Abstract
Introduction
Balance confidence and perception of task challenge is an important construct to measure in rehabilitation of people with lower-limb amputation (LLA). Measurement of electrodermal activity (EDA) captures physiological arousal responses reflecting an individual’s perceived challenge in a task. This study explores the feasibility of the use of EDA during outdoor walking tasks to capture task-specific physiological arousal changes associated with perception of challenge in people with amputation.
Objective
To develop and demonstrate feasibility of a portable EDA/GPS system mapping physiological arousal while challenging walking balance outdoors in individuals with LLA and controls.
Methods
Sixteen people (eight with LLA and eight age-/sex-matched controls) completed an outdoor walking course in the community (3 laps). A battery-powered portable device was developed containing EDA/GPS sensors with data logged on a microcontroller. Phasic EDA response was extracted from EDA signal to explore the physiological arousal response to walking tasks.
Results
Physiological arousal demonstrated task-specific modulation with ascending stairs without a handrail showing higher levels of phasic EDA than walking on a paved incline (p = 0.01) or a gravel decline (p = 0.01) in people with LLA. While evidence of habituation over repeated trials was shown in controls with lap 1 of walking down a gravel decline showing higher levels of phasic EDA than lap 3 (p = 0.01). Phasic EDA maps, representative of arousal levels throughout the walking course, showed individual-specific response.
Conclusion
Mapping of EDA during outdoor walking is feasible. Modulation of physiological arousal between outdoor walking tasks and over repeated trials is suggestive of clinical utility. Further research is warranted to explore how EDA may be incorporated into assessment of response to outdoor walking amongst individuals following LLA.
Keywords: Balance confidence, physiological arousal, community walking, lower-limb amputee
Introduction
Balance impairment following lower-limb amputation (LLA) is common and can be detrimental to independent community-level mobility. LLA significantly alters the biomechanics of gait and the associated balance reactions in response to a loss of balance, placing the individual at a higher risk of falls. 1 Accordingly, decreased balance confidence has been reported in people with LLA.2,3 While experiencing decreased balance confidence is somewhat expected following LLA, it is an important clinical consideration as impaired balance confidence can also lead to detrimental self-restriction of activities related to the perception of inability, that is not in-line with actual balance abilities. 4 Therefore, both balance-related functional abilities and balance confidence following LLA have been identified as critical components to address during rehabilitation to optimize physical function and quality of life.5–7
Balance confidence and perception of walking ability has previously been quantified using self-reported measurement tools that have been validated for use in people with LLA.8,9 There are many advantages to the self-reported approach of measuring balance confidence, such as ease of interpretation, motivation to report, causal force, and sheer practicality − namely cost-effectiveness and the ability to administer to large populations at once. However, self-reports are also susceptive to reporting error associated with responder bias and subjectivity that may be further impacted by unclear language, lack of understanding, misinterpretation, inaccurate self-presentation 10 or a conscious or unconscious desire to respond in a socially desirable way. 11
Use of objective measurement of physiological arousal may improve the clinicians understanding of a person’s response to balance challenges when introducing people with LLA to community-level balance challenges. Electrodermal activation (EDA) measures the skin’s electrical resistance that changes with the sympathetically-mediated release of sweat during heightened physiological arousal. 12 Measurements of EDA have been used to examine changes in physiological arousal associated with changes in level of attention, cognitive effort, and emotion during tasks. 13 The physiological arousal response associated with tasks that challenge balance is suggested to be influenced by the overarching construct of balance confidence.14,15
There are two main components of the EDA signal: phasic and tonic activity. The phasic signal activity is the most commonly evaluated component of the EDA when interested in physiological arousal response to psychological stress associated with discrete events.12,16,17 The phasic activity of the signal represents discrete event-stimulated changes in arousal that has been shown to occur in the frequency range of 0.08 Hz to 0.33 Hz.16,18 For example, when an individual is at rest and standing still, you can expect the phasic EDA to be unchanging and to visually create a straight horizontal line across time. However, if this same individual is asked to do something he or she perceives as challenging or dangerous, such as performing a task that challenges balance and poses a risk of falling, you can expect a sudden increase in the measured phasic EDA signal in units of conductance. The degree of this phenomenon can be quantified as the signal power or amplitude of the phasic response. The tonic activity of the EDA signal is slower to change over time and is associated with general levels of physiological arousal related to constructs such as general feelings of anxiety and required level of attentiveness. 19 The tonic signal is captured in the frequency range of 0.010 Hz to 0.033 Hz. 12
The use of EDA as a measure of physiological arousal during community-level balance and mobility challenges has not been explored in people with LLA. The objective of this study was to develop and demonstrate the feasibility of use of a portable EDA/GPS system to measure physiological arousal during completion of an outdoor walking course encompassing community-level walking balance challenges. Specifically, the focus of this study was the task-specific phasic response of physiological arousal to repetitions of outdoor walking challenges. Performance of the system was explored in people with LLA and age-matched controls.
Methods
Participants
Sixteen people, eight people with lower-limb amputation and eight age- and sex-matched controls, were recruited from the community. Inclusion criteria for participants with LLA were: unilateral lower-limb amputation, greater than one-year post-fitting for prosthesis, a healthy contralateral leg, between the ages 19 and 80 years old and independent ambulation in the community. Inclusion criteria for the control group ensured that the group was age-matched to, within ±5 years of the LLA group and that all controls had no history of impairment affecting balance and mobility. All participants were required to be fluent in English to understand instructions during testing and to complete self-reported outcome measures. Participants provided informed consent in adherence to Simon Fraser University’s Office of Research Ethics policies. Participant demographics are shown in Table 1.
