Abstract
Honey bees are important pollinators and take micronutrients from different natural floral resources and turbid water to adequately meet their nutritional requirements. But the role of micronutrients for honey bee health is not well understood. Here, the present study was conducted to determine honey bees' micronutrients preference in summer and winter seasons. Also, the impact of micronutrients on foraging behaviour and brood increase was studied in different honey bee colonies. The results elucidated that honey bees exhibited a strong preference for a salt solution compared to deionized water during the summer and winter seasons. However, there was a notable switch in salt preference between seasons. Overall, honey bees showed significantly more foraging activity, more pollen collection, and increased brood area after sodium consumption compared to other minerals in the summer season. Further, pollen collection and brood area were significantly higher after the use of potassium in the winter season. Thus, the food preference of honey bees is strongly linked with the seasons and the availability of the floral resources. Our data suggested that honey bees may seek specific nutrients during variation of the seasonal conditions.
Keywords: Honey bee, Minerals, Nutrition, Foraging behaviour, Pollen, Seasons
1. Introduction
Honey bees are economically most crucial eusocial insects to our ecosystem and food supply due to their pollination activities (Lawal and Banjo, 2010). They produce honey, royal jelly, bee wax, beebread, propolis, and bee venom. Adult bees and larvae depend on minerals and nutrients for development and reproduction (Michener, 2007, Brodschneider and Crailsheim, 2010, Ahmad et al., 2020a). Adult honey bees are under continuous stress and search for the appropriate nutrients from the environment for developing larvae, nurse bees, and queens in nests (Michener, 2007). The honey bees' nutrition is divided into two categories, including nectar and pollen collected from flowers. Floral nectar is the primary source of carbohydrates, while the pollen provides the proteins, lipids, vitamins, essential sterols, and some other micronutrients they need to survive (T’ai and Cane, 2000, Brodschneider and Crailsheim, 2010, Al-Kahtani et al., 2020). To get optimal nutrition, insects can balance food sources' intake, and mostly nutrient availability affects insects' foraging behaviour (Behmer, 2009). The bees' quantitative and qualitative requirements depend on the life history, including the social structure and brood size. Most previous studies related to nutritional needs are related to the honey bees and the bumblebees (Haydak, 1970, Brodschneider and Crailsheim, 2010, Danforth et al., 2013, Bonoan et al., 2017, Shakeel et al., 2020).
Most living organisms require a balanced diet in the form of both macronutrients (proteins and carbohydrates) and micronutrients (minerals and salts) for their daily activities (Simpson and Raubenheimer, 2012a, Hafeez et al., 2019, Adgaba et al., 2020). The balance between the nutrients requirement and the supply is challenging for the insects during the food source's varying composition. This difficulty is further enhanced by some biotic and abiotic factors like the temperature, humidity, and abundance of the predators (Mayntz, 2009, Chauhan et al., 2019, Saeed et al., 2019, Jaleel et al., 2020, Nawaz et al., 2020, Jamal et al., 2021). Micronutrients are also required for the physiological processes, such as precursors of moulting hormones and building blocks of the cellular membranes (Cohen, 2015, Rupp, 2015, Al-Ghamdi et al., 2020, Chakrabarti et al., 2020). The honey bees' nutritional requirement depends on the age, e.g., larvae's nutritional requirement is higher than the adult foragers (Haydak, 1970, Paoli et al., 2014, Ahmad et al., 2020b, Ghramh et al., 2020). The diversity of diet is essential for the honey bees' growth and development (Alaux et al., 2010). Bees raised on a multi-floral diet exhibit a stronger immunity than the bees raised on monofloral diets (Alaux et al., 2010). Honey bees need to have a more robust immune system than to get the right amount of protein. Much of the studies are related to the honey bees' requirements of macronutrients compared to the micronutrients (Haydak, 1970, Olejnícek, J.J.E.J.o.E, 2004).
