Abstract
Mood disorders represent a pressing public health issue and significant source of disability throughout the world. The classical monoamine hypothesis, while useful in developing improved understanding and clinical treatments, has not fully captured the complex nature underlying mood disorders. Despite these shortcomings, the monoamine hypothesis continues to dominate the conceptual framework when approaching mood disorders. However, recent advances in basic and clinical research have led to a greater appreciation for the role that amino acid neurotransmitters play in the pathophysiology of mood disorders and as potential targets for novel therapies. In this article we review progress of compounds that focus on these systems. We cover both glutamate-targeting drugs such as: esketamine, AVP-786, REL-1017, AXS-05, rapastinel (GLYX-13), AV-101, NRX-101; as well as GABA-targeting drugs such as: brexanolone (SAGE-547), ganaxolone, zuranolone (SAGE-217), and PRAX-114. We focus the review on phase-II and phase-III clinical trials and evaluate the extant data and progress of these compounds.
Keywords: depression, mood disorders, rapid-acting antidepressants, ketamine/esketamine, clinical trials
Introduction
Mood disorders, including major depressive disorder, are now the number one cause of disability worldwide. 1 Traditionally, the pathophysiology of mood disorders was thought to be caused by a deficiency in monoamines in the synaptic cleft: serotonin, dopamine, and norepinephrine. While the monoamine-based hypothesis of mood disorders has led to significant advances and is the theoretical foundation for all approved standard oral antidepressants, it does not adequately explain several features of mood disorders, including the lag time to clinical efficacy for monoamine-based drugs. 2
The last two decades have seen significant advances in a hypothesis of mood disorders based on abnormalities in the amino acid neurotransmitter system, including glutamate (the primary excitatory neurotransmitter) and GABA (the primary inhibitory neurotransmitter) 3 , 4 (Figures 1 and 2). Neuroimaging work consistently documents abnormalities in these systems in key brain regions of patients with mood disorders. 2 Abnormal levels of these neurotransmitters have also been seen in plasma, serum and cerebrospinal fluid of patients with mood disorders. 5 These developments have been spurred by the discovery that ketamine, an antagonist at the NMDA receptor, can produce rapid antidepressant effects.
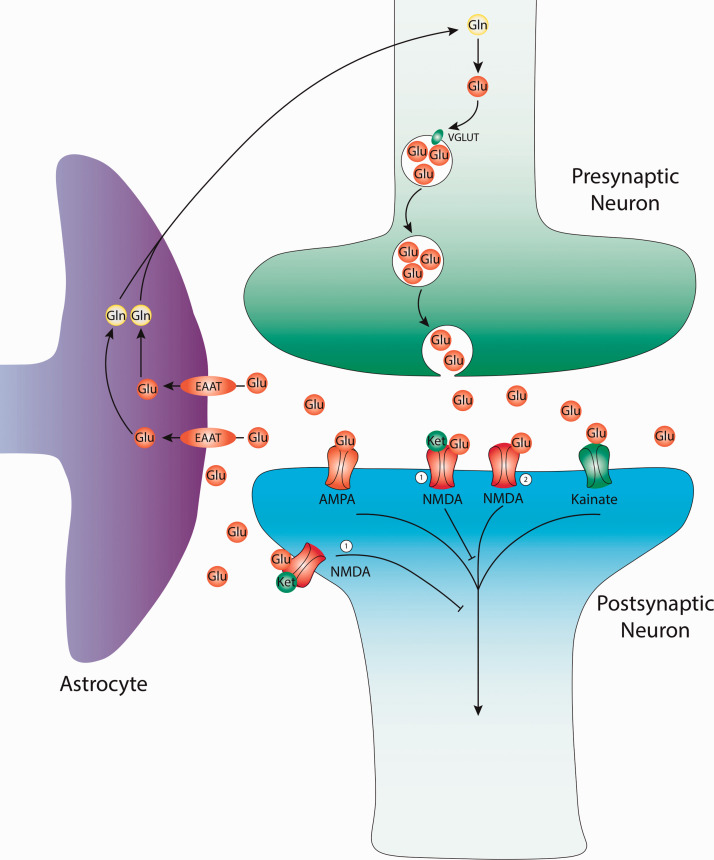
Figure 1.
Glutamatergic neurotransmission and current targets for therapy. Glutamate (Glu) is derived from glutamine (GLN) in the presynaptic terminal. Alternatively, Glu can be created from the TCA cycle (not depicted). Glu molecules are then packaged into presynaptic vesicles by the vesicular glutamate transporter (VGLUT). The packaged vesicles then release their contents from the presynaptic neuron into the synaptic cleft. Within the cleft, Glu can bind various receptors including: α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), Kainate, and N-methyl-D-aspartate (NMDA) receptors. Glutamate can be recycled from synaptic cleft by the excitatory amino-acid transporter (EAAT) of nearby astrocyte glial cells. Within glial cells glutamate is converted to glutamine before either being used by the glial cells or reused by nearby neurons for further glutamatergic transmission. 1) NMDA antagonist, site of action for: Ketamine, Esketamine, Lanicemine, AVP-786, AXS-05, Dextromethadone, MIJ821. 2) NMDA agonist, site of action for: Rapastinel, AV-101, NRX-100/NRX-101.
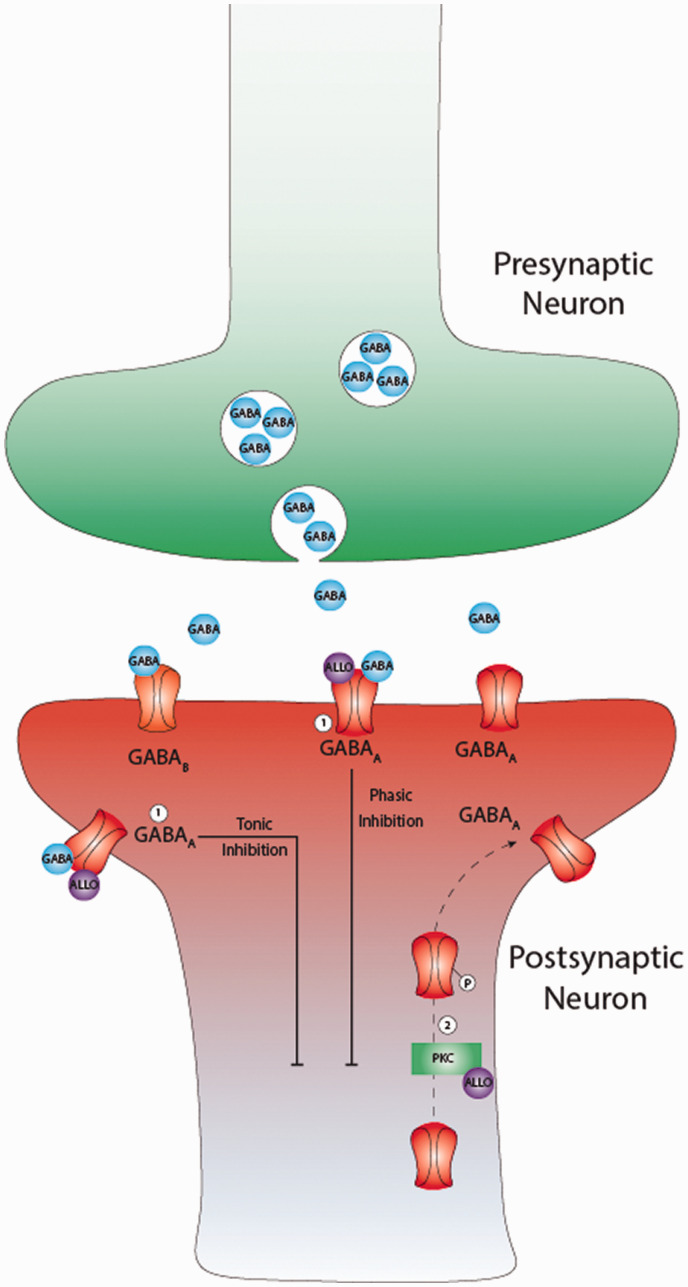
Figure 2.
Gamma-aminobutyric (GABA) neurotransmission and current targets for therapy. GABA neurotransmitters are the predominant source of inhibition in the adult mammalian brain. GABA molecules bind two main types of receptors on neurons to promote inhibition: GABAAand GABAB. The GABAA receptors are ionotropic which function by increasing inward chloride ion flux to hyperpolarize neurons while GABAB receptors which use metabotropic signaling via G-proteins to open potassium channels and hyperpolarize neurons. Inhibition mediated by GABAA receptors can come in two distinct forms: phasic inhibition from intrasynaptic receptors following synaptic events or tonic inhibition from extrasynaptic receptors that provides more a more persistent source of inhibition. Allopregnalone (ALLO) is a GABAergic neurosteroid that acts as a positive allosteric modulator (PAM) at GABAA receptors to increase both of these forms of inhibition. 1) PAM of GABAA receptors, site of action for: Brexanolone, Ganaxolone, Zuranolone. 2) Protein Kinase C (PKC) mediated phosphorylation and increased extrasynaptic GABAA receptor trafficking, site of action for: Brexanolone, Zuranolone.