Table 1.
Participant characteristics. (A) participants with lower limb amputation (LLA) and (B) control participants. The final row of each table shows the group means (SD).
| (A) Participants with LLA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex (M/F) | Age | Body mass | Amp. levela | Amp. etiology | ABC Score (%) | PLUS-M score (Adjusted %) | AMP PRO score/K-level | |||
| M | 64 | 108.9 | TF | Trauma | 87.50 | 79.90 | 43/K4 | |||
| M | 41 | 104.3 | TF | Cancer | 93.75 | 57.80 | 44/K4 | |||
| M | 76 | 90.7 | TF | Trauma | 73.75 | 70.20 | 41/K3 | |||
| M | 59 | 95.3 | TF | Trauma | 87.20 | 57.80 | 45/K4 | |||
| M | 49 | 59.0 | TT | Dysvasc. | 85.00 | 67.00 | 46/K4 | |||
| M | 60 | 99.8 | TT | Dysvasc. | 93.10 | 79.90 | 43/K4 | |||
| F | 63 | 58.1 | TT | Cancer | 97.50 | 89.90 | 46/K4 | |||
| F | 71 | 90.7 | TT | Trauma | 80.60 | 57.80 | 43/K4 | |||
| 6M/2F | 60.4 (±11.2) | 88.4 (±19.4) | 4TF/4TT | N/A | 87.30 (±7.68) | 69.99 (±12.1) | 43.89/K4 (±1.73) | |||
| (B) Control participants | ||||||||||
|
(Sex (M/F) |
Age |
Body mass |
ABC Score (%) |
|||||||
| M | 60 | 80.0 | 88.75 | |||||||
| M | 41 | 72.6 | 99.40 | |||||||
| M | 61 | 94.3 | 97.50 | |||||||
| M | 48 | 65.0 | 100.00 | |||||||
| M | 61 | 83.9 | 96.60 | |||||||
| M | 68 | 64.9 | 98.10 | |||||||
| F | 58 | 43.1 | 98.75 | |||||||
| F | 54 | 63.0 | 98.75 | |||||||
| 6 M/2F | 56.4 (±8.50) | 70.9 (±15.6) | 97.23 (±3.59) | |||||||
ABC: Activity-specific Balance Confidence Scale; PLUS-M: Prosthetic Limb User’s Survey of Mobility; AMP PRO: Amputee Mobility Predictor with prosthetics.
aAmp (amputation) level: TF = transfemoral amputation, defined as at or above knee; TT = transtibial amputation, defined as below knee.
Clinical measures of balance confidence and mobility
All subjects completed the Activity-specific Balance Confidence (ABC) Scale. The ABC has been validated for use in the LLA population. 8 It is a self-reported pen and paper form that has 16 descriptions of activities of daily living that may be challenging to one’s balance and mobility. The individual rates their confidence in not falling or losing their balance while performing each task (scale from 0% = not confident at all to 100% = very confident). 20
Participants with LLA also completed the Prosthetic Limb User’s Survey of Mobility (PLUS-M), as it is a measure of perceived ability for mobility-related activities and was developed specifically for self-reporting of mobility levels in people with LLA.21–23 Available literature for the PLUS-M have reported it to have comparable psychometric properties to the ABC. 21 While the two scales have slightly different directives, both are based on the overarching construct of perception of ability.
Functional mobility of each participant with LLA was also measured using the Amputee Mobility Predictor with Prosthesis test. 24 AMP PRO tasks include sitting, standing and walking balance challenges (total score/47).
Electrodermal activity measurement
The data collection device for EDA/GPS is a portable system, developed for this project by our lab (11 × 8 × 4 cm), that is mounted onto a backpack style frame with chest and waist straps, along with a GPS antenna attached to a hat. The system is battery-powered and contains an EDA sensor as well as a GPS sensor with data logged on a microcontroller. EDA and GPS signal collection was synchronized. A commercially purchased Arduino-compatible GPS shield was used to collect information of the latitude and longitude of a participant at a given time in order to map the participant’s position and associated EDA signal response. The EDA device was calibrated against a laboratory system (CED 2502 Skin Conductance Unit; Cambridge Electronics Design Ltd.), an established laboratory tool used for collection of EDA.14,25,26 A commonly used protocol of audio-visual stimuli (20 second fear evoking videos)27–30 was used to induce a physiological arousal response simultaneously measured by both EDA devices. EDA signal collected simultaneously with the EDA device and the CED Skin Conductance Unit, both at a sampling rate of 4 Hz, showed excellent between EDA signal agreement in six participants during each of the four tasks modulating physiological arousal (min. r = 0.85 to max r = 0.99). Collection of EDA together with GPS facilitates understanding of fluctuations of the EDA signal throughout a prescribed walking course.
