Abstract
Objective
Telemedicine has rapidly gained momentum in movement disorder neurology during the coronavirus disease (COVID-19) pandemic to preserve clinical care while mitigating the risks of in-person visits. We present data from the rapid implementation of virtual visits in a large, academic, movement disorder practice during the COVID-19 pandemic.
Methods
We describe the strategic shift to virtual visits and retrospectively examine elements that impacted the ability to switch to telemedicine visits using historical prepandemic in-person data as a comparator, including demographics, distance driven, and diagnosis distribution, with an additional focus on patients with deep brain stimulators.
Results
A total of 686 telemedicine visits were performed over a five-week period (60% of those previously scheduled for in-office visits). The average age of participants was 65 years, 45% were female, and 73% were Caucasian. Men were more likely to make the transition (p = 0.02). Telemedicine patients lived farther from the clinic than those seen in person (66.47 km vs. 42.16 km, p < 0.001), age was not associated with making the switch, and patient satisfaction did not change. There was a significant shift in the distribution of movement disorder diagnoses seen by telemedicine compared to prepandemic in-person visits (p < 0.001). Patients with deep brain stimulators were more likely to use telemedicine (11.5% vs. 7%, p < 0.001).
Conclusion
Telemedicine is feasible, viable and relevant in the care of movement disorder patients, although health care disparities appear evident for women and minorities. Patients with deep brain stimulators preferred telemedicine in our study. Further study is warranted to explore these findings.
Keywords: COVID-19, Movement disorders, Pandemic, Telemedicine
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease (COVID-19), has dramatically changed outpatient neurological care. The spread of the virus is reduced through social distancing, and healthcare providers are required to curtail the indications for face-to-face encounters to provide patient care.
Telemedicine is the use of real-time audio-visual technology to communicate with a patient and provide medical care when distance separates the participants. While telemedicine has been widely used in acute stroke and neurocritical care for years [1], it was gaining momentum in many other neurological subspecialties pre-COVID [2,3]. It is emerging in movement disorders, with the most common focus in Parkinson’s disease (PD) [4-8]; virtual care models for tremor and hyperkinetic movements have also been documented [9]. Policy changes loosening reimbursement restrictions, driven by the Centers for Medicare and Medicaid Services and followed by other payers, allowed organizations to rapidly implement telemedicine care models for evaluation and management (E&M) visits but not deep brain stimulation (DBS).
We report aspects of rapid initiation and scaling of telemedicine in a large academic movement disorder center during the COVID-19 pandemic, paying specific attention to the effects of patient age, sex, and race in transitioning from in-person to telemedicine appointments. We assessed the distribution of diagnoses seen through telemedicine appointments to ascertain whether patients with certain disorders were more likely to transition to telemedicine than the historical in-person visit population in the prior year. We reviewed DBS subsets to determine whether hardware implantation impacted the probability of establishing a telemedicine appointment. Finally, we evaluated patient satisfaction.
MATERIALS & METHODS
Study activities were approved by the Institutional Review Board at Emory University (IRB: e00002451). Written consent was not required by the ethical review board for this retrospective analysis.
Study design
The study period originated on March 23, 2020 (the first day of telemedicine visits, one week after the closure of outpatient clinics by the University) and terminated on April 28, 2020, to provide 5 weeks of data.
Operations
On March 16, 2020, an institutional announcement was made by Emory Healthcare, directing all outpatient practices to transition outpatient clinical care to remote care via telemedicine except for emergent, urgent, or time-sensitive visits. Each provider completed an online training program and received certification to practice via telemedicine, and workflows were established. Telemedicine clinic templates were created, and best practices were streamlined, including patient intake, scheduling, scheduling follow-up appointments, and billing/coding. Within one week on March 23, 2020, the Emory Movement Disorders division (14 faculty, 2 nurse practitioners, 3 fellows, 4 schedulers) was transitioned to a complete telemedicine clinical model with very few in-person clinical visits.
Patient population
Established patients who had scheduled appointments were contacted and offered to transition to a telemedicine visit or to wait for in-person visits to resume. Patients who opted for a telemedicine encounter were screened to ensure that they had the appropriate equipment to conduct the visit. Patients were excluded if they were located outside of Georgia or other states that did not provide a temporary waiver for our providers to practice telemedicine. Patients scheduled for botulinum toxin injections were also excluded, as this procedure cannot be performed remotely.