The optimal diet requirement of the honey bees includes resources from both plants and water sources. The floral resources contain only a trace amount of micronutrients (Somerville, 2005). In contrast, sodium specific behaviour is well known in social insects (Botch and Judd, 2011). To fulfil these micronutrient deficiencies, honey bees selectively feed on the soil and water sources for different minerals and salts absent in the floral diet (Lau and Nieh, 2016, Hakami et al., 2020). Honey bees prefer to feed on minerals for their physiological activities and functions like muscle movement (Day et al., 1990, Wang et al., 2013, Chakrabarti et al., 2020). For instance, iron accumulates at the periphery of the abdomen and plays a role in honey bee navigation (Wang et al., 2013), while sodium is required for osmoregulation (Nation, 2004). However, the role of micronutrients such as sodium, magnesium, and calcium in honey bee diets has rarely been studied (Black, 2006, Brodschneider and Crailsheim, 2010, Filipiak et al., 2017), the micronutrient requirement of honey bees is changed according to seasonal conditions,
The present study was performed to determine honey bees' micronutrient preference in the summer and winter seasons. Further, to underpin the effect of various micronutrients on foraging activity, pollen collection, and brood area in bee colonies during both seasons.
2. Materials and methods
The study was conducted in Langstroth hives equipped with 10 frames during the summer (March-August) and winter (October-February) seasons. The preference of honey bees was tested for general micronutrient requirements: Sodium chloride (NaCl), Potassium chloride (KCl), and Magnesium chloride (MgCl2) (Cohen, 2015). These salts were chosen based on what honey bees were likely to prefer soil and dirty water (Lau and Nieh, 2016).
2.1. Mineral preferences
After the training of bees, the preference assay was performed three times a week. For this purpose, three salt solutions were set up at 2-meter-long wooden table. Along with these salts solution, 10% sucrose solution and deionized water were also placed on the table for positive control and negative control, respectively (Chavarria Pizarro et al., 2012). A laboratory study indicated that the honey bees prefer to respond to 0.1–1.5% salt concentrations in NaCl, KCl, and MgCl2 (Lau and Nieh, 2016); thus, 1.5% salt solution was used in the experiments. Three tubes of each solution were randomly placed on the table, including sugar solution and deionized water; along with this, to calculate the loss of water due to evaporation, one tube as control was placed near the experiment. At the starting of each trial, plastic falcon tubes (50 mL) were selected and filled with the (30 mL) of the allocated solution; after that, the tubes were randomly placed on the table. At the end of each experiment, the total consumed volume was calculated from the remaining solution (mL) in the falcon tubes both in control and the treatments. The difference in the volume yielded the total volume consumed by the forager bees from each salt type. Overall, 12 preference assays were conducted in summer and 12 preference assays in winter. After each experiment, the bees were replaced with the new hives, so 12 hives used in this study.
2.2. Colony fitness
Along with this preference assay, the impact of 1.5% NaCl, 1.5% MgCl2, and 1.5% KCl was studies; the hives were placed at a distance of 1 Kilometer. After feeding on the specific salt solution, the hives were again placed together to study the foraging activity (number of bees leaving the hive in 30 min), pollen collection ability (pollen collected in grams) by foragers in 30 min), and also check their effect on brood rearing (increase in brood area (centimeter) in 7 days).
The honey bees on each salt type were also observed, indicating honey bees' preference towards the specific salt solution. To test the impact of these salt solutions on the honey bees, the foraging activity (number of bees lift the hive in 30 min), pollen collection ability (pollen collected (g) by foragers in 30 min), and also the effect on brood rearing (increase in brood area (cm) in 7 days) were measured. These three factors indicate the foraging ability and the colony's health status under different treatments, including the NaCl, MgCl2, KCl, and deionized water. The total area under capped brood was calculated by measuring the size before treatment and the area after treatment.
2.3. Statistical analysis
Results were obtained as mean ± Standard Error using SPSS software (version 26) according to variance (ANOVA). Graphs were prepared by using GraphPad Prism software (version 7.03). Statistically, significance was tested using Student's t-test between two groups and one-way ANOVA for more than two groups. Tukey post-hoc test was performed for multiple comparisons between groups. The data recorded the mineral preference, foraging activity, pollen collection, and brood area mean were compared at the 0.05 level.