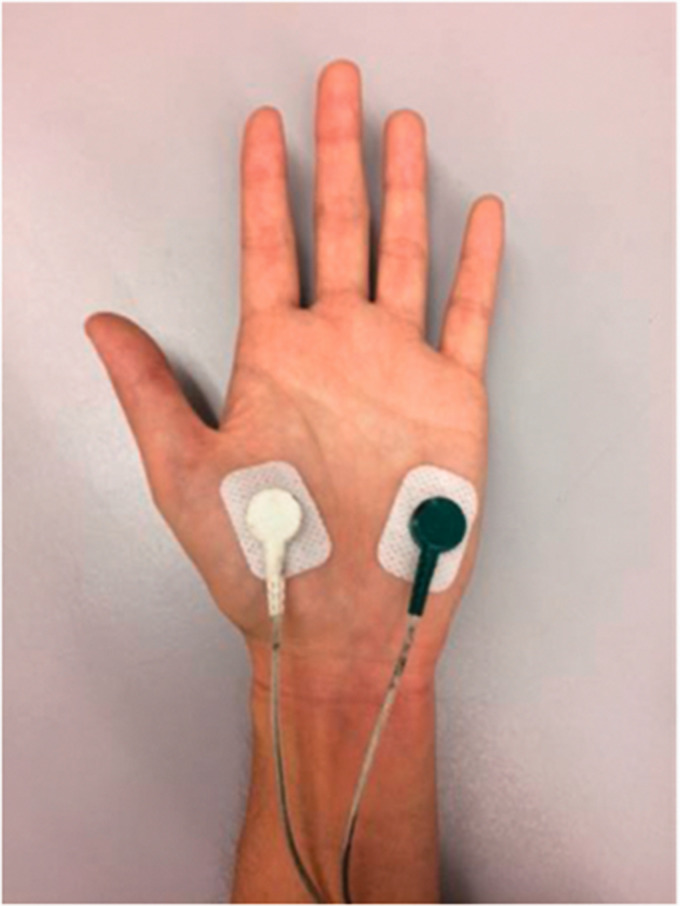
Bi-polar Ag/AgCl electrodes (10 mm diameter) were attached to the thenar and hypothenar eminences on palmar surface (Figure 1) where eccrine glands are found in the highest density, and therefore most likely to respond to emotional influences.31–33 This type of electrode has been shown to minimize electrode polarization during collection of EDA. 16 To minimize the risk for interference of the skin properties, the attachment site was washed with lukewarm water only. 34 The non-dominant hand was chosen for the attachment of the electrodes to minimize the chances of movement artifact in the case that, for safety reasons, the subject needed to grasp a handrail while completing the walking challenge. Video recordings of each participant completing the walking course were reviewed to identify any potential interference with EDA signal quality while walking (e.g. contact of hand with a surface, sneezing). Raw EDA signal was visually inspected to identify motion artifact. 35
Figure 1.
Electrode placement. Electrodes for EDA recording were placed on the palmar surface of the thenar and hypothenar eminences where primarily emotionally-stimulated eccrine sweat glands are most concentrated. To minimize electrode site deterioration, the electrodes were secured with additional breathable medical adhesive tape, 28 and wires to the forearm and upper arm with hook-and-loop closure straps.
To capture both tonic and phasic signal components, EDA was recorded at 4 Hz. The phasic response was resolved by convolving the EDA signal with a wavelet (frequency band of 0.08 Hz to 0.60 Hz), and calculating the power of the EDA within this band as a positive envelope (Figure 2). The phasic EDA was normalized to the median phasic level that occurred for each challenge task on the walking course, for each individual. Increases in the magnitude of the phasic EDA reflect increases in physiological arousal. The tonic response was calculated using a low-pass filter (cut-off 0.04 Hz) on the EDA signal and then normalizing the result to the tonic indoor baseline measurement of two minutes of flat-level walking preceding the outdoor course. These tonic and phasic responses were calculated from discrete frequency bands that concentrate on previously described ranges,16,18,19 but were selected to minimize cross-talk between the bands and thus ensure independence between the tonic and phasic measures.
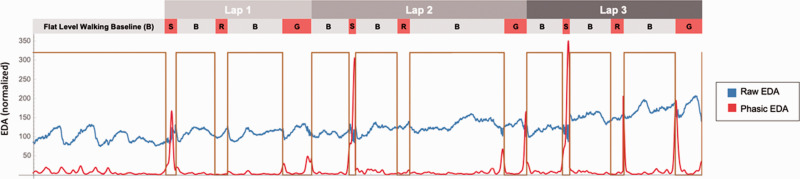
Figure 2.
Electrodermal activation (EDA) signal during completion of outdoor walking course. Laps 1-3 are indicated by the top-level shaded bars. The next level of bars shows the activity performed during the walking course. The initial grey bar is the flat level walking baseline (B) at the beginning of the course. Flat level walking baseline (B) is repeated between each of the walking tasks; walking up-stairs without a rail (S), paved incline ramp (R), and gravel decline (G). The traces show the participant’s raw EDA signal (blue, normalized) and superimposed red trace shows the corresponding phasic EDA while the participant completed the walking course (time to complete, approximately 40 minutes). Decreases in the raw EDA trace indicate increased sympathetic activity. Phasic EDA demonstrates upward deflections with increase in signal activity in the phasic frequency band.
Outdoor walking course
An outdoor walking course was developed to reflect realistic day-to-day community environments and included three tasks that clinicians (BP) identified as potentially challenging for people with LLA: 1) ascending stairs without a handrail, 2) paved incline, and 3) a gravel decline (Figure 3). Subjects first walked indoors on flat level ground for at least 2 minutes to record indoor baseline measures. Subjects then walked 3 laps of the course, in the established order from task 1 to task 3. Prior to each task, subjects walked for a minimum of 2 minutes on flat level ground outdoors to establish a between-task walking baseline. A signal from a handheld switch was synchronized with the EDA and GPS signal and was triggered by the experimenter during the course to indicate the start and end of each task. Standing breaks of 3-minutes were included at the end of each obstacle.