Data acquisition
Telemedicine appointments were identified by filtering for a current procedural terminology (CPT) E&M code (99202–99205 and 99212–99215) that was linked to a 95 modifier, denoting “synchronous telemedicine service rendered via real-time interactive audio and video telecommunications system.” [10] Unique DBS subsets were identified by filtering for CPT 95983 and/or 95984 for the 24 months prior to the scheduled encounter. Demographic information, including age, sex, race, distance from the clinic site, and provider Press Ganey scores pre-COVID, were obtained during the study period.
Telemedicine platform
Patients were seen by the providers from their own respective remote locations treating patients within the same state as the provider’s medical licensure allowed. The Emory Movement Disorders division utilized an Emory University/Healthcare Zoom Enterprise (Zoom Video Communications, Inc., San Jose, CA, USA), a Health Insurance Portability and Accountability Act (HIPAA) compliant video conferencing application, to conduct the visits. A few days prior to a scheduled telemedicine visit, patients were contacted by a “prep team,” whose purpose was to guide the patient in the use of the technology and provide the opportunity for a test appointment if/when applicable. Approximately 30–60 minutes prior to a scheduled visit, patients were contacted by a medical assistant for the intake process, which included documenting estimated weight and height, calculating body mass index, documenting the review of systems, and completing medication history. Patients were given a private link to access the encounter at the time of their scheduled visit. The Zoom link directed the patients into the provider’s virtual “waiting room.” Following the intake process, the movement disorders specialist joined the video conference to conduct the visit. The provider led the visit in the same manner as an in-person encounter, with the sole difference of utilizing audio-visual technology to document the examination. At the conclusion of the visit, the movement disorders specialist ended the call, thereby ending the encounter. Follow-up instructions were documented by the provider in the Electronic Health Record, and the patient was contacted by a scheduler the next day to coordinate follow-up appointments and/or orders. Patients who were located in states where the provider was not licensed or did not have access to audio-visual equipment received continued, standard care through telephone or patient portal communication with our care teams but were not billed for these visits. For telephone visits, patients underwent the same intake process as audio-visual visits and were given a specific appointment for the telephone interview. Staff documented preferred telephone numbers in visit comments accessible to clinicians. The visit was documented in the same manner as an audio-visual visit, with the exception of not documenting a physical examination.
Statistical analysis
To describe the characteristics of the telemedicine patient population, we performed descriptive analysis of demographic and clinical characteristics for all patients seen via telemedicine from March 23, 2020 to April 28, 2020 (n = 686) and compared them to historical controls of all patients seen in person over the last year (n = 6,433). Demographics included age, sex, race, and distance from the clinic. Clinical characteristics included diagnosis and the presence of DBS implantation. The telemedicine subgroup was also analyzed for demographics and diagnoses of patients who opted for telemedicine audiovisual (n = 546) vs. telephone (n = 126) visits. Comparisons between groups were made using two-tailed t-tests and chi-square tests. A subset analysis was performed for the same variables to investigate how DBS patients seen via telemedicine (n = 79) differed from the population typically followed in person in our DBS clinic (n = 454).
RESULTS
Feasibility
Our group performed 686 telemedicine visits of 1140 that were originally scheduled in the study period (60%). Of these, 546 (79.6%) were telemedicine audio-visual visits, 126 (18.4%) were telephone visits, and 14 (2.0%) were undetermined (Table 1). Over the course of 5 weeks, the number of telemedicine visits and the distribution of audio-visual visits vs. telephone calls steadily increased over time (Figure 1). By the 5th week of implementation, our group was able to achieve a clinic volume of 60% of normal.
Table 1.
Weekly progression of telemedicine encounters
| Week | Telemedicine arrivals |
|||
|---|---|---|---|---|
| Telephone | Audio-visual | Other | Grand total | |
| March 23, 2020 – March 27, 2020 | 30 | 62 | 1 | 93 |
| March 30, 2020 – April 3, 2020 | 18 | 94 | 3 | 115 |
| April 6, 2020 – April 10, 2020 | 20 | 102 | 2 | 124 |
| April 13, 2020 – April 17, 2020 | 29 | 120 | 3 | 152 |
| April 20, 2020 – April 24, 2020 | 25 | 124 | 4 | 153 |
| April 27, 2020 – April 28, 2020 (Monday/Tuesday) | 4 | 44 | 1 | 49 |
| Grand total | 126 | 546 | 14 | 686 |
Figure 1.
