotensin converting enzyme (ACE) inhibitory activities of ethanol extract and pure flavonoids from Adinandra nitida leaves. Pharm. Biol. 2010;48:1432–1438. doi: 10.3109/13880209.2010.490223. [DOI] [PubMed] [Google Scholar]
- 23.Zuo W, Xu J, Zhou C, Gan L. Simultaneous determination of five flavonoid compounds in leaves of Adinandra nitida by HPLC-PAD. China J. Chin. Mater. Med. 2010;35:2406–2409. [PubMed] [Google Scholar]
- 24.Daniell H, Lin CS, Yu M, Chang WJ. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer JD. Comparative organization of chloroplast genomes. Annu. Rev. Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- 26.Jansen RK, et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA. 2007;104:19369–19374. doi: 10.1073/pnas.0709121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X, et al. Comparative chloroplast genomes of Paris Sect. Marmorata: Insights into repeat regions and evolutionary implications. BMC Genomics. 2018;19:878. doi: 10.1186/s12864-018-5281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng J, et al. Comparative analysis of the complete chloroplast genomes of four aconitum medicinal species. Molecules. 2018 doi: 10.3390/molecules23051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J, et al. Complete chloroplast genomes of Papaver rhoeas and Papaver orientale: Molecular structures, comparative analysis, and phylogenetic analysis. Molecules. 2018 doi: 10.3390/molecules23020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen RK, Ruhlman TA. Plastid genomes of seed plants. In: Bock R, Knoop V, editors. Genomics of chloroplasts and mitochondria. Advances in Photosynthesis and Respiration (Including Bioenergy and Related Processes) Springer; 2012. pp. 103–126. [Google Scholar]
- 31.Kawata M, Harada T, Shimamoto Y, Oono K, Takaiwa F. Short inverted repeats function as hotspots of intermolecular recombination giving rise to oligomers of deleted plastid DNAs (ptDNAs) Curr. Genet. 1997;31:179–184. doi: 10.1007/s002940050193. [DOI] [PubMed] [Google Scholar]
- 32.Medgyesy P, Fejes E, Maliga P. Interspecific chloroplast recombination in a nicotiana somatic hybrid. Proc. Natl. Acad. Sci. USA. 1985;82:6960–6964. doi: 10.1073/pnas.82.20.6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan AR, Schiffthaler B, Thompson SL, Street NR, Wang XR. Interspecific plastome recombination reflects ancient reticulate evolution in picea (pinaceae) Mol. Biol. Evol. 2017;34:1689–1701. doi: 10.1093/molbev/msx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He S, Wang Y, Volis S, Li D, Yi T. Genetic diversity and population structure: Implications for conservation of wild soybean (Glycine soja Sieb. et Zucc) based on nuclear and chloroplast microsatellite variation. Int. J. Mol. Sci. 2012 doi: 10.3390/ijms131012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, et al. The complete chloroplast genome sequences of five epimedium species: Lights into phylogenetic and taxonomic analyses. Front. Plant Sci. 2016;7:306. doi: 10.3389/fpls.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong W, et al. Discriminating plants using the DNA barcode rbcLb: An appraisal based on a large data set. Mol. Ecol. Resour. 2014;14:336–343. doi: 10.1111/1755-0998.12185. [DOI] [PubMed] [Google Scholar]
- 37.Souza HAV, Muller LAC, Brandão RL, Lovato MB. Isolation of high quality and polysaccharide-free DNA from leaves of Dimorphandra mollis (Leguminosae), a tree from the Brazilian Cerrado. Genet. Mol. Res. 2012;11:756–764. doi: 10.4238/2012.March.22.6. [DOI] [PubMed] [Google Scholar]
- 38.Johnson M, et al. NCBI BLAST: A better web interface. Nucl. Acids Res. 2008;36(Web Server Issue):W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chin CS, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat. Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, et al. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genomics. 2012;13:715. doi: 10.1186/1471-2164-13-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowe TM, Eddy SR. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohse M, Drechsel O, Bock R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007;52:267–274. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 43.Beier S, Thiel T, Münch T, Scholz U, Mascher M. MISA-web: A web server for microsatellite prediction. Bioinformatics. 2017;33:2583–2585. doi: 10.1093/bioinformatics/btx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurtz S, Schleiermacher C. REPuter: Fast computation of maximal repeats in complete genomes. Bioinformatics. 1999;15:426–427. doi: 10.1093/bioinformatics/15.5.426. [DOI] [PubMed] [Google Scholar]
- 45.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayor C, et al. VISTA: Visualizing global DNA sequence alignments of arbitrary length. Bioinformatics. 2000;16:1046–1047. doi: 10.1093/bioinformatics/16.11.1046. [DOI] [PubMed] [Google Scholar]
- 47.Amiryousefi A, Hyvönen J, Poczai P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34:3030–3031. doi: 10.1093/bioinformatics/bty220. [DOI] [PubMed] [Google Scholar]
- 48.Rozas J, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 49.Okonechnikov K, Golosova O, Fursov M, Team U. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics. 2012;28:1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- 50.John PH, Keith AC. Phylogeny estimation and hypothesis testing using maximum likelihood. Annu. Rev. Ecol. Syst. 1997;28:437–466. doi: 10.1146/annurev.ecolsys.28.1.437. [DOI] [Google Scholar]
- 51.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]