Figure 3.
Outdoor walking tasks completed by participants during the walking course. Participants walked; up stairs without the use of the handrail (task 1), up a paved incline (task 2), and down a gravel decline with no use of a handrail.
EDA is also known to be sensitive to several environmental factors in addition to personal factors.31,36 Testing was conducted in the late mornings during the summer months where the ambient temperature was close to the thermal neutral zone of 22 to 24 °C in order to minimize the effects of sweating or shivering on the EDA. 35 Importantly, previous research has shown that sweating associated with physical activity does not significantly affect electrodermal activity collected on the palm.36–38 Subjects were also made aware that there would be no talking apart from the directions of where to go in the course given by the experimenter. Synchronized video recording was used to allow for identification of periods of activity (e.g. physically touching the electrodes, excessive talking, coughing or sneezing) that may need to be excluded in the post-processing of the data.
Feasibility
Participants were asked to report their comfort level with completion of the walking tasks while wearing the EDA/GPS device. EDA/GPS signals were inspected for any instances of signal loss that would be reflective of inability of the device to capture data throughout the outdoor walking course. Temperature and humidity were measured at the beginning of each testing session.
Statistical analyses
Kruskal-Wallis tests were conducted separately for each of the amputee group and the control group to determine whether there were differences in phasic EDA depending on the task; ascending stairs without a handrail, walking up a paved incline, and down a gravel decline. Friedman tests were conducted separately for each of the amputee group and the control group to determine whether there were differences in phasic EDA levels between laps (1–3) in each task. Planned post-hoc comparisons with Wilcoxon Signed Ranks Test were run to identify specific between lap differences and Mann-Whitney Tests were run to identify specific between task differences in each group. Between group differences in phasic EDA were explored using Mann-Whitney Tests within each task, collapsed across laps. Finally, to explore the slower signal changes over time associated with more generalized levels of physiological arousal, Friedman tests were conducted separately for each of the amputee group and the control group to determine whether there were differences in tonic EDA levels between laps (1-3) in each task. This was followed by post-hoc comparisons with Wilcoxon Signed Ranks Tests to identify specific between lap differences in tonic EDA levels. Effects were considered significant at p ≤ 0.05. Statistical analysis was conducted using IBM SPSS Statistics Version 24.0. Individual mappings of phasic EDA during performance of the course were inspected to identify any individual variability that may direct future research.
Results
Participants
Descriptions of the participants with LLA and control subjects are shown in Table 1. There was no significant difference in age between controls and people with LLA. The average time since amputation for participants with LLA was 19.4 years (SD ± 15.7). Three out of the four participants with transfemoral amputation used a microprocessor knee (Otto bock C-Leg©), while the other individual used a hydraulic-based mechanical knee (OrtoPed Hydracadence Knee©).
Participants with LLA reported a lower score than controls on the ABC (p < 0.01), indicative of decreased confidence in their balance and mobility in the community. Five participants with LLA reported falls over the past year (5 participants reported 1–2 falls, one participant reported a damaged prosthetic knee that resulted in 6 falls prior to repair). Controls reported no falls in the past year.
Feasibility
All participants could complete the course without any reported concerns or adverse effects. In addition, all participants reported being comfortable with the course and the wearing of the EDA/GPS device. Subjectively, participants reported no difficulty with any of the tasks and that the tasks were reflective of day-to-day mobility in their community. The outdoor data collection was conducted in late mornings, with the mean ambient temperature of 21.87 °C (SD ± 2.70) and mean humidity of 56.56% (SD ± 16.29).
EDA and GPS signals were successfully collected over the entire course using the portable system with no loss of signal at any point of the walking course (Figure 4). The synchronization of EDA and GPS signals facilitated the development of individualized phasic EDA maps that allow for ease of interpretation of the individual experience during specific outdoor walking tasks.
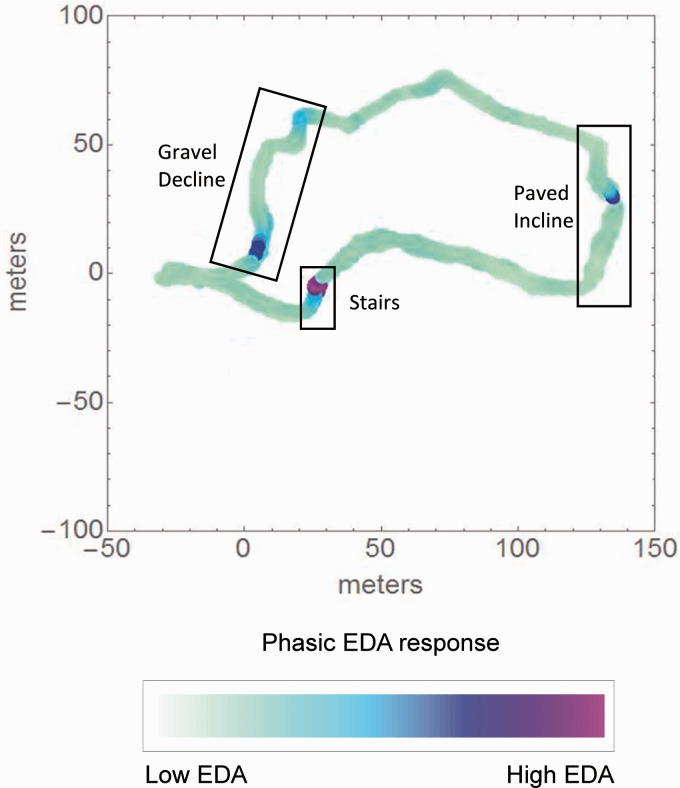
Figure 4.