3. Results
3.1. Mineral preferences
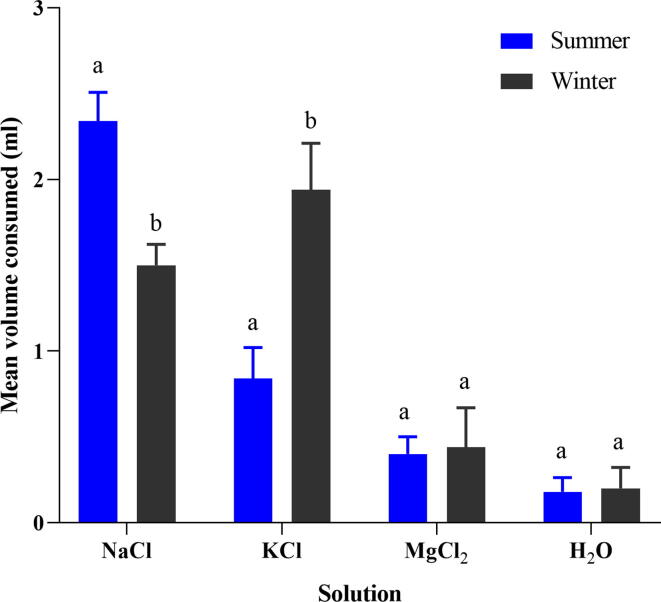
The results indicated that honey bees consumed significantly different amounts of all salt solutions from all falcon tubes during the summer (F (3, 16) = 242.667, p = 0.001). Similarly, honey bees consumed significantly different amounts of all salt during the winter season (F (3,16) = 89.128, p = 0.001). Hence, honey bees consumed various salt solutions in both the summer and winter seasons (Fig. 1). Honey bees consumed a significantly higher salt solution than the deionized water in both seasons (P < 0.001). The bees drunk significantly more sodium in summer as a comparison to winter (t = 9.058, p = 0.001), which was 2.34 ± 0.07 mL and 1.5 ± 0.05 mL, respectively. In contrast, honey bees preferred to drink KCl solution significantly more in the winter season than the summer season (t = −7.555, p = 0.001). The bees consumed 1.92 ± 0.12 mL KCl solution in the winter season, whereas that was 0.84 ± 0.08 mL in the summer season. However, no significant difference was observed during the MgCl2 salt solution consumption in summer and winter (t = −0.356, p = 0.731). Similarly, no significant difference was seen during the consumption of deionized H2O solution in both seasons (t = −0.302, p = 0.771).
Fig. 1.
Mean volume (ml) of various salts (NaCl, KCl, and MgCl2) and deionized water consume by honey bees in summer and winter seasons. Different small letters “a, b” indicated the significant difference at (p < 0.001).
3.2. Colony fitness
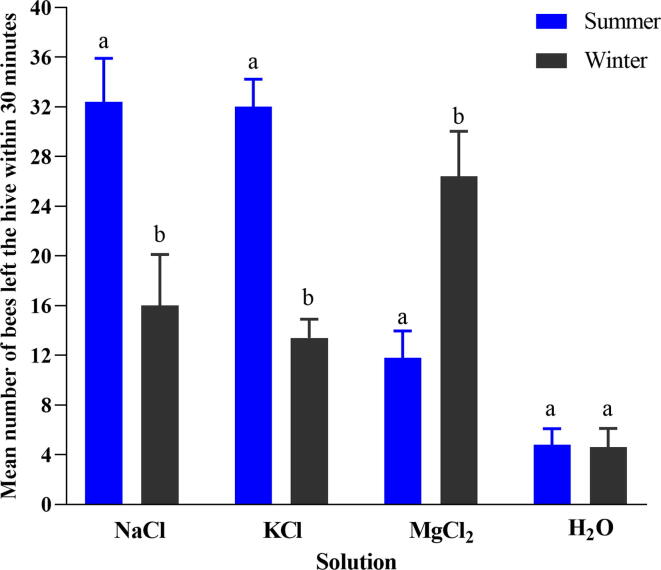
The results indicated that the different salts NaCl, KCl, and MgCl2 impact the honey bees' foraging behaviour during the summer and winter seasons (Fig. 2). Honey bees fed on different salt solutions had significantly higher foraging activity during the summer season (F (3,16) = 167.592, p = 0.001). Similarly, the salt significantly impacted the honey bees' foraging activity in the winter season (F (3,16) = 46.159, p = 0.001). In the summer and winter seasons, the honey bees fed on different salt solutions had shown significantly higher foraging activity than the deionized water (P < 0.001). The honey bees fed on sodium had shown a considerably higher foraging activity (t = 6.775, p = 0.00), the number of visits made by foragers was 32.4 ± 1.57 in the summer season and 16 ± 1.84 in the winter season. Similarly, KCl had shown significantly higher foraging activity during both seasons (t = 15.393, p = 0.00), the number of bees left the hive was 32.00 ± 1.00 in the summer and 13.4 ± 0.68 in the winter season. However, foraging activity significantly higher in the winter season than in summer after using MgCl2 (t = −7.695, p = 0.001); the mean number of bees left the hive was 26.40 ± 1.63 and 11.80 ± 0.97 in winter and summer season, respectively. There was no significant difference observed in the bee foraging activity after using deionized water during both seasons (t = 0.224, p = 0.829).