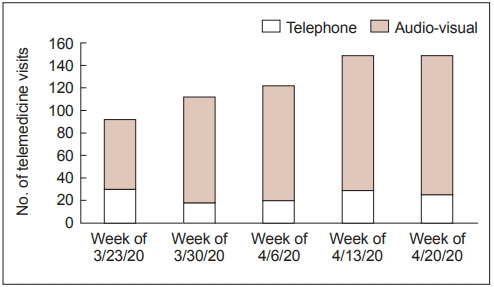
Weekly telemedicine encounters by type.
Demographics
Table 2 summarizes the demographics of our patient population, prepandemic (March 1, 2019 to February 29, 2020) and those seen via telemedicine (March 23, 2020 to April 28, 2020) during COVID-19. The average age of our telemedicine population was 64.9 (± 14.9) years, and 55% were male. Patients in the 70–79 age bracket had the largest percentage of telemedicine appointments (36.5%), followed by patients in the 60–69 age bracket (27.2%). Age distribution did not differ between inclinic and telemedicine visits; hence, age did not play a role in whether patients established a telemedicine visit (p = 0.17). Men were more likely than women to transition to a telemedicine visit (p = 0.02). The racial demographics of the population of patients who elected to participate in telemedicine encounters differed compared to the demographics of patients seen in person over the last year (p = 0.01). The majority of patients evaluated by telemedicine were Caucasian (72.9%), followed by Other (12.5%), Black (11.7%), and Asian (2.92%). The percentage of telemedicine compared to in-person visits prepandemic increased for Caucasians by 4%, while the percentage decreased by 3% for Blacks. On average, patients who participated in telemedicine were located farther from the clinic than the population of patients evaluated in person over the last year (66.47 km vs. 42.16 km, p < 0.001).
Table 2.
Baseline characteristics of study population
| Characteristics | Pre-pandemic (in person) (n = 6,433) | COVID-19 (telemedicine) (n = 686) | p-value |
|---|---|---|---|
| Age (yr) | 63.99 ± 15.75 | 64.86 ± 14.88 | 0.17 |
| Age group (%) | |||
| 10–19 yrs | 1.3 | 0.4 | |
| 20–29 yrs | 3.2 | 4.0 | |
| 30–39 yrs | 4.9 | 4.2 | |
| 40–49 yrs | 7.2 | 5.4 | |
| 50–59 yrs | 13.2 | 12.0 | |
| 60–69 yrs | 25.8 | 27.2 | |
| 70–79 yrs | 32.4 | 36.5 | |
| 80–89 yrs | 11.1 | 9.4 | |
| ≥ 90 yrs | 0.9 | 0.7 | |
| Sex | 0.02 | ||
| Male | 3,229 (50.19) | 376 (54.81) | |
| Female | 3,204 (49.81) | 310 (45.19) | |
| Race | 0.01 | ||
| Caucasian | 4,370 (67.93) | 500 (72.89) | |
| Black | 945 (14.69) | 80 (11.66) | |
| Asian | 167 (2.60) | 20 (2.92) | |
| Other | 951 (14.80) | 86 (12.50) | |
| Distance (km) | 42.16 ± 71.87 | 66.47 ± 137.97 | < 0.001 |
| Diagnosis | < 0.001 | ||
| PD | 2,723 (42.46) | 421 (61.37) | |
| ET | 475 (7.41) | 30 (4.37) | |
| Dystonia | 677 (10.6) | 33 (4.8) | |
| HD | 82 (1.28) | 10 (1.46) | |
| Other | 2,476 (38.5) | 192 (27.99) | |
| DBS | 454 (7.06) | 79 (11.52) | < 0.001 |
Data are presented as mean ± SD or n (%) unless otherwise indicated.
PD: Parkinson’s disease, ET: essential tremor, HD: Huntington’s disease, DBS: deep brain stimulation.
Table 3 summarizes the demographic characteristics of the telemedicine audiovisual group compared to the telephone group. Only age revealed a statistically significant shift towards telephone visits compared to other factors, such as race, distance, and diagnosis; older patients preferred telephone visits compared with audio-video visits (p < 0.001).
Table 3.
Baseline characteristics of audiovisual vs. telephone visits
| Audiovisual visit (n = 546) | Telephone visit (n = 126) | p-value | |
|---|---|---|---|
| Age (yr) | 64 ± 15 | 70 ± 11 | < 0.001 |
| Gender | 0.78 | ||
| Male | 302 (55) | 68 (54) | |
| Female | 244 (45) | 58 (46) | |
| Race | 0.42 | ||
| Caucasian | 404 (74) | 86 (68) | |
| Black | 58 (11) | 21 (17) | |
| Asian | 16 (3) | 4 (3) | |
| Other | 68 (12) | 15 (12) | |
| Distance (km) | 62.8 ± 144.8 | 82.1 ± 112.7 | 0.42 |
| Diagnosis | 0.61 | ||
| PD | 328 (60) | 87 (69) | |
| ET | 26 (5) | 4 (3) |
Data are presented as mean ± SD or n (%). PD: Parkinson’s disease, ET: essential tremor.