Representative sample of a phasic electrodermal activation (EDA) map constructed in synchronization with coordinates from GPS signal collected over the walking course. The locations of the tasks are indicated by boxed labels on the figure. The colour gradient at yellow-green represents the lowest phasic EDA level observed in all subjects, and as the colour transforms to a more blue-purple the colour, it represents the highest phasic EDA level observed in all subjects.
Electrodermal activation
Kruskal-Wallis tests were used to compare phasic EDA responses during tasks (collapsed across laps) within each group. Between task differences in phasic EDA were found in participants with LLA (p = 0.01) but not in controls (p = 0.69). Mann-Whitney tests revealed that in participants with LLA, phasic EDA during the ascending stairs without a handrail task was significantly greater than each of the paved incline and the gravel decline tasks (Table 2, p = 0.01).
Table 2.
Phasic electrodermal activity for each lap of each walking task in participants with lower limb amputation (LLA) and control participants. Data presented as median (IQR), significant within group findings indicated.
| Walking task | Participants with amputation | Age-matched controls |
|---|---|---|
| Up stairs no rail | ||
| Lap 1 | 1.16 (1.11–2.00)† | 1.15 (1.01–1.26) |
| Lap 2 | 1.02 (0.83–1.19) | 0.85 (0.64–1.11) |
| Lap 3 | 1.16 (0.90–1.30)† | 1.00 (0.64–1.24) |
| Paved incline | ||
| Lap 1 | 0.97 (0.58–1.44) | 1.01 (0.85–1.12) |
| Lap 2 | 0.90 (0.72–1.13) | 0.97 (0.69–1.12) |
| Lap 3 | 0.92 (0.76–1.08) | 0.95 (0.83–1.19) |
| Gravel decline | ||
| Lap 1 | 0.94 (0.77–1.23) | 1.12 (0.93–1.25)* |
| Lap 2 | 0.96 (0.82–1.07) | 1.01 (0.84–1.07)* |
| Lap 3 | 0.92 (0.77–1.08) | 0.80 (0.65–0.84)* |
*within task, between lap, p = 0.01.
†Between task collapsed across laps, p = 0.01.
Friedman tests were used to explore task-specific between lap differences in phasic EDA in each group. Only the gravel decline demonstrated within task effects of lap in controls (p = 0.01). Wilcoxon Signed Rank Tests showed that levels of EDA were significantly higher in lap 1 than lap 3 (Table 2, p = 0.01).
Between group comparison of phasic EDA response to performing each task (collapsed across laps) showed no significant difference between participants with LLA and age matched controls while ascending stairs without a handrail (p = 0.13), walking up a paved incline (p = 0.58), or down a gravel decline (p = 0.97).
Friedman tests were used to explore task-specific between lap differences in slower changing tonic EDA in each group. In controls, all three tasks demonstrated between lap effects (p ≤ 0.05). In participants with LLA, ascending stairs without a handrail and walking up a paved incline demonstrated between lap effects (p ≤ 0.05). Wilcoxon Signed Rank Tests showed that levels of tonic EDA were decreased significantly with each lap in controls (Table 3, p ≤ 0.05). In participants with LLA, tonic EDA was higher in lap 1 than lap 3 when ascending stairs without a handrail and walking up a paved incline (Table 3, p ≤ 0.05).
Table 3.
Tonic electrodermal activity for each lap of each walking task in participants with lower limb amputation (LLA) and control participants. Data presented as median (IQR), significant within group findings indicated.
| Walking task | Participants with amputation | Age-matched controls |
|---|---|---|
| Up stairs no rail | ||
| Lap 1 | 0.93 (0.77–1.03)* | 1.00 (0.94–1.07)* |
| Lap 2 | 0.66 (0.53–0.87)* | 0.88 (0.79–0.98)* |
| Lap 3 | 0.59 (0.56–0.80)* | 0.83 (0.76–0.90)* |
| Paved incline | ||
| Lap 1 | 0.79 (0.65–1.20)* | 0.96 (0.85–1.09)* |
| Lap 2 | 0.72 (0.56–0.96)* | 0.84 (0.77–0.95)* |
| Lap 3 | 0.66 (0.52–0.86)* | 0.81 (0.73–0.93)* |
| Gravel decline | ||
| Lap 1 | 0.73 (0.62–1.15) | 0.90 (0.77–0.98)* |
| Lap 2 | 0.68 (0.51–0.97) | 0.81 (0.76–0.95)* |
| Lap 3 | 0.65 (0.47–0.95) | 0.73 (0.60–0.90)* |
*between lap, p ≤ 0.05.