Given the rapidly changing state of the field, we undertook an updated systematic review of key compounds in development with mechanisms of action based on the amino acid neurotransmitter systems. We systematically searched MEDLINE (no start date cutoff; end date cutoff of March 31, 2020), the US Clinical Trials Registry (www.clinicaltrials.gov), and Sponsor websites for the latest clinical trial updates for the following compounds: esketamine (Spravato), AVP-786, REL-1017, AXS-05, Rapastinel (formerly GLYX-13), AV-101, NRX-101, AGN-241751, brexanlone (Zulresso, SAGE-547), ganaxolone, zuranolone (SAGE-217), MIJ821, PRAX-114.
As no updates were found in the last two years, 6 the following drugs were omitted: EVT-101, Traxoprodil/CP-101,606, Lanicemine/AZD-6765, Rislenemdaz/CERC-301, basimglurant, riluzole.
We limit the scope of this review to compounds in Phase II or III human trials. Two compounds, esketamine (Spravato) and brexanlone (Zulresso) received FDA approval for treatment-resistant depression and post-partum depression, respectively, in March 2019. We also review what is known about the mechanisms of action of these compounds. Where reported in study results, we use Cohen’s d effect size measure (between group comparison). Where not given and where sufficient data were available, we estimated the effect size.
Ketamine
Ketamine is the prototypical rapid-acting antidepressant that was discovered to have powerful antidepressant effects over two decades ago. 7 Traditionally an anesthetic, its antidepressant effects are most pronounced at subanesthetic doses, most commonly 0.5 mg/kg given intravenously over 40 minutes. 8 There are many small, academic studies using this treatment paradigm, though the vast majority are single-dose studies. 7 ,9–11 Hence, there is little long-term data on the use of IV racemic ketamine as a treatment for psychiatric disorders. Given the lack of industry sponsorship, it is unlikely that ketamine will be approved by the US FDA.
Esketamine
Treatment-Resistant Depression
Esketamine is the S-enantiomer of ketamine. It was approved by the US FDA for treatment-resistant depression (TRD), in conjunction with an oral antidepressant in March 2019. In August 2020, it was also approved by the FDA for treatment of depressive symptoms in adults with MDD who have acute suicidal ideation or behavior.
The new drug application leading to approval for TRD consisted of 5 Phase-III trials and 2 Phase-II trials. The phase-III trials included a fixed-dose short-term trial in adults ages 18-64 (TRANSFORM-1), a flexible-dose short-term trial in adults ages 18-64 (TRANSFORM-2), a flexible-dose short-term trial in geriatric patients ages 65 or older (TRANSFORM-3), a randomized withdrawal study (SUSTAIN-1), and a long-term, open-label (non-randomized) study (SUSTAIN-2). Notably, all subjects, regardless of treatment assignment, started a new oral antidepressant on the first study day. Hence, the comparison was between esketamine nasal spray plus a new oral antidepressant versus placebo nasal spray plus a new oral antidepressant.
The short-term fixed-dose trial (TRANSFORM-1, n = 346) did not meet its primary outcome (change in depression severity at week 4, p = 0.088, d = 0.22 for 84 mg; d = 0.28 for 56 mg). 12 The short-term flexible-dose trial (TRANSFORM-2, n = 236) met its primary outcome (change in depression severity at week 4, p = 0.02), with an effect size of d = 0.30. 13 The short-term flexible dose geriatric study (TRANSFORM-3;n = 139) did not meet its primary outcome (p = 0.059, d = 0.31 in favor of esketamine). 14
In the randomized withdrawal study (SUSTAIN-1), patients who had achieved stable response (n = 121) or remission (n = 176) were maintained on a stable dose of esketamine dosing for 12 weeks. At this point, half were randomized to placebo nasal spray (plus antidepressant) in lieu of the esketamine (discontinuation), while the other half continued to receive esketamine (plus antidepressant). A significant difference in the primary outcome (time to relapse) was seen between those continuing esketamine and those who were withdrawn among both remitters (hazard ratio [HR] = 0.49, p<0.05) and stable responders (HR = 0.30, p<0.05). 15
The SUSTAIN-2 study was a long-term (up to 52 weeks) open-label, non-randomized study that included both new participants and those who previously completed the TRANSFORM-3 study. 16 Of 802 enrolled patients, 9.5% discontinued esketamine due to treatment emergent adverse events. The most common side effects included dizziness, dissociation, nausea, and headache. With regards to secondary outcomes, 76.5% maintained response and 58.2% maintained remission at study endpoint. 16 A long-term extension study of SUSTAIN-2 (SUSTAIN-3; NCT02782104) is ongoing, with plans to follow patients for several years.
Several additional Phase-III studies are ongoing. One is comparing esketamine to quetiapine (50 mg, 100 mg, and 150 mg doses) in addition to SSRI/SNRIs (NCT04338321; expected n = 622). The third is similar in design to TRANSFORM-2 and is recruiting primarily from Chinese sites (NCT03434041).
Major Depression With Suicidal Ideation (MDSI)
Esketamine is FDA approved for the rapid reduction of depressive symptoms in adults with major depressive disorder who have active suicidal ideation with intent. This application is based on one positive Phase-II trial and two positive Phase-III trials, all of which had similar designs. In these studies, patients with MDD who were judged to be at imminent risk of suicide and in need of hospitalization were recruited from emergency departments or similar settings. All subjects received standard of care (including hospitalization and medications, but excluding ECT), with half receiving esketamine nasal spray and the other half receiving placebo nasal spray. The primary outcome was the change in depression severity (MADRS) at 4 hours following the first dose. The Phase-II trial (n = 68) (NCT02133001) demonstrated an effect size of d = 0.61 while the Phase-III trials (ASPIRE-I, N 226; ASPIRE-II, N = 230) demonstrated effect size of d = 0.33 and d = 0.30, respectively). 17 , 18,40
AVP-786
AVP is an oral formulation that combined dextromethorphan and quinidine. Dextromethorphan is an antagonist at the NMDA receptor that, similar to ketamine, may have antidepressant properties. Quinidine is added to this formulation to increase the bioavailability by inhibiting the action of cytochrome P450-2D6 and thereby blocking metabolism of dextromethorphan. 19 This compound is similar to dextromethorphan HBr and quinidine sulfate (manufacturer: Avanir), which is approved for the treatment of pseudobulbar affect. AVP-786 differs because it contains deuterium, a heavier isotope of hydrogen, which may further extend the half-life of dextromethorphan. 20 A single Phase-II study in patients with MDD or TRD (n = 206) was completed by February 2016 but results are not published (NCT02153502). The compound isnow in development primarily for agitation in Alzheimer’s dementia or for schizophrenia (see NCT03896945, NCT04464564, NCT04408755, NCT03393520, NCT02477670, NCT02446132). As of March 2021, no additional trials for mood disorders are registered in the US.
AXS-05
AXS-05 (Axsome) is a formulation of dextromethorphan and bupropion. In addition to its antidepressant effects,bupropion is added to increase the bioavailability of dextromethorphan through inhibition of CYP2D6. The compound is currently being developed as a potential treatment for both MDD and TRD.
Major Depressive Disorder (MDD)
Several trials were recently completed with a positive Phase-II and a positive Phase-III trial investigating AXS-05 for the treatment of MDD. The phase-II ASCEND (NCT03595579) trial compared the effects of AXS-05 with the active comparator, bupropion (210 mg) for 6 weeks (n = 97). Results indicated that there was a statistically significant improvement (p < 0.001, d = 0.50) in the depression severity scores between the AXS-05 group and the comparator group (primary endpoint). Additionally, remission was attained in 47% of AXS-05 patients, compared to 16% of the bupropion group (p = 0.004). 21
The phase-III GEMINI (NCT04019704) study showed a statistically significant difference between AXS-05 and placebo after 6 weeks (p = 0.002, primary endpoint), with an effect size of d = 0.38. 22 Additionally, the phase-III COMET (NCT04039022) open-label, long-term safety study of AXS-05 in patients with MDD or TRD found safety profiles consistent with these previous trials. In this trial, 40% of patients met response criteria by week 2 and 73.2% by week 6. This initial response was retained by 84.6% of patients at 6 months and 82.8% of patients at 12 months. Similarly, remission criteria were met by 21.5% of patients by week 2 and 52.5% of patients by week 6. Remission was retained in 68.7% of patients at 6 months and 69% of patients at 12 months.