Movement disorder diagnosis
The distribution of diagnoses seen is summarized in Table 2. Among telemedicine encounters, there was a significant shift in the distribution of all diagnoses (p < 0.001) compared to our prepandemic population. The percentage of patients with PD seen by our practice showed an increasing trend when compared with prepandemic in-person visits, while the percentage decreased for patients with essential tremor (ET), dystonia, and other disorders. The shift towards or away from telemedicine visits for each disease alone did not meet statistical significance. The DBS cohort did, however, demonstrate a shift towards telemedicine, with 11.5% of telemedicine encounters comprised of DBS patients, compared to 7% during baseline encounters (p < 0.001). This suggests that patients with DBS were more likely to convert their visit type to telemedicine when asked by schedulers compared to patients without DBS.
We further analyzed the distribution of diagnoses conducted among the audiovisual vs. telephone visits (Table 3); we did not identify statistically significant differences (p = 0.61). For reference, the frequencies of two of the most common diagnoses are shown.
Patient satisfaction
Press Ganey results were obtained for all providers (n = 19). The average Press Ganey score prepandemic (September 1, 2019 to March 16, 2020) was 89.6% (n = 974) vs. 92.9% (n = 113) during the COVID-19 pandemic, demonstrating that patient satisfaction during telemedicine encounters was not compromised during the pandemic.
Provider satisfaction
While we did not obtain provider telemedicine satisfaction data during this study period, provider surveys were distributed soon thereafter from the Brain Health Center, which includes the Movement Disorders division, as well as the entire Emory Physician Group, which includes all subspecialties. In the two surveys, approximately 83–89% of clinicians were either satisfied or very satisfied with telemedicine visits.
DISCUSSION
In this study, we demonstrated the successful and rapid implementation of a telemedicine healthcare delivery model across our Movement Disorders division, transitioning to virtual visits within one week and demonstrating a continuous and steady rise in the number of visits. Several factors contributed to this, including the availability of centralized training modules, the documentation of standard work for all individuals participating in the visit process, and iteration over best practices within our group. The 60% conversion rate of patients who were scheduled for in-person visits during this time was on par with all neurology subdivisions. Among the reasons that it was not higher are patient desire to wait for in-person appointment, lag in scheduling new patient visits to be able to accommodate existing patients of the practice and to minimize new testing (e.g., MRI) due to social distancing restrictions, being unable to contact the patient or surrogate, and not scheduling procedural visits that were not able to be performed by telemedicine.
Contrary to prior reports [11,12], age did not play a role in converting to a telemedicine visit. In fact, the older age groups (70–79 years) and (60–69 years) comprised the largest patient populations, at 32.4% and 25.8%, respectively. This may be in part due to the older age of movement disorder populations. However, when the telemedicine subgroup was analyzed further, patients opting for a telephone visit were older than those opting for an audiovisual visit (p < 0.001). This finding may reflect the discomfort or inexperience of older patients with the audiovisual platform. Further studies regarding the impact of age in establishing telemedicine visits are warranted.
Men were more likely to transition to a telemedicine visit. This trend has been previously demonstrated in a number of publications [13-16], particularly in developing countries where gender disparities may be more profound. It has been shown that some women face social barriers that may inhibit their participation in telemedicine [13], while in more rural settings, engaging in telemedicine visits can influence gender relations in a positive way by providing new modes of communication for a couple’s health as well as enabling greater male participation in health areas typically targeted towards women [14]. Further studies on gender disparities in telemedicine in developing countries would be of interest.
We observed apparent health care disparities in access to telemedicine visits, as Blacks and minorities were less likely to engage in virtual care than Caucasians. It is well documented in the literature that racial/ethnic minorities who are socioeconomically disadvantaged face significant barriers to receiving healthcare [17-19]. Much of this disparity is thought to be due to lack of timely access to appropriate healthcare [13,20], and this is likely further compounded with subspecialty care that many movement disorders patients require. Additional problems may include access to technology to conduct visits, including both devices and broadband or cellular capability. We anticipate further study to define the reasons for these disparities so they can be mitigated.