Fig. 2.
Mean a number of bees left the hive within 30 min after using (NaCl, KCl, and MgCl2) and deionized water in summer and winter seasons. Different small letters “a, b” indicated the significant difference at (p < 0.001).
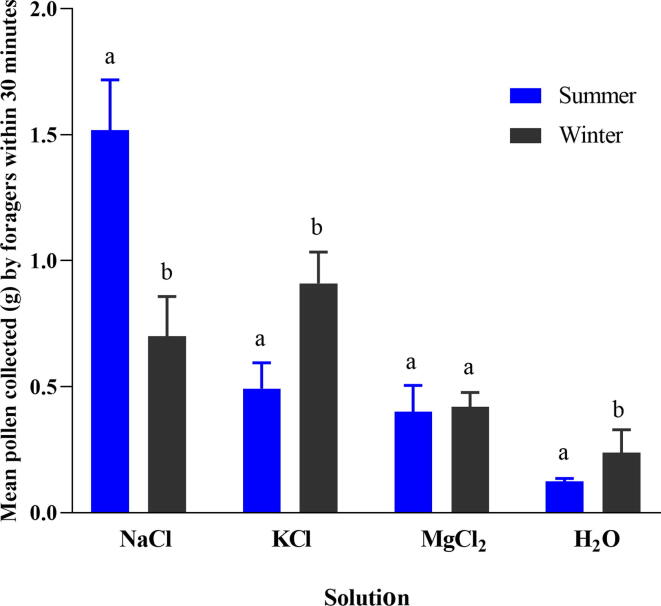
The results revealed that the forager bee collected the various pollen (g) after various salts during the summer and winter seasons (Fig. 3). The forager honey bees collected a significantly different amount of pollen (g) after the consumption of various salts during summer seasons (F (3,16) = 120.890, p = 0.001). Similarly, the foragers collected a significantly different amount of pollen (g) by consuming various salts in the winter season (F (3,16) = 34.183, p = 0.001). Pollen collected by foragers significantly increased the consumption of salts than deionized water (p < 0.001). In the case of sodium, forager bees collected pollen (g) differed significantly in summer and winter seasons (t = 7.190, p = 0.001), the amount was 1.52 ± 0.09 g and 0.70 ± 0.07 g, respectively. However, using KCl salt, the amount of pollen collected by foragers significantly more in the winter season than in the summer seasons (t = −5.806, p = 0.001). The pollen amount was 0.91 ± 0.06 g in the winter season, while 0.49 ± 0.05 g in the summer season. In MgCl2, there was no significant difference (t = −0.374, p = 0.718) was observed in a collection of pollen by foragers bees in summer (0.4 ± 0.04 g) and winter seasons (0.4 ± 0.03 g), respectively. In deionized water, there was a significant difference in the amount of pollen (g) collected by foragers during both seasons (t = −2.790, p = 0.048).
Fig. 3.
Mean weight (g) of pollen collected by forager bees after using (NaCl, KCl, and MgCl2) and deionized water in summer and winter seasons. Different small letters “a, b” indicated the significant difference at (p < 0.001).