Treatment Resistant Depression (TRD)
In addition to being pursued as a treatment for MDD, AXS-05 is also being pursued as a treatment for TRD. The STRIDE-1 phase-III study did not show a statistically significant difference between active (AXS-05) and control (bupropion) in depression improvement after 6 weeks (p = 0.12, d = 0.21). 23
Two additional phase-II trials for TRD are currently ongoing. The MERIT study (anticipated n = 50; NCT04608396) is a randomized withdrawal study that will investigate the effect AXS-05 on preventing relapse. Another ongoing study (NCT04634669) examines safety and adverse events over 12 months of treatment with secondary endpoints examining change in MADRS score in an open-label design.
Relmada-1017
Dextromethadone (REL-1017), the d-stereoisomer of methadone, acts as a noncompetitive NMDA receptor antagonist. A Phase-II fixed-dose study (NCT03051256) evaluated the safety and efficacy of 25 mgand 50 mg doses of REL-1017 over a 7-day period in individuals with TRD. The study was primarily a safety study and was thus not powered to detect efficacy outcomes. 24 Unlike ketamine, a drug that acts on the same binding site as REL-1017, there was no evidence of dissociative effects during treatment. The currently ongoing RELIANCE-I study (NCT04688164) is a Phase-III, two-arm RCT that will test for changes in MADRS at 4 weeks in a larger sample (anticipated n = 400). REL-1017 is also currently in the preclinical research stage for uses in treating Rett Syndrome, mitochondrial diseases/ALS, and various uses in ophthalmology.
MIJ821
MIJ821 is an NR2B inhibitor being developed by Novartis Pharmaceuticals. The intravenously-administered compound is currently in Phase-II clinical trials investigating the use of the drug in the treatment of treatment-resistant depression. According to clinicaltrials.gov, one 6-arm RCT (low dose weekly, high dose weekly, low dose bi-weekly, high dose bi-weekly, placebo, and ketamine (active comparator) 0.5 mg/kg weekly) has completed, however, results have yet to be made publicly available as of March 2021 (NCT03756129). As of January 2021, there is also a 7-arm RCT (very low dose, low dose, high dose, very high dose, placebo, high dose followed by placebo, and very high dose followed by placebo) described on clinicaltrials.gov that is not yet recruiting (NCT04722666). Both studies will use the MADRS at 24 hours as the primary endpoint.
AV-101
AV-101 (L-4-chlorokyurenine or 4-CI-KYN) is a prodrug converted via the kyurenine pathway to an antagonist (7-chlorokynurenic acid) at the glycine binding site of the NMDAR NR1 subunit that has been tested for the treatment of MDD. Results from a Phase-II study of AV-101 (ELEVATE) showed that the AV-101 treatment arm did not differentiate from the placebo group on the primary endpoint (MADRS at 2 weeks). 25 An additional small (n = 19) Phase-II crossover of AV-101 in individuals with TRD (NCT02484456) was also negative (p = 0.71, d = 0.22). 26
Rapastinel
Rapastinel (Glyx-13) is a partial functional agonist at the glycine site of the NMDAR. Despite previous positive findings in a Phase-II trial, 27 three recent phase-III studies on the use of Rapastinel as adjunctive treatment of MDD did not show a significant difference between treatment and placebo (see Table 1 for effect size estimates).28–30 A fourth phase III study using a randomized withdrawal design also did not achieve its primary outcome. 31
Table 1.
Phase II and III trials of glutamate/GABA-based compounds in development for the treatment of mood disorders.
| NCT identifier/ trial acronym | Trial design | Condition | Expected/ actual completion date | Projected/ actual recruitment | Phase | Primary outcome measure and results if reported | Effect size (Cohen’s d unless otherwise noted; between-group) |
|---|---|---|---|---|---|---|---|
| Esketamine, Janssen Pharmaceuticals*, Celon Pharma SA**, and China Medical University, China*** | |||||||
|
NCT04425473 (Beijing Hospital) |
Prospective, 2-arm, RCT for in patients undergoing major risk surgery | Post-operative depression | Dec 2023 | 564 | II/III | Percentage of participants with remission (MADRS total score ≤ 10) 3 days after major surgical operation | Trial not complete. |
|
NCT02782104* SUSTAIN-3 |
Open label, single group. | TRD | Dec 2022 | 1150 | III | Long-term safety outcomes. | Trial not complete. |
|
NCT04338321* (ESCAPE-TRD) |
RCT, flexible dose study of esketamine vs. quetiapine extended release. Conducted in multiple countries. | Elderly TRD | Dec 2022 | 622 | III | Percentage of participants with remission (MADRS total score ≤ 10). | Trial not complete. |
| NCT04599855 | RCT, multiple fixed-dose, 3 arm study of inhaled nasal esketamine alone without additional AD. | TRD | June 2022 | 450 | IV | Change in MADRS at 4 weeks. | Trial not complete. |
| NCT03185819* | 4-arm RCT, fixed dose. | Suicidal MDD, ages 12-17 | Feb 2022 | 145 | II | Change from baseline in CDRS-R at 24 hours. | Trial not complete. |
|
NCT04414943*** (Peking) |
Prospective, 2-arm IV esketamine, RCT for parturients with prenatal depression. | Postpartum depression | Aug 2021 | 364 | II | Postpartum depression prevalence at 42 days as measured by Mini-International Neuropsychiatric Interview | Trial not complete. |
| NCT03434041* | RCT, 2-arm plus initiation of new oral antidepressant. | TRD | Aug 2021 | 234 | III | Change in MADRS at 4 weeks. | Trial not complete. |
| NCT03927378*** (Peking) | Prospective, 2-arm IV esketamine, RCT for parturients with prenatal depression. | Postpartum depression | June 2021 | 364 | II | Postpartum depression prevalence at 42 days as measured by Mini-International Neuropsychiatric Interview | Trial not complete. |
| NCT03965871** | RCT, multiple dose, 4-arm study of inhaled dry powder esketamine. Conducted in Poland. | Bipolar Depression | Feb 2021 | 88 | II | Change from baseline in MADRS total score at Day 14. | Not enough data for Cohen’s d. |
|
NCT04303325*** (ESPOD-BI) |
Prospective RCT in MDD patients undergoing breast cancer operation. Conducted in China. | Post-operative Depression | Dec 2020 | 36 | IV | Changes of MADRS total score (baseline to postoperative 24hrs). | Not enough data for Cohen’s d. |
| NCT03965858** | RCT, multiple dose, 4-arm study of inhaled dry powder esketamine. Conducted in Poland. | TRD | Apr 2020 | 88 | II | Change from baseline in MADRS total score at Day 14. | Not enough data for Cohen’s d. |
| NCT02918318* | RCT, multiple fixed-dose, 4-arm, study. Conducted in Japan. | TRD | Dec 2019 | 202 | II | Change from baseline in MADRS at 4 weeks. Primary outcome was not achieved at any dose level. | 0.01 (28 mg) 0.07 (58 mg) 0.02 (84 mg) |
|
NCT03097133* ASPIRE-II |
RCT, recruiting from emergency settings. | Suicidal MDD | Apr 2019 | 230 | III | Change in MADRS at 24 hours post-dose. Positive primary outcome for reduction in depressive symptoms at 24hrs (p=0.006). 20 | 0.30 (84 mg) |
|
NCT03039192* ASPIRE-1 |
RCT, recruiting from emergency settings. | Suicidal MDD | Dec 2018 | 226 | III | Change in MADRS at 24 hours post-dose. Positive primary outcome for reduction in depressive symptoms at 24hrs (p=0.006). 17 | 0.32 (84mg) |
|
NCT02493868* SUSTAIN-1 |
Randomized withdrawal study. | TRD in remission | Feb 2018 | 719 | III | Time to relapse in remitters (ESK+AD v. PBO+AD). Primary endpoint was met; remitters and responders who continued esketamine had a lower risk of relapse compared to those who discontinued the drug (p=0.003). 15 | HR= 0.49 (remission), HR = 0.3 (response) |