Patients who chose to participate in a telemedicine visit were from a farther average distance than our prepandemic in-person patient population. This is of particular interest in movement disorder patients, where the combination of disease progression often leads to limited mobility and subsequent challenges with in-person visits, as well as limited access to specialty care, is paramount. Current care models often require travel to tertiary medical centers, increasing the burden on patients as well as caregivers [4,21]. Telemedicine improves access to trained subspecialists, allowing earlier diagnosis as well as skilled management of movement disorders [3,22]. Outcome studies for various movement disorder diseases using telemedicine care models would be an important area of future research.
While the feasibility of telemedicine in PD has been previously demonstrated [5-8,23-26], our study implemented telemedicine across all movement disorders. The top diagnoses seen in our study included PD, ET, dystonia, and Huntington’s disease. As previously discussed, while no significant differences were observed among the diagnoses of patients opting for audiovisual vs. telephone visits, there was a significantly different shift in the distribution of diagnoses seen in the telemedicine period overall. However, this is likely partly driven by a longer baseline clinical period (12 months), which included many diseases that are infrequently seen and may have not been amenable to a telemedicine visit. This is of particular importance in dystonia patients, as many require botulinum toxin injections and were not included in this study. A longer study period would be indicated to determine if this finding is maintained over time.
For PD, the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [27] is commonly used to document key features of the examination, including tremor, rigidity, bradykinesia, gait, and postural instability. While previous studies have confirmed the validity of remote assessments in PD [5,28-30], there are limitations. Specifically, rigidity cannot be assessed without touching the patient, and postural instability cannot be measured without a trained health professional. Most recently, Goetz et al. [31] reassessed their prior publication regarding handling missing values in the MDS-UPDRS [32] and concluded that the motor examination can accommodate the consistent loss of 3 values on any given visit to maintain a validated total score. While the instance of a telemedicine visit will result in 6 missing values (5 rigidity, 1 postural reflex), a very recent letter by the same authors in the setting of COVID-19 stated “We are very comfortable providing video-based or telemedicine care of high quality and compassion in PD.” Abdolahi et al. [33] conducted a study to compare whether administering the UPDRS without rigidity and postural instability would make a significant difference in trial outcomes. The results indicated that the same clinical outcomes were demonstrated, and high internal consistency was found. Another advantage of telemedicine in PD is the ability to observe patients in their own natural home environment [4], which can potentially help eliminate fall risks or other contributing factors.
In our study, a higher percentage of patients with DBS chose to convert to telemedicine than patients without DBS. These findings may hint at the influence of disease severity, although we did not measure this variable directly. DBS requires both clinical and surgical expertise, which requires advanced infrastructure and highly specialized skills and is usually limited to large urban centers. Additionally, of note, the population of DBS patients who converted to a telemedicine visit type was more representative of the typical historical prepandemic clinic population without a disparity in gender or race. This may reflect that to obtain DBS, patients must overcome typical barriers to care. In addition, the rapid cessation of DBS therapy due to battery failure in a time of limited surgical procedures during a pandemic can be life-threatening in certain cases [34-36]. Telemetry monitoring and remote care for device-based therapies are well established in cardiac pacemakers [24,37]; however, this technology is lacking in DBS programming devices. Two studies [38,39] confirmed the feasibility of using telemedicine for DBS in patients with PD. Moreover, both patients and physicians reported a high degree of satisfaction with telemedicine [38]. However, with the current technology, the changes allowed are limited to only checking the DBS settings via the patient programmer, as well as limited setting adjustments if patient parameters are set in advance, which is not always the case. This was of importance in our patient population during the pandemic, as in-person visits were unavailable and/or limited. Our providers were able to assess both PD and ET patients via telemedicine. Advanced technology in DBS hardware to truly program patients remotely is necessary in the future to fully implement a DBS telemedicine visit. It remains to be seen whether the shift towards telemedicine in DBS patients will continue to be seen as in-person visits resume.
Our finding that patient satisfaction was not compromised with the implementation of telemedicine as a care model is consistent with findings from multiple studies [8,26,29,40] that reported greater satisfaction in PD patients with telemedicine due to convenience and accessibility. Previous studies have also supported greater patient satisfaction with internet-based UPDRS assessment compared to in-person assessments [28].
The limitations of our study include a short study period of 5 weeks. A longer follow-up period would be helpful in adequately evaluating the benefit/limitations of telemedicine in movement disorders. In addition, further details regarding why certain patients opted out of a telemedicine visit would be useful to see if we can overcome whatever barriers exist and provide better specialized care to our patients. Last, more detailed patient and provider satisfaction variables are needed to adequately assess quality and patient satisfaction in a telemedicine movement disorder model. We plan to implement these changes to follow our patient population in a long-term prospective study.