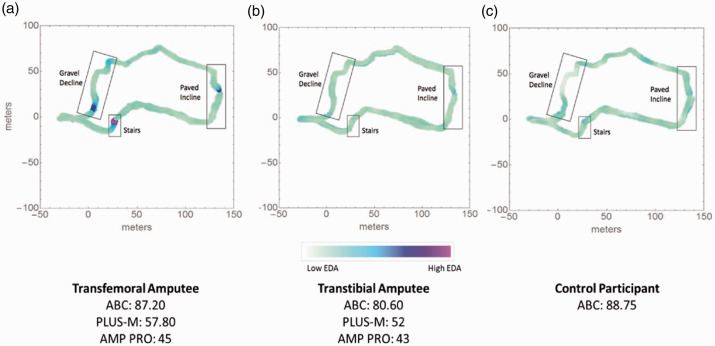
Inspection of the individual phasic EDA maps revealed that participants with transfemoral amputation showed consistent rise of phasic EDA while performing each of the three mobility tasks of the walking course (Figure 5(a)). This task-specific fluctuation of phasic EDA was less apparent in participants with transtibial amputation and control subjects (participants with transtibial amputation and controls with the lowest reported balance measures chosen as comparators for these groups, Figure 5(b) and (c)).
Figure 5.
Phasic EDA maps over the complete walking course for participants with (A) transfemoral LLA or (B) transtibial LLA and, (C) a control participant. The participant for the transfemoral group (the group with the highest level of amputation) shows scores on clinical measures (ABC, PLUS-M, and AMP PRO) that are representative of the transfemoral group mean score in each clinical measure. In contrast, the representative participants for both the transtibial and control groups have the lowest ABC score of each respective group, suggestive of the lowest reported balance confidence (ABC) and balance performance of each group. Representative of the transfemoral group, the participant with transfemoral LLA demonstrated more notable phasic EDA responses during tasks compared to the participant with transtibial LLA or controls (despite similarly rated balance confidence). The locations of each walking tasks are indicated by boxed labels on each of the maps. Only the participant with transfemoral LLA demonstrates heightened phasic activity (blue-purple colour) during each of the three walking tasks during the walking course.
Discussion
Participants with lower limb amputation report lower community level balance confidence than age-matched peers. 2 Our findings suggest that measurement of EDA together with GPS is feasible and successfully captures the physiological arousal response to specific outdoor mobility challenges in individuals with lower-limb amputations and age-matched controls. Initial findings of between task and lap differences in levels of physiological arousal in people with LLA are promising for further exploration of the use of these measures to explore levels of physiologic arousal during real-life community mobility challenges that people with amputation identify as challenging.
Collection of EDA outside of the lab setting has been shown to be feasible in variable levels of exercise, temperature and during prolonged data collection. 36 This study is the first to demonstrate successful implementation of a portable EDA/GPS measurement system to capture task-specific response to performing specific community-level outdoor mobility activities. The resulting activity-linked physiological arousal maps show seamless data collection and support further investigation into the clinical use and applicability of EDA in ‘real-life’ outdoor settings that are reflective of environments that challenge balance and mobility in people with LLA.
Between task differences in the level of task-specific physiological arousal captured by phasic EDA were noted only in people with LLA. Heightened phasic EDA during walking upstairs without the use of a handrail occurred compared to walking up a paved incline and walking down a gravel decline, suggesting that this task was perceived to be more challenging for these participants with LLA. Methodologically, this finding demonstrates that the portable EDA system was successful in quantifying levels of arousal in response to tasks of various levels of challenge. Interestingly, control participants demonstrated significant decreasing levels of phasic EDA with repetition of the walking down the gravel decline task that is suggestive of habituation/adaptation, not noted in participants with LLA. This lack of habituation/adaptation of physiological arousal with repeated exposure to a balance challenge task has been previously noted in people with stroke compared to age-matched controls. 14 Although the motor impairment is notably different between people with LLA and people with stroke, a common clinical feature of both disability groups is decreased balance performance and decreased balance confidence.2,14 In the current study, the notable increased phasic EDA in people with LLA during stair climbing compared to the other two tasks, together with the lack of habituation with repeated practice of tasks, are promising findings for further exploration of the utility of measurement of physiological arousal in rehabilitation. These task-specific measurements of physiological arousal during outdoor mobility may serve as an indicator of clinically meaningful change in self-perception of abilities or task challenge during repeated practice of community level mobility tasks during rehabilitation.
The slower changing tonic EDA levels, representative of more general levels of physiological arousal levels of individuals, showed further suggestion of decreasing levels of physiological arousal in controls with repetition of laps of the walking course. This was less apparent in people with LLA in which decrease in tonic EDA levels was only significant between laps 1 and 3 in walking upstairs without the use of a handrail and walking up a paved incline. These findings are suggestive of habituation of general levels of physiological arousal in controls more so than in people with LLA and are in-line with the more discrete task-specific responses of phasic EDA while completing the walking challenges in the current walking course.
The limited responsiveness of phasic EDA levels to the walking challenges of walking on a gravel ramp and paved incline in people with LLA are likely a reflection of the nature of the mobility challenges included in the current walking course. Of the three tasks, walking up the stairs is likely the item with the least amount of variability in design in the community. It is likely that the other two tasks used in the current study did not present sufficient challenge. The main objective of the study was to demonstrate the feasibility of the use of the portable system and therefore, the challenges of the walking tasks on the ramp were somewhat tempered with a goal of ensuring that all participants could complete the course without assistance. Future studies investigating outdoor mobility tasks that present greater challenges to walking balance are warranted to further explore the physiological arousal responses, both on initial exposure and with repeated practice.