|
NCT02417064* TRANSFORM-1 |
3-arm RCT, fixed dose study. | TRD | Feb 2018 | 346 | III | Change in MADRS at 4 weeks (ESK+AD v. PBO+AD). Primary outcome was not met (p=0.088). 12 | 0.28 (56mg) 0.22 (84mg) |
|
NCT02418585* TRANSFORM-2 |
2-arm RCT, flexible dose. | TRD | Nov 2017 | 236 | III | Change in MADRS at 4 weeks (ESK+AD v. PBO+AD). Primary endpoint was achieved (p=0.02). 13 | 0.30 |
|
NCT02497287* SUSTAIN-2 |
Open label, single group. | TRD | Oct 2017 | 802 | III | Long-term (up to 12-months) efficacy and safety study. Common AEs included nausea, dizziness, dissociation, and headache; 9.5% of subjects discontinued esketamine due to treatment emergent AEs. Response and remission rates at study endpoint were 76.5% and 58.2%, respectively. 16 | Not enough data for Cohen’s d. |
|
NCT02422186* TRANSFORM-3 |
2-arm RCT, flexible dose. | TRD, elderly | Aug 2017 | 139 | III | Change in MADRS at 4 weeks (ESK+AD v. PBO+AD). Primary outcome was not achieved (p=0.059). 14 | 0.31 |
| NCT02133001* | RCT, recruiting from emergency settings | Suicidal MDD | Feb 2016 | 68 | II | Change in MADRS at 4 hours post-dose (ESK+AD v. PBO+AD). Primary outcome was achieved. 40 p=0.015 | 0.61 (MADRS), 0.67 (MADRS-SI) at 4 hours |
|
NCT01998958* SYNAPSE |
RCT, 4-arm, dose fiding study. | TRD | Sep 2015 | 108 | II | Change in MADRS at 1 week (placebo vs. 28mg, 56mg, and 84mg). The study achieved its primary outcome (p<0.05 for all esketamine groups compared to placebo). 41 | Phase 1, Phase 2: 28mg: 0.51, 0.44 56mg: 0.78, 0.60 84mg: 1.08, 1.04 |
| NCT01640080* | RCT, multiple-dose, 3-arm study of IV esketamine. Conducted in multiple countries. | TRD | Jun 2013 | 30 | II | Change in MADRS at 24 hours. Both doses (0.2mg/kg and 0.4mg/kg) showed significant improvement compared to placebo on secondary outcomes (HAM-D, BDI), but not the primary outcome (MADRS)42. |
0.2mg/kg: 1.54 0.4mg/kg: 1.70 |
| AVP-786 (Avanir/Otsuka), oral formulation | |||||||
| NCT02153502 | 2-arm RCT | TRD | Feb 2016 | 206 | II | MADRS score at week 10; results are not publicly available. No further trials currently planned. | Not enough data for Cohen’s d. |
| AXS-05 (Axsome Therapeutics) | |||||||
| NCT04634669 | Open label | TRD | May 2022 | 150 | II | Safety outcomes: TEAEs within 12 months of AXS-05 daily dosing. Additional non-primary endpoint will measure change in MADRS. | Trial not complete |
|
NCT04608396 (MERIT) |
2-arm RCT, withdrawal study | TRD (remitted) | June 2021 | 50 | II | Time to relapse from randomization (up to 26 weeks) | Trial not complete |
|
NCT04039022 (COMET) |
Open label | MDD, TRD | Oct 2020 | 876 | III | Safety outcomes: types and rates of adverse events (up to 12 months). Safety profile consistent with prior trials. Of all participants, 39.7% met clinical response by Week 2 and 73.2% of patients by Week 6. Response was retained in 84.6% and 82.8% of patients at 6 and 12 months, respectively. Remission was achieved by 21.5% of patients at Week 2 and 52.5% of patients at Week 6. Remission was retained in 68.7% and 69.0% of patients at 6 and 12 months, respectively. | N/A |
|
NCT02741791 (STRIDE-1) |
2-arm RCT (active comparator: bupropion) | TRD | March 2020 | 312 | III | Did not achieve primary endpoint (MADRS at week 6, p=0.12). 23 | 0.21 |
|
NCT04019704 (GEMINI) |
2-arm RCT (inert placebo) | MDD | Dec 2019 | 327 | III | Safety outcomes: types and rates of adverse events (6 weeks), and MADRS at Week 6. Primary endpoint was met. 22 | 0.38 |
|
NCT03595579 (ASCEND) |
2-arm RCT (active comparator: bupropion) | MDD | Jan 2019 | 97 | II | Primary outcome was safety. Change in MADRS at 6 weeks (secondary outcome) was statistically different in favor of AXS-05 (p<0.001). 21 | 0.50 |
| REL-1017 (Relmada) | |||||||
|
NCT04688164 (RELIANCE-I) |
2-arm RCT, fixed dose | MDD | Jun 2022 | 400 | III | Change in MADRS at 4 weeks. | Trial not complete |
| NCT03051256 | 3-arm RCT (25 mg, 50mg, placebo) | TRD | Aug 2019 | 62 | IIa | Safety and tolerability: Incidence of treatment emergent adverse events (AEs). No significant difference in AEs were found between placebo and treatment groups. 24 | Secondary outcome (MADRS): 25mg, 50mg dose: Day 2: 0.3, 0.0 Day 4: 0.9, 0.8 Day 7: 0.8, 0.7 Day 14: 0.9, 1.0 |
| MIJ821 (Novartis Pharmaceuticals), intravenous formulation | |||||||
| NCT04722666 | 7-arm RCT, dose finding study | MDD with suicidal intent | Dec 2023 | 195 | II | Change from baseline in the total score of the MADRS at 24 hours (and up to 52 weeks). | Trial not complete |
| NCT03756129 | 6-arm RCT, placebo- and active- (ketamine) controlled | TRD | March 2020 | 72 | II | MADRS score 24 hours after infusion. Results submitted, but not posted as of March 2021. | Not available |
| AV-101 (VistaGen), oral formulation designed for once daily dosing | |||||||
| NCT02484456 | 2-week crossover RCT | MDD | Dec 2019 | 22 | II | Change from baseline in HAM-D. No statistically significant treatment effect was detected. 26 p=0.71 | 0.261 |
|
NCT03078322 ELEVATE |
2-arm RCT | MDD | Oct 2019 | 180 | II | MADRS at 2 weeks. Primary endpoint was not achieved. 35 | Not enough data for Cohen’s d. |
| Rapastinel (GLYX-13, Allergan); unless otherwise noted, rapastinel is delivered intravenously | |||||||
| NCT03614156 | 3-arm RCT, (clinically driven dosing, weekly dosing, placebo) | MDD (relapse) | July 2019 | 363 | III | Time to first relapse during the 52 weeks. Study terminated due to business reasons. | Not enough data for Cohen’s d. |
| NCT03675776 | 3-arm RCT, (225mg, 450mg, placebo) | MDD | July 2019 | 50 | III | Change from baseline MADRS at 6 weeks. Study terminated due to business reasons. Did not meet primary endpoint. | 1.03 (225mg) 0.16 (450mg) |
| NCT03560518 | 3-arm RCT, (450mg, 900mg, placebo) | MDD | July 2019 | 439 | III | Change from baseline MADRS at 6 weeks. Study terminated due to business reasons. Did not meet primary endpoint. | 0.05 (450mg) 0.21 (900mg) |
| NCT03668600 | Open label (flexible dose) | MDD | July 2019 | 230 | III | Safety outcomes: Patients experiencing one or more TEAEs within 45 weeks. Study terminated due to business reasons. | Not enough data for Cohen’s d. |
| NCT03352453 | 2-arm RCT, recruiting from emergency settings | Suicidal MDD | June 2019 | 138 | II | Change from baseline in MADRS at 1 day. (Study terminated due to business reasons.) Results were not significant | 0.03 |
| NCT02951988 | 3-arm, randomized withdrawal | MDD | Feb 2019 | 604 | III | Time to first relapse during 52 weeks since randomization. Results were not significant. 31 | Not enough data for Cohen’s d. |
| NCT03002077 | Open label, single group | MDD | Dec 2018 | 617 | III | Safety outcomes over 52 weeks. No safety concerns identified (Clinicaltrials.gov, June 2020) | Not enough data for Cohen’s d. |
|
NCT02943564 RAP-MD-02 |
3-arm, fixed-dose RCT | MDD | Dec 2018 | 638 | III | Change from baseline in MADRS at 1 day Did not differentiate from placebo. 30 |
0.01 (225mg), 0.07 (450mg) |
|
NCT02943577 RAP-MD-03 |
2-arm RCT | MDD | Nov 2018 | 415 | III | Change from baseline in MADRS at 1 day Did not differentiate from placebo. 28 |
0.12 (450mg) |
|
NCT02932943 RAP-MD-01 |
2-arm RCT | MDD | Nov 2018 | 457 | III | Change from baseline in MADRS at 1 day Did not differentiate from placebo. 29 |
0.04 (450mg) |