In summary, telemedicine is both a viable and relevant care model in the treatment of movement disorder patients. If the policy factors that enabled telemedicine payments persist, it is likely that virtual care will become a mainstay of diagnosis and ongoing treatment. Additional models including provider-toprovider telemedicine for more rapid diagnosis and institution of therapy will become options as well. This would enable appropriate care and treatment sooner in the continuum of movement disorders with the opportunity to affect disease outcomes.
Acknowledgments
We would like to thank all of the Emory movement providers for their participation in our telemedicine program. We would also like to thank Ambar Kulshreshtha and Lindsey Wells for their assistance with the surveys.
Footnotes
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee. The Emory University School of Medicine Institutional Review Board approved this study. Informed patient consent was not necessary for this work. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Conflicts of Interest
C.D. Esper receives honorarium from NeuroOne Medical Technologies, Inc., royalties from UpToDate. L. Scorr reports no disclosures. S. Papazian reports no disclosures. D. Bartholomew reports no disclosures. G.J. Esper serves on the advisory board for NeuroOne Medical Technologies and receives remuneration for medicolegal consulting. S.A. Factor receives honoraria from Lundbeck, Sunovion, Biogen, Acadia, Impel, Acorda, CereSpir; royalties from Demos, Blackwell Futura, Springer for textbooks, UpToDate; grants from Medtronics, Boston Scientific, Biohaven, Impax, Lilly, US World Meds, Sunovion Therapeutics, Vaccinex, Voyager, Jazz Pharmaceuticals, CHDI Foundation, Michael J. Fox Foundation, NIH(U10 NS077366), Parkinson Foundation, and other: Bracket Global LLC, CNS Ratings LLC.
Author Contributions
Conceptualization: Christine Doss Esper, Gregory Jacob Esper, Stewart Alan Factor. Data curation: Daniel Bartholomew. Formal analysis: Laura Scorr. Investigation: all authos. Methodology: Christine Doss Esper, Laura Scorr, Sosi Papazian, Gregory Jacob Esper, Stewart Alan Factor. Visualization: Christine Doss Esper, Daniel Bartholomew. Writing—original draft: Christine Doss Esper. Writing—review & editing: all authors.
REFERENCES
- 1.Hess DC, Wang S, Hamilton W, Lee S, Pardue C, Waller JL, et al. REACH: clinical feasibility of a rural telestroke network. Stroke. 2005;36:2018–2020. doi: 10.1161/01.STR.0000177534.02969.e4. [DOI] [PubMed] [Google Scholar]
- 2.Wechsler LR, Tsao JW, Levine SR, Swain-Eng RJ, Adams RJ, Demaerschalk BM, et al. Teleneurology applications: report of the Telemedicine Work Group of the American Academy of Neurology. Neurology. 2013;80:670–676. doi: 10.1212/WNL.0b013e3182823361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatcher-Martin JM, Adams JL, Anderson ER, Bove R, Burrus TM, Chehrenama M, et al. Telemedicine in neurology: Telemedicine Work Group of the American Academy of Neurology update. Neurology. 2020;94:30–38. doi: 10.1212/WNL.0000000000008708. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Pazi H, Browne P, Chan P, Cubo E, Guttman M, Hassan A, et al. The promise of telemedicine for movement disorders: an interdisciplinary approach. Curr Neurol Neurosci Rep. 2018;18:26. doi: 10.1007/s11910-018-0834-6. [DOI] [PubMed] [Google Scholar]
- 5.Dorsey ER, Venkataraman V, Grana MJ, Bull MT, George BP, Boyd CM, et al. Randomized controlled clinical trial of “virtual house calls” for Parkinson disease. JAMA Neurol. 2013;70:565–570. doi: 10.1001/jamaneurol.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanson RE, Truesdell M, Stebbins GT, Weathers AL, Goetz CG. Telemedicine vs office visits in a movement disorders clinic: comparative satisfaction of physicians and patients. Mov Disord Clin Pract. 2018;6:65–69. doi: 10.1002/mdc3.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider RB, Biglan KM. The promise of telemedicine for chronic neurological disorders: the example of Parkinson’s disease. Lancet Neurol. 2017;16:541–551. doi: 10.1016/S1474-4422(17)30167-9. [DOI] [PubMed] [Google Scholar]