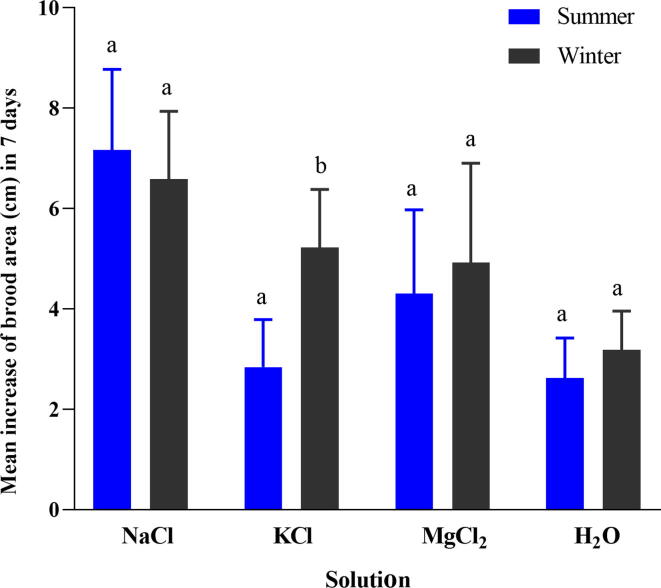
Moreover, results revealed that the brood area increased (cm) after various salt during the summer and winter seasons (Fig. 4). Brood area increased significantly after using various salt by the bees in the summer season (F (3,16) = 12.628, p = 0.001). Similarly, the brood area increased significantly after the use of various salt in the winter season (F (3,16) = 5.076, p = 0.012). There was no significant difference observed in the brood area after using sodium salt in the summer and winter seasons (t = 0.617, p = 0.554), which was 7.16 ± 0.72 cm and 6.58 ± 0.61 cm, respectively. In the case of KCl, the brood area increased significantly more in the winter season than the summer season (t = −3.556, p = 0.007). It was increased (5.22 ± 0.52 cm) in the winter season, whereas (2.84 ± 0.42 cm) in the summer season. However, the brood area did not differ significantly after using MgCl2 (t = −0.535, p = 0.607), the brood area was 4.3 ± 0.75 cm in summer, while 4.92 ± 0.89 cm in the winter season. Similarly, no significant difference was observed in the brood area after using deionized water during both seasons (t = −1.125, p = 0.293).
Fig. 4.
Mean increased of brood area (cm) after the use of (NaCl, KCl, and MgCl2) and deionized water in summer and winter seasons. Different small letters “a, b” indicated the significant difference at (p < 0.001).
4. Discussion
The present study revealed that honey bees showed different micronutrient preferences in the summer and winter seasons. Overall, the honey bee exhibited a strong preference for salt nutrients than deionized water in both seasons. In summer season, honey bees showed a strong sodium preference, followed by potassium and magnesium. But in winter seasons, honey bees consumed more potassium salts as a comparison to sodium and magnesium. Lau and Nieh (2016) reported a similar result that the forager bees exhibited a strong preference for the specific concentration of sodium, magnesium, potassium, and phosphate compared to deionized water. The switch of minerals potassium and magnesium preference is particularly interesting because they are present in pollens (Herbert Jr and Miller-Hill, 1987). These minerals in pollen are directly related to the seasons (Nation, 2004, Bonoan et al., 2017).
As such, most commercial beekeepers have become reliant on an artificial diet to maintain the nutritional requirement of bee and colony health during pollen scarcity (Donkersley et al., 2014, Ricigliano et al., 2018, Ricigliano, 2020). Moreover, our results indicated that availability of specific micronutrient effect on the foraging activity, pollen collection, and brood area in bee colonies. Honey bees collected more pollen and increased the brood area after sodium in the summer season. However, forager bees collected more pollen after the consumption of potassium in the winter seasons. The forager bees provide carbohydrates, protein, lipids, mineral elements, and water to maintain the bee colony's nutritional requirement (Lihoreau et al., 2015, Wright et al., 2018). So the foraging on different resources at the same time is the best strategy adapted by social insects like bees to get a balanced diet (McLellan, 1978). Insects can detect the level of amino acid and could reject the diet that is deficient amino acids (Abisgold and Simpson, 1987, Simpson et al., 1990, Ribeiro and Dickson, 2010, Toshima and Tanimura, 2012). The balance of nutrients is a complex phenomenon in which alimentary cues are integrated with the prerecorded information about food quality (Simpson and Raubenheimer, 2012b).