| NCT02192099 | Open label extension, single group | MDD | Nov 2018 | 61 | II | Safety outcomes. Study Terminated. | N/A |
| NCT01684163 | 3-arm, fixed-dose RCT | MDD | Jun 2014 | 369 | II | Change in HAM-D at 6, 12, and 16 weeks | N/A |
| NCT01234558 | 4-arm, fixed-dose RCT | MDD | Jul 2012 | 115 | II | Change in HAM-D score at various timepoints within a 14-day period. A clear dose response relationship was observed and primary outcome measures was positive for the 5mg and 10mg doses (at days 3 and 7), but not the 1mg and 30mg doses. 43 | 5mg, 10mg dose: Day 3: 0.41, 0.36 Day 7: 0.39, 0.60 |
| NRX-100 (single-dose IV Ketamine) followed by NRX-101 (D-cycloserine and lurasidone, NeuroRx) | |||||||
| NCT03395392 | 2-arm RCT, control arm is placebo + lurasidone | Suicidal BPD | Mar 2022 | 150 | II/III | Change in MADRS from baseline at 6 weeks. | Trial not complete. |
|
NCT03396068 SBP-ASIB |
2-arm RCT, control arm is placebo + lurasidone | Suicidal BPD | Dec 2021 | 72 | II/III | Change in MADRS from baseline at 6 weeks. | Trial not complete. |
|
NCT03396601 SevereBD |
2-arm RCT of NRX-100 (ketamine) v. saline | Suicidal BPD | Aug 2021 | 150 | III | Suicidal ideation at 24 hours. | Trial not complete. |
| NCT03402152 | Part A: NRX-101 vs. Placebo, RCT Part B: NRX-101 vs. lurasidone HCl, RCT |
Bipolar MDD | Feb 2021 | 24 | II/III | Mean change in Glx (Glutamate+Glutamine) area under the curve (AUC) measured in 15min increments over 2hrs at following experimental drug vs. comparator. | Trial not complete. |
|
NCT02974010 STABIL-B |
2-arm RCT, control arm is placebo + lurasidone | Suicidal BPD | Nov 2018 | 20 | III | Change in MADRS from baseline at 6 weeks. Primary endpoint was achieved. 44 | 2.94* (resultssubmitted but not yet QC reviewed) |
| AGN-241751 (Allergan), oral formulation | |||||||
| NCT03726658 | Part A: 1x/day (3mg vs. 10mg vs. placebo) RCT Part B: 2x/day (3mg vs. 25mg vs. placebo) RCT |
MDD | Oct 2019 | 223 | I/II | Part A: MADRS at 1-day post first dose Part B: MADRS at 7 days post first dose Completed, but no results posted as of March 2021. |
Not available |
| NCT03586427 | 5-arm, fixed-dose RCT | MDD | Aug 2019 | 251 | II | Change in MADRS at 1 day. Completed, but no results posted as of March 2021. | Not available |
| Zulresso™, Brexanolone (SAGE-547) (Sage Therapeutics) which is delivered intravenously over 60 hours | |||||||
| NCT03924492 | Expanded Access (compassionate use) | PPD | N/A | N/A | N/A | This is an expanded access/compassionate use study intended to provide access to ZULRESSO™ (brexanolone) injection for the treatment of a limited number of eligible women with PPD during the period prior to commercial availability. | Trial not complete |
| NCT04273191 | Open label | PPD | Aug 2021 (withdrawn) |
10 | IV | Change from baseline on the HAMD-17 total score and change from baseline in functional connectivity (fMRI). This study was withdrawn (Sage has decided not to proceed with this study). | N/A |
| NCT03665038 | Open label | Adolescent PPD |
Jan 2021 | 20 | III | Percentage of participants with treatment-emergent adverse events (TEAEs). Completed, but no results posted as of March 2021. | Not available |
| NCT02942017 | 2-arm RCT | Moderate PPD |
Oct 2017 | 108 | III | Change from baseline in HAM-D at 3 days. This study met the primary endpoint (p=0.0160). 33 | 0.43 (90μg) |
| NCT02942004 | 2-arm RCT | Severe PPD | Oct 2017 | 138 | III | Change from baseline in HAM-D at 60 hours. This study met the primary endpoint (p=0.0013 for the BRX60 group; p=0.0252 for the BRX90 group). 33 |
0.73 (60μg) 0.89 (90μg) |
| NCT02614547 | 2-arm RCT | PPD | Jul 2016 | 21 | II | Change from baseline in HAM-D at 3 days. This trial met its primary endpoint (p=0.0075). 32 | 1.2 |
|
NCT02285504 547-PPD-201 |
Open label, single group | PPD | Jun 2015 | 4 | II | Safety measures were primary outcomes45. | N/A |
| Ganaxolone (Marinus), oral and intravenous formulations | |||||||
|
NCT03228394 (Magnolia) |
Part 1: 4-arm RCT, IV dose-finding study Part 2: 2-arm RCT of IV followed by oral medicine |
PPD | May 2020 | 91 | II | Part 1: Primary outcomes were adverse events and other safety outcomes. Part 2: No data available as of March 2021. |
Not available |
|
NCT03460756 (Amaryllis) |
2-arm RCT, oral | PPD | July 2019 | 84 | II | Primary outcome was HAM-D scores at 38 days and safety outcomes. Completed, but primary outcome is not data available as of March 2021. | Not available |
| NCT02900092 | Open label, single group | Postmenopausal MDD | Jan 2018 | 10 | N/A | The primary endpoint was change in depression severity (MADRS) using within-group comparison. A significant decrease was seen by Week 8 (p=.015). 34 | N/A |
| Zuranolone SAGE-217 (SAGE), which is an oral formulation designed for once daily dosing | |||||||
|
NCT04476030 CORAL |
2-arm RCT (Sage-217+Sertraline vs. Placebo+Sertraline) | MDD | Dec 2021 | 424 | III | Change from baseline in the HAM-D total score at day 15. | Trial not complete |
| NCT04442503SKYLARK | 2-arm RCT | PPD | Dec 2021 | 192 | III | Change from baseline in the HAM-D total score at day 15. | Trial not complete |
| NCT04007367, MDD-302 (REDWOOD) | 2-arm RCT | MDD | Dec 2021 (suspended) | 546 | III | Time to relapse during the double-blind Phase (HAM-D). This trial is listed as suspended as of March 2021 (evaluating potential amendments to the study). | N/A |
|
NCT03864614 SHORELINE |
Open label, single group | MDD | Nov 2021 | ∼777 | III | Safety and tolerability study of the initial treatment and retreatment cycles. Interim results in an SEC report state that Zuranolone was generally well-tolerated, with adverse events consistent with prior trials; 71.6% and 39.8% achieved response and remission, respectively, at day 15. Additionally, it was reported that 44.5% of participants with a positive initial response did not need an additional treatment course after up to one-year of follow-up. 37 | Trial not complete |
|
NCT04442490 WATERFALL |
2-arm RCT | MDD | June 2021 | 575 | III | Change from baseline in the HAM-D total score at day 15. | Trial not complete |
| NCT03771664, MDD-304 (RAINFOREST) | 2-arm RCT | MDD | May 2020 (suspended) | 102 | III | Change from baseline in sleep efficiency (SE) as assessed by polysomnography (PSG). This trial is listed as suspended as of March 2021 (evaluating potential amendments to the study). | N/A |
|
NCT03672175, MDD-301 (MOUNTAIN) |
3-arm RCT | MDD | March 2020 | 581 | III | This study did not meet its primary endpoint (p = 0.115) (change from baseline in HAM-D at Day 15). 36 | 0.04 (20mg) 0.16 (30mg) |
| NCT03692910, BPD-201 (ARCHWAY) | Part A: open-label Part B: 2-arm, parallel group |
Bipolar depression | March 2019 | 35 | II | Part A: Safety and tolerability of SAGE-217 as assessed by the frequency and severity of adverse events. All treatment-emergent adverse events were mild or moderate; there was a 45% response rate by Day 15. No results for Part B have been reported. |
Part A: N/A Part B: Not available |
| NCT02978326, PPD-201 (ROBIN) | 2-arm RCT | PPD | Dec 2018 | 153 | III | The primary endpoint (change from baseline at day 14 in HAM-D) was met (p=0.0029), with a 17.8-point improvement in active v. 13.6 improvement in placebo on the HAM-D. 38 | 0.40 |
|
NCT03000530, MDD-201 |
Part A: open-label (N=14) Part B: 2-arm RCT (N=89) |
MDD | Nov 2017 | 89 | II | Efficacy: Primary outcome (Part B, change in HAM-D at day 15) was achieved (p<0.001).