- 8.Venkataraman V, Donohue SJ, Biglan KM, Wicks P, Dorsey ER. Virtual visits for Parkinson disease: a case series. Neurol Clin Pract. 2014;4:146–152. doi: 10.1212/01.CPJ.0000437937.63347.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan R, Ben-Pazi H, Dekker M, Cubo E, Bloem B, Moukheiber E, et al. Telemedicine for hyperkinetic movement disorders. Tremor Other Hyperkinet Mov (N Y) 2020 Feb 17; doi: 10.7916/tohm.v0.698. [Epub]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Medicare & Medicaid Services . Baltimore: CMS; c2020. Telehealth services [Internet] [cited 2020 March 6]. Available from: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-NetworkMLN/MLNProducts/Downloads/TelehealthSrvcsfctsht.pdf. [1. Title of the document corrected. Please confirm the change. 2. And please add the cited year, month, and date.] [Google Scholar]
- 11.Albright KC, Boehme AK, Mullen MT, Wu TC, Branas CC, Grotta JC, et al. The effect of telemedicine on access to acute stroke care in Texas: the story of age inequalities. Stroke Res Treat. 2015;2015:813493. doi: 10.1155/2015/813493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mobley LR, Root E, Anselin L, Lozano-Gracia N, Koschinsky J. Spatial analysis of elderly access to primary care services. Int J Health Geogr. 2006;5:19. doi: 10.1186/1476-072X-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George AS, Morgan R, Larson E, LeFevre A. Gender dynamics in digital health: overcoming blind spots and biases to seize opportunities and responsibilities for transformative health systems. J Public Health (Oxf) 2018;40(suppl_2):ii6–ii11. doi: 10.1093/pubmed/fdy180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings L, Gagliardi L. Influence of mHealth interventions on gender relations in developing countries: a systematic literature review. Int J Equity Health. 2013;12:85. doi: 10.1186/1475-9276-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khairat S, Liu S, Zaman T, Edson B, Gianforcaro R. Factors determining patients’ choice between mobile health and telemedicine: predictive analytics assessment. JMIR Mhealth Uhealth. 2019;7:e13772. doi: 10.2196/13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steege R, Waldman L, Datiko DG, Kea AZ, Taegtmeyer M, Theobald S. ‘The phone is my boss and my helper’-a gender analysis of an mHealth intervention with Health Extension Workers in Southern Ethiopia. J Public Health (Oxf) 2018;40(suppl_2):ii16–ii31. doi: 10.1093/pubmed/fdy199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George SM, Hamilton A, Baker R. Pre-experience perceptions about telemedicine among African Americans and Latinos in South Central Los Angeles. Telemed J E Health. 2009;15:525–530. doi: 10.1089/tmj.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips KA, Mayer ML, Aday LA. Barriers to care among racial/ethnic groups under managed care. Health Aff (Millwood) 2000;19:65–75. doi: 10.1377/hlthaff.19.4.65. [DOI] [PubMed] [Google Scholar]
- 19.Williams DR. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann N Y Acad Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 20.Baker RS, Watkins NL, Wilson MR, Bazargan M, Flowers CW., Jr Demographic and clinical characteristics of patients with diabetes presenting to an urban public hospital ophthalmology clinic. Ophthalmology. 1998;105:1373–1379. doi: 10.1016/S0161-6420(98)98015-0. [DOI] [PubMed] [Google Scholar]
- 21.Velasquez SE, Chaves-Carballo E, Nelson EL. Pediatric teleneurology: a model of epilepsy care for rural populations. Pediatr Neurol. 2016;64:32–37. doi: 10.1016/j.pediatrneurol.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Shore J, Vo A, Yellowlees P, Waugh M, Schneck C, Nagamoto H, et al. Antipsychotic-induced movement disorder: screening via telemental health. Telemed J E Health. 2015;21:1027–1029. doi: 10.1089/tmj.2014.0242. [DOI] [PubMed] [Google Scholar]
- 23.Stillerova T, Liddle J, Gustafsson L, Lamont R, Silburn P. Antipsychotic-induced movement disorder: screening via telemental health. Remotely assessing symptoms of Parkinson’s disease using videoconferencing: a feasibility study. 2016 Dec 26; doi: 10.1155/2016/4802570. [Epub]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Achey M, Aldred JL, Aljehani N, Bloem BR, Biglan KM, Chan P, et al. The past, present, and future of telemedicine for Parkinson’s disease. Mov Disord. 2014;29:871–883. doi: 10.1002/mds.25903. [DOI] [PubMed] [Google Scholar]