Following LLA, a priority goal of rehabilitation is independent walking to facilitate independence in the community and enhanced quality of life. While treatment of physical abilities is of the upmost importance to achieving this goal, assessing and treating balance confidence impairments may further contribute successful independent access to the community in people with LLA. Inspection of individual activity-specific physiological arousal maps aid in the understanding of future applications of this measurement. In persons with transfemoral amputations, higher levels of physiological arousal are noted during performance of all three tasks. On the other hand, representative physiological arousal maps from participants with transtibial amputation and a control show the phasic EDA levels remain essentially unchanged regardless of the task. It is important to note the similarity between all three participants in self-reported balance confidence. This suggests that regardless of self-reported levels of balance confidence, people with transfemoral amputation may demonstrate altered levels of physiological arousal during balance and mobility tasks than people with transtibial amputation and controls. Further investigation into the effect of level LLA level on the modulation of physiological arousal during mobility challenges and the interplay with self-reported balance confidence is warranted.
Limitations and future directions
The current study established the feasibility of outdoor measurement of EDA together with GPS during community-level walking challenges. Future studies with larger sample size are required to explore the effect of LLA on physiological arousal during community-level walking tasks of various levels of challenge to balance presented in random order. Variability within the current data warrants further exploration of possible covariates such as level of amputation, age and types of prosthetic knees used among people with TF amputation.
The current study included people with LLA who have been living independently in the community for more than 10 years post LLA. Future work directed towards people with LLA at an earlier stage in their rehabilitation and individuals reporting more restricted community-level ambulation may further our understanding of physiological arousal and self-reported balance and mobility confidence over the course of rehabilitation and with mobility restrictions. Importantly, future research with a more functionally varied group of people with LLA is required to explore physiological arousal responses that may be associated with maladaptive patterns of perceptual response to repeated walking challenges during rehabilitation of community level walking.
Finally, techniques to make the extraction of information from EDA signal more computationally efficient are rapidly advancing39–41 and have potential to contribute to future developments in portable EDA sensors.
Conclusion
The presented methods of using portable EDA/GPS measurement during community mobility tasks are promising. It may help clinicians determine individualized, task-specific treatment plans in-line with tasks their client may perceive challenging in their community setting. Further research is required to explore how EDA may be incorporated into clinical research and clinical settings to assist clinicians in assessing balance and mobility confidence in people with LLA.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Sciences and Engineering Research Council (EC and JMW) and the Canadian Institutes for Health Research (CLP).
Contributorship: EC performed data collection, analysis and drafted the manuscript. All authors contributed to research design, analysis and revision of this manuscript.
ORCID iD: Courtney L Pollock https://orcid.org/0000-0003-1720-3472
References
- 1.Sagawa Y, Jr, Turcot K, Armand S, et al. Biomechanics and physiological parameters during gait in lower-limb amputees: a systematic review. Gait Posture 2011; 33: 511–526. [DOI] [PubMed] [Google Scholar]
- 2.Miller WC, Speechley M, Deathe AB. Balance confidence among people with lower-limb amputations. Phys Ther 2002; 82: 856–65. [PubMed] [Google Scholar]
- 3.Kulkarni J, Wright S, Toole C, et al. Falls in patients with lower limb amputations: prevalence and contributing factors. Physiotherapy 1996; 82: 130–136. [Google Scholar]
- 4.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehab Med 2010; 46: 239. [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R, van den Heuvel WJ, Arokiasamy P. Factors affecting quality of life in lower limb amputees. Prosthet Orthot Int 2011; 35: 90–96. [DOI] [PubMed] [Google Scholar]
- 6.Zidarov D, Swaine B, Gauthier-Gagnon C. Quality of life of persons with lower-limb amputation during rehabilitation and at 3-month follow-up. Arch Phys Med Rehab 2009; 90: 634–645. [DOI] [PubMed] [Google Scholar]
- 7.Asano M, Rushton P, Miller WC, et al. Predictors of quality of life among individuals who have a lower limb amputation. Prosthet Orthot Int 2008; 32: 231–243. [DOI] [PubMed] [Google Scholar]
- 8.Miller WC, Deathe AB, Speechley M. Psychometric properties of the activities-specific balance confidence scale among individuals with a lower-limb amputation1. Arch Phys Med Rehab 2003; 84: 656–661. [DOI] [PubMed] [Google Scholar]
- 9.Hafner BJ, Morgan SJ, Askew RL, et al. Psychometric evaluation of self-report outcome measures for prosthetic applications. J Rehab Res Dev 2016; 53:797, 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt SM, Bhopal R. Self report in clinical and epidemiological studies with non-English speakers: the challenge of language and culture. J Epidemiol Commun Health 2004; 58: 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulhus DL, Vazire S. The self-report method. In: Robins RW, Fraley RC and Krueger RF (eds) Handbook of research methods in personality psychology. New York: Guilford Press, 2007, pp.224–239.
- 12.Boucsein W. Electrodermal activity. Berlin: Springer Science & Business Media, 2012. [Google Scholar]
- 13.Critchley HD, Elliott R, Mathias CJ, et al. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci 2000; 20: 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollock CL, Carpenter MG, Hunt MA, et al. Physiological arousal accompanying postural responses to external perturbations after stroke. Clin Neurophysiol 2017; 128: 935–944. [DOI] [PubMed] [Google Scholar]
- 15.Shan H, Mason P. A neuroscience framework for psychophysiology. In: Cacioppo JT, Tassinary LG and Berntson GG (eds) Handbook of psychophysiology. 4th ed. Cambridge: Cambridge University Press, 2017, pp.16–25.