35
Safety: adverse events, laboratory values, vital signs, ECG, and suicidal ideation (C-SSRS). |
Part A: N/A Part B: 0.81 (30mg) |
| PRAX-114 (Praxis Precision Medicines), oral formulation | |||||||
| ACTRN12619000575134 PRAX-114-202 |
Randomized 3-arm fixed-dose study (no placebo arm) conducted in Australia. | MDD | Not listed | 36 | IIa | Safety and tolerability study. Secondary outcomes were improvement in depression outcomes. Interim results released in an SEC report showed improvements of 15 to 19 in HAM-D after 1 week of therapy across the three dose groups. 39 | N/A |
“*,” “**,” and “***” under the Esketamine section refers to the location/sponsor of the study: Janssen Pharmaceuticals*, Celon Pharma SA**, and China Medical University, China***.BPD – bipolar depression; MDD – major depressive disorder; PPD – post-partum depression.Unless otherwise noted, the data here is taken from www.clinicaltrials.gov. Compounds are grouped by purported mechanism of action and are listed in the same order they appear in Table 2 and different studies for the same compound are listed in chronological order of estimated or actual completion date. Some completed studies did not have publicly available data.
Table 2.
Potential antidepressant compounds in development.
| Compound, route of administration | Pharmacology | Sponsor | Phase | Comments |
|---|---|---|---|---|
| Ketamine, various | Non-selective, non-competitive NMDA receptor antagonist | Multiple | -- | Several small trials from academia2; unlikely to be studied in phase III clinical trials required to receive FDA approval. |
| Esketamine, IN | Non-selective, non-competitive NMDA receptor antagonist | Janssen | III | Granted FDA approval in 2019 for TRD in conjunction with an oral antidepressant, with supplemental indication approved for major depression with suicidal ideation (Aug 2020). Esketamine has 4-5 times the NMDAR binding potency compared to (R)-ketamine. 46 |
| AVP-786, oral | Non-selective antagonist of NMDAR | Avanir/ Otsuka | II | Combination of dextromethorphan and quinidine. Phase II trial completed in Feb 2016 (results are not published); no additional studies for mood disorders registered as of March 2021. |
| AXS-05, oral | Non-selective antagonist of NMDAR | Axsome | III | Combination of dextromethorphan/ bupropion. Primary outcome was attained in Phase-II and Phase-III studies for MDD, but not in Phase-III study for TRD. Additional studies are ongoing. |
| REL-1017, oral | Noncompetitive NMDAR antagonist | Relmada | II | Being pursued for MDD. One phase-II study found statistically significant improvement in depression compared to placebo. A Phase-III study is ongoing. |
| MIJ821 | NMDAR NR2B antagonist | Novartis Pharmaceuticals | II | Two phase-II trials are completed (MDD, MDSI) but have not publicly released data. |
| AV-101, oral | Selective antagonist at glycine site of NMDA receptor NR1 subunit | VistaGen | II | The primary endpoint was not met in a recent phase-II trial. Another phase-II completed in Dec 2019 also did not meet primary endpoint. |
| Rapastinel/ GLYX-13, IV | Partial functional agonist at glycine site of NMDA receptor | Allergan/Abbvie | III | At least three phase-III studies did not differentiate from placebo on the primary outcome. A relapse-prevention study also did not meet primary endpoint. |
| NRX-100/NRX-101, oral | Partial NMDAR agonist at glycine-site | NeuroRx | III | Ketamine (NRX-100) followed by D-cycloserine plus lurasidone (NRX-101) to sustain effects in suicidal bipolar depression. Primary endpoint was positive for a phase-II trial.* Three Phase-II/III trials (suicidal BPD) and one biomarker trial are ongoing. |
| AGN-241751 | NMDAR modulator | Allergan | II | Being pursued for the treatment of MDD. Two phase-II trials completed in 2019 but data is not available publicly. |
| Brexanolone/ SAGE-547, Zulresso™.IV | PAM of GABAA receptor | Sage | III | Approved by FDA for PPD. Two phase-III trials for the treatment of PPD met their primary endpoints There is one ongoing open-label phase-III study in adolescents with PPD. |
| Ganaxolone, IV and oral | PAM of GABAA receptor | Marinus | II | Being pursued as a treatment for post-partum depression. The primary endpoint was met in an initial open label pilot study in postmenopausal MDD. Two phase-II studies (one IV study in moderate PPD and one oral study in severe PPD) have completed but full data are not publicly available. |
| Zuranolone, SAGE-217, oral | PAM of GABAA receptor | Sage | III | Being pursued as a treatment for MDD, BPD, and PPD. One Phase-II trials (MDD) and one Phase-III trials (PPD) achieved the primary endpoint, while another phase-III trial (MDD) did not meet the primary endpoint. A phase-II trial (BPD) recently completed but data is not available publicly. There are currently four active phase-III trials (3 in MDD, 1 in PPD). In July 2019, Sage announced that it would begin to investigate SAGE-217 in TRD, but no trials for TRD have been posted on clinicaltrials.gov. |
| PRAX-114 | PAM of GABAA receptor | Praxis Precision Medicines | II | There is one ongoing phase-II trial (MDD). IND submission for Prax-114 as treatment for MDD is on clinical hold by the FDA. |
Drugs are grouped according to purported mechanism of action. Abbreviations: AMPA – α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GABA – gamma-aminobutryic acid; mGluR – metabotropic glutamate receptor; NAM – negative allosteric modulator; PAM – positive allosteric modulator; NMDAR – N-methyl-D-aspartate receptor; Route of administration: IV – intravenous; IN – intranasal. MDD – major depressive disorder; MDSI – major depression with suicidal ideation; IND – investigational new drug; PPD – post-partum depression; BPD – bipolar depression; TRD – treatment-resistant depression
*This data is still awaiting quality control at the US Clinical Trials Registry.
NRX-100/NRX-101
This compound is a sequential treatment regimen, where a single-dose of intravenous ketamine (NRX-100) is followed by an oral formulation of D-cycloserine combined with lurasidone (NRX-101). This compound is being investigated for the treatment of bipolar depression with acute suicidal ideation or behavior. D-cycloserine is a partial agonist at the NMDA receptor and lurasidone is an antagonist at the serotonin 2a receptor as well as at the D2 dopamine receptor.
NRX-100/NRX-101 recently completed a Phase-II trial (STABIL-B), in which subjects with bipolar disorder and recent suicidal ideation or behavior who responded to an initial, single dose of intravenous ketamine were randomized to receive NRX-101 or lurasidone alone for 6 weeks. Preliminary data (posted on the US Clinical Trials Registry) demonstrate that patients who received NRX-101 exhibited lower levels of depression (MADRS, p < 0.02, d = 2.94) compared to those that received only lurasidone (NCT02974010). No participants in the NRX-101 group (n = 12) relapsed, while 2 lurasidone patients (n = 5) relapsed (NCT02974010).
NRX-101 is also currently in phase-IIb/III clinical trials (SBP-ASIB) for the treatment of severe bipolar depression and acute suicidal ideation and behavior, which is expected to complete in December 2021. One study currently recruiting is investigating NRX-100 versus placebo for the rapid stabilization of suicidal ideation and behavior in bipolar depression (SevereBD), while another phase-II/III study is investigating NRX-101 for the treatment of moderate bipolar depression and suicidal ideation (NCT03395392). Plans are also in place for a NRX-101 biomarker validation phase-II/III study investigating the effects of the compound on Glutamate+Glutamine (Glx) levels in the anterior cingulate cortex (ACC), an area that has been shown to have low levels of Glx in patients with depression and PTSD (NCT03402152). Researchers plan to compare Glx levels in patients with bipolar depression receiving lurasidone or placebo, and plan to study the effects of Glx modulation on depression.
AGN-241751 (Allergan)
AGN-241751 is an NMDA receptor modulator that is being pursued for the treatment of MDD. There is one 2-part (Part A: once daily dosing (3 mg vs. 10 mg vs. placebo) trial; Part B: twice daily dosing (3 mg vs. 25 mg vs. placebo) RCT) Phase-I/II study examining the change in MADRS score at 1-day post first dose (Part A), and at 7 days post first dose (Part B) (NCT03726658). Another Phase-II 5-arm, fixed-dose RCT is investigating AGN-241751’s effect on the MADRS at 1-day (NCT03586427). Both trials have completed, but no results have been posted as of March 2021.
Partial Allosteric GABAA Modulators
Brexanolone (SAGE-547/Zulresso™)
Brexanolone is an intravenous formulation of allopregnanolone, an endogenous neuroactive steroid, that acts as a positive allosteric modulator of GABAAreceptors through the δ subunit. 47 It is given intravenously over a 60-hour period. Allopregnanolone is the major metabolite of progesterone and its levels rise rapidly during pregnancy, followed by a precipitous drop after parturition. As the drop in progesterone levels following parturition has been linked with the pathophysiology of postpartum depression, 32 it is likely that brexanolone uses this pathway of GABAergic modulation as a therapeutic for PPD. 48
Following two positive phase-III trials, Brexanolone was approved by the FDA for the treatment of moderate to severe postpartum depression in 2019. At 60 hours, reductions in depressive symptom (HAM-D) scores in response to Brexanolone treatment were seen in the study investigating severe PPD (60 µg: p = 0.0013, d = 0.73; 90 µg: p = 0.0252, d = 0.48) and in the study investigating moderate PPD (90 µg: p = 0.0160, d = 0.43). 33 Brexanolone is the first FDA-approved compound for post-partum depression. 33 In the phase-III study of severe PPD (doses 60 and 90mcg), both doses retained statistical significance at 30 days; in the phase-III study of moderate PPD (90mcg), statistical significance was not retained at 30 days.