- 25.Barbour PJ, Arroyo J, High S, Fichera LB, Staska-Pier MM, McMahon MK. Telehealth for patients with Parkinson’s disease: delivering efficient and sustainable long-term care. Hosp Pract (1995) 2016;44:92–97. doi: 10.1080/21548331.2016.1166922. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson JR, Spindler M, Wood SM, Marcus SC, Weintraub D, Morley JF, et al. High patient satisfaction with telehealth in Parkinson disease: a randomized controlled study. Neurol Clin Pract. 2016;6:241–251. doi: 10.1212/CPJ.0000000000000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 28.Cubo E, Gabriel-Galán JM, Martínez JS, Alcubilla CR, Yang C, Arconada OF, et al. Comparison of office-based versus home Web-based clinical assessments for Parkinson’s disease. Mov Disord. 2012;27:308–311. doi: 10.1002/mds.24028. [DOI] [PubMed] [Google Scholar]
- 29.Dorsey ER, Deuel LM, Voss TS, Finnigan K, George BP, Eason S, et al. Increasing access to specialty care: a pilot, randomized controlled trial of telemedicine for Parkinson’s disease. Mov Disord. 2010;25:1652–1659. doi: 10.1002/mds.23145. [DOI] [PubMed] [Google Scholar]
- 30.Goetz CG, Stebbins GT, Wolff D, DeLeeuw W, Bronte-Stewart H, Elble R, et al. Testing objective measures of motor impairment in early Parkinson’s disease: feasibility study of an at-home testing device. Mov Disord. 2009;24:551–556. doi: 10.1002/mds.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetz CG, Stebbins GT, Luo S. Movement Disorder Society-Unified Parkinson’s Disease Rating Scale use in the Covid-19 Era. Mov Disord. 2020;35:911. doi: 10.1002/mds.28094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetz CG, Luo S, Wang L, Tilley BC, LaPelle NR, Stebbins GT. Handling missing values in the MDS-UPDRS. Mov Disord. 2015;30:1632–1638. doi: 10.1002/mds.26153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdolahi A, Scoglio N, Killoran A, Dorsey ER, Biglan KM. Potential reliability and validity of a modified version of the Unified Parkinson’s Disease Rating Scale that could be administered remotely. Parkinsonism Relat Disord. 2013;19:218–221. doi: 10.1016/j.parkreldis.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azar J, Elinav H, Safadi R, Soliman M. Malignant deep brain stimulator withdrawal syndrome. BMJ Case Rep. 2019;12:e229122. doi: 10.1136/bcr-2018-229122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miocinovic S, Ostrem JL, Okun MS, Bullinger KL, Riva-Posse P, Gross RE, et al. Recommendations for deep brain stimulation device management during a pandemic. J Parkinsons Dis. 2020;10:903–910. doi: 10.3233/JPD-202072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuter S, Deuschl G, Falk D, Mehdorn M, Witt K. Uncoupling of dopaminergic and subthalamic stimulation: life-threatening DBS withdrawal syndrome. Mov Disord. 2015;30:1407–1413. doi: 10.1002/mds.26324. [DOI] [PubMed] [Google Scholar]
- 37.Boyne JJ, Vrijhoef HJ, Crijns HJ, De Weerd G, Kragten J, Gorgels AP, et al. Tailored telemonitoring in patients with heart failure: results of a multicentre randomized controlled trial. Eur J Heart Fail. 2012;14:791–801. doi: 10.1093/eurjhf/hfs058. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Hao H, Chen H, Li L. The study on a telemedicine interaction mode for deep brain stimulation postoperative follow-up. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:186–189. doi: 10.1109/EMBC.2015.7318331. [DOI] [PubMed] [Google Scholar]
- 39.Jitkritsadakul O, Rajalingam R, Toenjes C, Munhoz RP, Fasano A. Telehealth for patients with deep brain stimulation: the experience of the Ontario Telemedicine Network. Mov Disord. 2018;33:491–492. doi: 10.1002/mds.27230. [DOI] [PubMed] [Google Scholar]
- 40.Arora S, Venkataraman V, Zhan A, Donohue S, Biglan KM, Dorsey ER, et al. Detecting and monitoring the symptoms of Parkinson’s disease using smartphones: a pilot study. Parkinsonism Relat Disord. 2015;21:650–653. doi: 10.1016/j.parkreldis.2015.02.026. [DOI] [PubMed] [Google Scholar]