Regarding the different salts and colony fitness, a strong correlation was identified. The preference for different salt is also affected by the season. In the present study, the honey bees prefer more NaCl and KCl salts, while in the case of winters, they like the MgCl2 and consumed more volume than the deionized water. This study indicated that the honey bees preferred to drink the dirty water because of the deficiency of mineral results in line with the previous study (Bonoan et al., 2017). Different minerals' preference is directly linked with the floral resources' availability; more will be flowers lesser preference will be given to the salts (Butler, 1940). Although the micronutrients are not required in large quantities, they are essential for the balance for nutrition and the pollinators' health.
Results of this study have broader implications, including the basic and applied sciences. It is crucial to know about the honey bees' seasonal micronutrient requirement on the applied side because it leads to developing an artificial diet for the honey bees based on their requirements. The knowledge about the mineral need of the honey bees increases the honey bee's foraging activity, which is important for the pollination of different crops and improve colony health. This study may support to help formulate a complete diet for honey bees and improve the beekeeping industry.
5. Conclusions
This results indicated that honey bee showed a strong preference to salt solution compared to deionized water during summer and winter seasons. Moreover, honey bees exhibited significantly more foraging activity, pollen collection, and brood area after using sodium salt compared to other minerals, including potassium and magnesium in the summer season. The amount of collected pollen and brood area was significantly more after potassium in the winter season. The result elucidated that honey bee may show specific nutrient preference during changing of the seasonal conditions. The authors acknowledge Saboor Ahmad for assistance and preparing the manuscript.
Declaration of Competing Interest
All authors declare that they have no known competing financial interests or personal relations that could have appeared to influence the work reported in this paper.
Acknowledgements
The author extends his appreciation to the Scientific Research Deanship at King Khalid University and the Ministry of Education in KSA for funding this research work through the project number IFP-KKU-2020/5.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abisgold J., Simpson S. The physiology of compensation by locusts for changes in dietary protein. J. Exp. Biol. 1987;129(1):329–346. [Google Scholar]
- Adgaba N., Al-Ghamdi A., Sharma D., Tadess Y., Alghanem S.M., Khan K.A. Physico-chemical, antioxidant and anti-microbial properties of some Ethiopian mono-floral honeys. Saudi J. Biol. Sci. 2020;27(9):2366–2372. doi: 10.1016/j.sjbs.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Campos M.G., Fratini F., Altaye S.Z., Li J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020;21(2):382. doi: 10.3390/ijms21020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Khan S.A., Khan K.A., Li J. Novel insight into the development and function of hypopharyngeal glands in honey bees. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.615830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi A.A., Al-Ghamdi M.S., Ahmed A.M., Mohamed A.S.A., Shaker G.H., Ansari M.J. Immune investigation of the honeybee Apis mellifera jemenitica broods: a step toward production of a bee-derived antibiotic against the American foulbrood. Saudi J. Biol. Sci. 2020 doi: 10.1016/j.sjbs.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kahtani S.N., Taha E.-K., Khan K.A., Ansari M.J., Farag S.A., Shawer D.M. Effect of harvest season on the nutritional value of bee pollen protein. PLoS ONE. 2020;15(12):e0241393. doi: 10.1371/journal.pone.0241393. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alaux C., Ducloz F., Crauser D., Le Conte Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010;6(4):562–565. doi: 10.1098/rsbl.2009.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmer S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009;54(1):165–187. doi: 10.1146/annurev.ento.54.110807.090537. [DOI] [PubMed] [Google Scholar]
- Black, J., 2006. Honeybee nutrition: review of research and practices. Rural Industries Research Development Corporation. Canberra.