A phase-III study examining the drug’s safety, tolerability, and pharmokinetics in adolescent females (ages 15-17) with PPD has completed but has yet to post results as of March 2021 (NCT03665038). One planned phase-IV study was to evaluate the relationship between changes in depressive symptoms in response to brexanolone and related changes in resting state functional imaging (NCT04273191), but the company has decided not to proceed with the study at this time. Additionally, a compassionate use expanded access protocolfor brexanolone for adults with PPD was available until the drug was approved (NCT03924492).
Ganaxolone
Ganaxolone is a neuroactive steroid, similar to Brexanolone, that modulates the GABAA receptor. It is currently under development for the treatment of PPD. In one open label, pilot study in postmenopausal women, a significant decrease in MADRS score at week 8 (n = 10). 34 There are also two active Phase-II studies that have completed, but not yet published official results: one study is a multiple-dose escalation design in individuals with severe PPD that is utilizing the orally bioavailable form of the drug (NCT03460756); the other study is being conducted in individuals with moderate PPD and is utilizing the intravenous form of the drug (Magnolia study; NCT03228394). Some study results have been shared through press releases but, to our knowledge, the primary outcome data of both studies are not yet reported.
Zuranolone (SAGE-217)
Zuranolone is an oral formulation of a positive allosteric modulator of the GABAA receptor. The formulation is being investigated as a treatment for major depressive disorder, postpartum depression, and bipolar disorder. The treatment paradigm has been designed such that patients take the medicine for a 2-week course.
Major Depressive Disorder (MDD)
A Phase-II trial in patients with MDD (n = 89) achieved its primary endpoint (p = 0.0005, d = 0.81), measured as the change in HAMD-17 score at 2 weeks post randomization (NCT03000530). 35 There were no serious adverse events; those in the treatment group were more likely to experience headache, dizziness, nausea, and somnolence.
Sage has launched several Phase-III trials of zuranolone in MDD. A fixed-dose Phase-III trial (MOUNTAIN study, NCT03672175) in patients with MDD did not meet its primary endpoint (change in HAMD-17) at day 15 (p = 0.115). 36 A one-year, open-label study is ongoing and is assessing the safety, tolerability, and need for re-treatment with zuranolone in subjects with MDD (SHORELINE, NCT03864614).Interim results were released in October 2020 stating that 71.6% of patients responded and 39.8% of patients remitted following a 2-week course of treatment with 30 mg dose. Adverse event profiles were generally consistent with earlier trials. 37 Of responding patients who continued in the protocol, 44.5% did not receive an additional Zuranolone treatment course for up to one-year of follow-up. Two studies (RAINFOREST [NCT03771664] and REDWOOD [NCT04007367]) are listed as suspended to “evaluate potential amendments to the study” according to clinicaltrials.gov as of March 2021. Additionally, two new phase-III studies were recently launched in June 2020 that will evaluate the efficacy of Zuranolone in the treatment of MDD through the use of the 17-item HAM-D scale comparing Day 15 to baseline. One study will evaluate the efficacy of zuranolone monotherapy compared to placebo (NCT04442490), and the other will measure zuranolone + sertraline compared to placebo+ sertraline (NCT04476030). It should be noted that some later trials of zuranolone are looking at a higher dose (50 mg) than earlier trials (20 or 30 mg).
Sage Therapeutics also announced in a 2019 press release that zuranolone may be investigated as a potential therapy for treatment-resistant depression (TRD), however, no trial has been registered on clinicaltrials.gov as of the time of writing. 49
Post-partum Depression (PPD)
Zuranolone is also being developed as treatment for postpartum depression (PPD). According to a PowerPoint presentation at the International Marcé Society in October 2020, the Phase III trial, ROBIN (PPD-201, NCT02978326) achieved its primary endpoint of reduction in HAMD-17 scores at 2 weeks when investigating the effect of a 30 mg dose of zuranolone compared to placebo in women with severe PPD (Cohen’s d = 0.40; p = 0.0029). 38 A 2-arm, phase-III RCT investigating the effects of zuranolone on depressive symptoms in females with severe postpartum depression (PPD) as compared to placebo, is currently recruiting. This study will examine depressive symptoms using the 17-item HAM-D, with the primary endpoint being the change from baseline in the HAM-D total score at day 15 (NCT04442503).
Bipolar Depression (BPD)
In addition to being pursued as a treatment for MDD and PPD, Zuranolone is also being pursued as a treatment for bipolar depression in a two-part, phase-II study (NCT03692910). This study of zuranolone in subjects with Bipolar I/II Disorder with a current major depressive episode reported that all treatment-emergent adverse events were mild or moderate during the open-label trial (Part A), and that there was a 45% response rate by Day 15. The study has completed, but results from Part B of the study (2-arm parallel group) have not been reported as of March 2021.
PRAX-114
PRAX-114 is a GABAA positive allosteric modulator being developed by Praxis Precision Medicines that is currently in Phase-II trials for the treatment of MDD. One non-placebo-controlled study is investigating the safety and tolerability of PRAX-114 over a 14-day treatment period. According to the Australian New Zealand Clinical Trials Registry (ANZCTR), the study began enrolling in May 2019. As of January 2021, no official end date or results have been posted, but Praxis reported interim results for this study in their report to the SEC in September 2020. Secondary outcomes were improvement in depression severity; change from baseline in the Hamilton Depression Rating Scale were seen in the 45-mg, 60-mg, and 80-mg doses over an 8-day period. A reduction of 15 to 19 points from baseline were noted across the three dose groups. 39
In October 2020, Praxis submitted the Investigational New Drug (IND) submission to the US FDA for the initiation of a randomized, placebo-controlled Phase II/III clinical trial for PRAX-114 in MDD, but as of November 2020, this IND submission has been placed on full clinical hold by the FDA pending the resolution of nonclinical pharmacology and toxicology matters. 50
Future Challenges and Conclusions
Despite the fact that several large pharmaceutical companies have disinvested from the development of CNS drugs, 51 there have been several exciting developments in the treatment of mood disorders recently. Nonetheless, this novel approach targeting the amino acid neurotransmitter systems also brings new challenges. In contrast to traditional antidepressants that are generally designed to maintain a relatively steady-state serum drug level, several new antidepressants are designed to be delivered in a pulsatile fashion. Ketamine, for instance, which has a half-life of 2-4 hours, has downstream effects on the brain that outlast the drug’s persistence in the body. For many of these newer agents in the pipeline, questions of the optimal dose schedule remain unanswered.
Another challenge in the development of drugs targeting the amino acid neurotransmitter systems, many of which are purportedly rapid-acting, is the way that changes in mood are assessed. Most clinical trials rely upon the Hamilton Depression Rating Scale (HAM-D) 52 the Mongtomery-Asberg Rating Scale (MADRS) 53 to measure changes in symptom severity. Both of these scales were designed to evaluate symptoms over a period of weeks, not hours to days. For instance, both the HAM-D and the MADRS evaluate sleep and appetite as core features of depression, but it is unrealistic to expect these symptoms to change realibly over a period of hours. The development of improved measurements to assess changes in symptoms severity for rapid-acting agents should be considered.
Finally, the heterogeneity of mood disorders and our incomplete understanding of their pathophysiology makes it critical to recruit well defined clinical cohorts. Related to this, placebo response in clinical trials for mood disorders can be difficult to predict, rendering it challenging to show a placebo-adjusted improvement. Given that some agents still under investigation may be using a non-parenteral route of administration, these non-specific therapeutic effects can be even further enhanced in a given clinical context. Careful attention to these non-specific effects are critical to conduct successful clinical trials.
These challenges notwithstanding, it is an exciting time in the development of novel treatments for mood disorders. Two agents recently received FDA approval and several additional promising therapeutics are being evaluated in later stage clinical trials.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Wilkinson has received contract funding from Janssen, Sage Therapeutics, and Oui Therapeutics for the conduct of clinical trials administered through Yale University; he has received consulting fees from Biohaven Pharmaceuticals, Sage Therapeutics, Janssen, Eleusis, and Oui Therapeutics. The other authors report no financial relationships with commercial interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: P.A.D was supported by an NIH Medical Scientist Program Training Grant T32GM136651. There was no direct support for this work.