- 16.Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. USA: Oxford University Press, 2001. [Google Scholar]
- 17.Kilpatrick DG. Differential responsiveness of two electrodermal indices to psychological stress and performance of a complex cognitive task. Psychophysiology 1972; 9: 218–226. [DOI] [PubMed] [Google Scholar]
- 18.Dawson M, Schell A, Filion D. The electrodermal system. In: Cacioppo JT and Tassinary LG (eds) Principles of psychophysiology: physical, social, and inferential elements. Cambridge: Cambridge University Press, 1990.
- 19.Nagai Y, Critchley HD, Featherstone E, et al. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage 2004; 22: 243–251. [DOI] [PubMed] [Google Scholar]
- 20.Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol Series A: Biol Sci Med Sci 1995; 50A: M28–M34. [DOI] [PubMed] [Google Scholar]
- 21.Hafner BJ, Gaunaurd IA, Morgan SJ, et al. Construct validity of the prosthetic limb users survey of mobility (plus-M) in adults with lower limb amputation. Arch Phys Med Rehab 2017; 98: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amtmann D, Abrahamson DC, Morgan S, et al. The plus-M: item bank of mobility for prosthetic limb users. Qual Life Res 2014; 1: 39–40. [Google Scholar]
- 23.Morgan SJ, Amtmann D, Abrahamson DC, et al. Use of cognitive interviews in the development of the plus-M item bank. Qual Life Res 2014; 23: 1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gailey RS, Roach KE, Applegate EB, et al. The amputee mobility predictor: an instrument to assess determinants of the lower-limb amputee's ability to ambulate. Arch Phys Med Rehab 2002; 83: 613–27. [DOI] [PubMed] [Google Scholar]
- 25.Horslen BC, Carpenter MG. Arousal, valence and their relative effects on postural control. Exp Brain Res 2011; 215: 27–34. [DOI] [PubMed] [Google Scholar]
- 26.Cleworth TW, Horslen BC, Carpenter MG. Influence of real and virtual heights on standing balance. Gait Posture 2012; 36: 172–176. [DOI] [PubMed] [Google Scholar]
- 27.Hubert W, de Jong-Meyer R. Autonomic, neuroendocrine, and subjective responses to emotion-inducing film stimuli. Int J Psychophysiol 1991; 11: 131–140. [DOI] [PubMed] [Google Scholar]
- 28.Kappeler-Setz C, Gravenhorst F, Schumm J, et al. Towards long term monitoring of electrodermal activity in daily life. Person Ubiquitous Comput 2013; 17: 261–271. [Google Scholar]
- 29.Greco A, Valenza G, Citi L, et al. Arousal and valence recognition of affective sounds based on electrodermal activity. IEEE Sensors J 2017; 17: 716–725. [Google Scholar]
- 30.Sokhadze EM. Effects of music on the recovery of autonomic and electrocortical activity after stress induced by aversive visual stimuli. Appl Psychophysiol Biofeedback 2007; 32: 31–50. [DOI] [PubMed] [Google Scholar]
- 31.Shaffer F, Combatalade D, Peper E, et al. A guide to cleaner electrodermal activity measurements. Biofeedback 2016; 44: 90–100. [Google Scholar]
- 32.Hugdahl K. Psychophysiology: the mind-body perspective. Cambridge, MA: Harvard University Press, 1995. [Google Scholar]
- 33.Critchley HD. Electrodermal responses: what happens in the brain. Neuroscientist 2002; 8: 132–142. [DOI] [PubMed] [Google Scholar]
- 34.Boucsein W, Fowles DC, Grimnes S, et al. Society for psychophysiological research ad hoc committee on electrodermal measures. publication recommendations for electrodermal measurements. Psychophysiology 2012; 49: 1017–1034. [DOI] [PubMed] [Google Scholar]
- 35.Braithwaite JJ, Watson DG, Jones R, et al. A guide for analysing electrodermal activity (EDA) & skin conductance responses (SCRs) for psychological experiments. Psychophysiology 2013; 49: 1017–1034. [Google Scholar]
- 36.Doberenz S, Roth WT, Wollburg E, et al. Methodological considerations in ambulatory skin conductance monitoring. Int J Psychophysiol 2011; 80: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts LE, Young R. Electrodermal responses are independent of movement during aversive conditioning in rats, but heart rate is not. J Comparat Physiol Psychol 1971; 77: 495–512 [DOI] [PubMed] [Google Scholar]
- 38.Turpin G, Shine P, Lader M. Ambulatory electrodermal monitoring: effects of ambient temperature, general activity, electrolyte media, and length of recording. Psychophysiology 1983; 20: 219–224 [DOI] [PubMed] [Google Scholar]
- 39.Bach DR, Staib M. A matching pursuit algorithm for inferring tonic sympathetic arousal from spontaneous skin conductance fluctuations. Psychophysiology 2015; 52: 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernando-Gallego F, Luengo D, Artés-Rodríguez A. Feature extraction of galvanic skin responses by nonnegative sparse deconvolution. IEEE J Biomed Health Inform 2018; 22: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 41.Greco A, Valenza G, Lanata A, et al. cvxEDA: a convex optimization approach to electrodermal activity processing. IEEE Trans Biomed Eng 2016; 63: 797–804. [DOI] [PubMed] [Google Scholar]