ORCID iDs: Pasha A. Davoudian https://orcid.org/0000-0002-5096-7610
Samuel T. Wilkinson https://orcid.org/0000-0002-3483-9168
References
- 1.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392(10159): 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkinson ST, Ostroff RB, Katz RB, Krystal JH. Ketamine: a promising rapid-acting antidepressant. In: Kim Y-K, ed. Understanding Depression: Volume 2. Clinical Manifestations, Diagnosis and Treatment. Singapore: Springer Singapore; 2018: 223–239. [Google Scholar]
- 3.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012; 13(1): 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011; 16(4): 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012; 62(1): 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today. 2019; 24(2): 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000; 47(4): 351–354. [DOI] [PubMed] [Google Scholar]
- 8.Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020; 25(7): 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archiv Gen Psychiatry. 2010; 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archiv Gen Psychiatry. 2006; 63(8): 856–864. [DOI] [PubMed] [Google Scholar]
- 11.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013; 170(10):1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019; 22(10): 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanina P, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019; 176: 428–438. [DOI] [PubMed] [Google Scholar]
- 14.Ochs-Ross R, Daly EJ, Zhang Y, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression—TRANSFORM-3. Am J Geriatr Psychiatry. 2020; 28(2): 121–141. [DOI] [PubMed] [Google Scholar]
- 15.Daly EJ, Trivedi MH, Janik A, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019; 76(9): 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wajs EAL, Holder R, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. 2020; 81(3): e1–e10. [DOI] [PubMed] [Google Scholar]
- 17.Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020; 81(3): e1–e9. [DOI] [PubMed] [Google Scholar]
- 18.Ionescu DF, Fu DJ, Qiu X, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021; 24(1): 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murrough JW, Wade E, Sayed S, et al. Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: a proof of concept clinical trial. J Affect Disord. 2017; 218: 277–283. [DOI] [PubMed] [Google Scholar]
- 20.Gant TG. Using deuterium in drug discovery: leaving the label in the drug. J Med Chem. 2014; 57(9): 3595–3611. [DOI] [PubMed] [Google Scholar]
- 21.Axsome Therapeutics. ASCEND phase 2 trial of AXS-05 in MDD topline results. https://axsometherapeuticsinc.gcs-web.com/static-files/229c52a8-ab22-4dd3-b36f-08904c22cd4b. Accessed April 8, 2021. Published January 7, 2019.
- 22.Axsome Therapeutics. GEMINI phase 3 trial of AXS-05 in MDD topline results. https://axsometherapeuticsinc.gcs-web.com/static-files/974bbc2e-c3d9-4917-96b3-7cc59267807b. Accessed April 8, 2021. Published December 16, 2019.
- 23.Axsome Therapeutics. Topline results of the STRIDE-1 phase 3 trial in treatment resistant depression. https://axsometherapeuticsinc.gcs-web.com/node/9176/pdf. Accessed April 8, 2021. Published March 30, 2020.
- 24.Relmada Therapeutics. Top-line results from REL-1017 phase 2 study in individuals with treatment resistant depression. https://www.relmada.com/investors/news/press-releases/detail/200/relmada-therapeutics-announces-top-line-results-from. Accessed April 8, 2021. Published October 15, 2019.
- 25.VistaGen Therapeutics. VistaGen reports topline phase 2 results for AV-101 as an adjunctive treatment of major depressive disorder. https://www.vistagen.com/news-media/press-releases/detail/130/vistagen-reports-topline-phase-2-results-for-av-101-as-an. Accessed April 8, 2021. Published November 14, 2019.
- 26.Park LT, Kadriu B, Gould TD, et al. A randomized trial of the N-methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int J Neuropsychopharmacol. 2020; 23(): 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preskorn S, Preskorn S, Macaluso M, et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015; 21(2): 140–149. [DOI] [PubMed] [Google Scholar]
- 28.A study of rapastinel as adjunctive therapy in major depressive disorder (RAP-MD-03) (NCT02943577). https://clinicaltrials.gov/ct2/show/results/NCT02943577?term = NCT02943577&draw = 2&rank = 1. Published November 2019.
- 29.A study of rapastinel as adjunctive therapy in major depressive disorder (RAP-MD-01) (NCT02932943). https://clinicaltrials.gov/ct2/show/results/NCT02932943?term = NCT02932943&draw = 2&rank = 1. Published October 2019.
- 30.A study of rapastinel as adjunctive therapy in major depressive disorder (RAP-MD-02) (NCT02943564). https://clinicaltrials.gov/ct2/show/results/NCT02943564?term = NCT02943564&draw = 2&rank = 1. Published December 2019.
- 31.A study of rapastinel as adjunctive therapy in the prevention of relapse in patients with major depressive disorder (RAP-MD-04) (NCT02951988). https://clinicaltrials.gov/ct2/show/results/NCT02951988?term = RAP-MD-04&draw = 1&rank = 1&view = results. Published March 2020.
- 32.Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017; 390(10093): 480–489. [DOI] [PubMed] [Google Scholar]
- 33.Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018; 392(10152): 1058–1070. [DOI] [PubMed] [Google Scholar]
- 34.Dichtel LE, Nyer M, Dording C, et al. Effects of open-label, adjunctive ganaxolone on persistent depression despite adequate antidepressant treatment in postmenopausal women: a pilot study. J Clin Psychiatry. 2020; 81(4): e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunduz-Bruce H, Silber C, Kaul I, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. 2019; 381(10): 903–911. [DOI] [PubMed] [Google Scholar]
- 36.Lasser R, Gunduz-Bruce H, Bullock A, et al. The neuroactive steroid (NAS) zuranolone in major depressive disorder: the landscape development program of an investigational, oral, positive allosteric modulator (PAM) of GABAA receptors. In: EPA 28th European Congress of Psychiatry; March 2020; Madrid, Spain.
- 37.Sage Therapeutics. Sage therapeutics announces positive interim, topline zuranolone safety and tolerability data from open-label SHORELINE study in patients with MDD. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-positive-interim-topline-zuranolone. Accessed April 8, 2021. Published October 15, 2020.
- 38.Meltzer-Brody S. Insights on GABAergic mechanism of PPD from pivotal studies of brexanolone injection and zuranolone (SAGE-217). In: International Marcé Society Biennial Virtual Meeting; October 2019.
- 39.United States Securities and Exchange Commission. Form S-1 registration statement, praxis precision medicines, Inc. https://www.sec.gov/Archives/edgar/data/1689548/000119312520254741/d945651ds1.htm. Accessed April 12, 2021. Published September 25, 2020.
- 40.Canuso CM, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018; 175(7): 620–630. [DOI] [PubMed] [Google Scholar]
- 41.Daly EJ, Singh JB, Fedgchin M, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018; 75(2): 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016; 80(6): 424–431. [DOI] [PubMed] [Google Scholar]
- 43.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015; 21(2): 140–149. [DOI] [PubMed] [Google Scholar]
- 44.NeuroRx. NeuroRx announces phase 2 data from NRX-101, initiates pivotal study for severe bipolar depression with acute suicidal ideation & behavior (ASIB). https://www.neurorxpharma.com/nrx.neurorxpharma.com/assets/9.04.2018-neurorx-stabil-b-data-release-(002)-1-.pdf. Accessed April 8, 2021. Published September 4, 2018.
- 45.Kanes S, Colquhoun H, Doherty J, et al. Open‐label, proof‐of‐concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol Clin Exp. 2017; 32: e2576–e2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997; 16(5): 357–372. [DOI] [PubMed] [Google Scholar]
- 47.Schumacher M, Mattern C, Ghoumari A, et al. Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Progr Neurobiol. 2014; 113: 6–39. [DOI] [PubMed] [Google Scholar]
- 48.Lüscher B, Möhler H. Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience [version 1; peer review: 4 approved]. F1000Research. 2019; 8: 751. [DOI] [PMC free article] [PubMed]
- 49.Sage Therapeutics. Sage therapeutics announces clinical updates and progress across neuroscience pipeline during “Sage FutureCast.” https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-clinical-updates-and-progress-across. Accessed April 8, 2021. Published July 24, 2019.
- 50.Praxis Precision Medicines. Praxis precision medicines provides update on Prax-114 ind submission for the treatment of major depressive disorder. praxismedicines.com. Accessed April 8, 2021. Published November 17, 2020.
- 51.Choi DW, Armitage R, Brady LS, et al. Medicines for the mind: policy-based “pull” incentives for creating breakthrough CNS drugs. Neuron. 2014; 84(3): 554–563. [DOI] [PubMed] [Google Scholar]
- 52.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960; 23(1): 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]