- Bonoan, R.E., Tai, T.M., Tagle Rodriguez, M., Feller, L., Daddario, S.R., Czaja, R.A., et al. (2017). Seasonality of salt foraging in honey bees (Apis mellifera). Ecol. Entomol. 42(2), 195-201.
- Botch P.S., Judd T.M. Effects of soil cations on the foraging behavior of Reticulitermes flavipes (Isoptera: Rhinotermitidae) J. Econ. Entomol. 2011;104(2):425–435. doi: 10.1603/ec10118. [DOI] [PubMed] [Google Scholar]
- Brodschneider R., Crailsheim K. Nutrition and health in honey bees. Apidologie. 2010;41(3):278–294. [Google Scholar]
- Butler C. The choice of drinking water by the honeybee. J. Exp. Biol. 1940;17(3):253–261. [Google Scholar]
- Chakrabarti P., Lucas H.M., Sagili R.R. Evaluating effects of a critical micronutrient (24-methylenecholesterol) on honey bee physiology. Ann. Entomol. Soc. Am. 2020;113(3):176–182. doi: 10.1093/aesa/saz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A., AbuAmarah B.A., Kumar A., Verma J., Ghramh H.A., Khan K.A. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi J. Biol. Sci. 2019;26(6):1298–1304. doi: 10.1016/j.sjbs.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarria Pizarro, L., Mccreery, H.F., Lawson, S.P., Winston, M.E., and O’donnell, S., 2012. Sodium‐specific foraging by leafcutter ant workers (Atta cephalotes, Hymenoptera: Formicidae). Ecol. Entomol. 37(5), 435-438.
- Cohen A.C. CRC Press; 2015. Insect diets: science and technology. [Google Scholar]
- Danforth B.N., Cardinal S., Praz C., Almeida E.A., Michez D. The impact of molecular data on our understanding of bee phylogeny and evolution. Annu. Rev. Entomol. 2013;58:57–78. doi: 10.1146/annurev-ento-120811-153633. [DOI] [PubMed] [Google Scholar]
- Day S., Beyer R., Mercer A., Ogden S. The nutrient composition of honeybee-collected pollen in Otago, New Zealand. J. Apic. Res. 1990;29(3):138–146. [Google Scholar]
- Donkersley P., Rhodes G., Pickup R.W., Jones K.C., Wilson K. Honeybee nutrition is linked to landscape composition. Ecol. Evol. 2014;4(21):4195–4206. doi: 10.1002/ece3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipiak M., Kuszewska K., Asselman M., Denisow B., Stawiarz E., Woyciechowski M. Ecological stoichiometry of the honeybee: pollen diversity and adequate species composition are needed to mitigate limitations imposed on the growth and development of bees by pollen quality. PLoS ONE. 2017;12(8):e0183236. doi: 10.1371/journal.pone.0183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghramh H.A., Khan K.A., Ahmed Z., Ansari M.J. Quality evaluation of Saudi honey harvested from the Asir province by using high-performance liquid chromatography (HPLC) Saudi J. Biol. Sci. 2020;27(8):2097–2105. doi: 10.1016/j.sjbs.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeez M., Liu S., Jan S., Gulzar A., Fernández-Grandon G.M., Qasim M. Enhanced effects of dietary tannic acid with chlorantraniliprole on life table parameters and nutritional physiology of Spodoptera exigua (Hübner) Pesticide Biochem. Physiol. 2019;155:108–118. doi: 10.1016/j.pestbp.2019.01.012. [DOI] [PubMed] [Google Scholar]
- Hakami A.R., Khan K.A., Ghramh H.A., Ahmad Z., Al-Zayd A.A.A. Impact of artificial light intensity on nocturnal insect diversity in urban and rural areas of the Asir province, Saudi Arabia. Plos One. 2020;15(12):e0242315. doi: 10.1371/journal.pone.0242315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haydak M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970;15(1):143–156. [Google Scholar]
- Herbert Jr, E., Miller-Hill, N., 1987. Seasonal variation of seven minerals in honey bee collected pollen. Am. bee J.
- Jaleel W., Saeed S., Naqqash M.N., Sial M.U., Ali M., Zaka S.M. Effects of temperature on baseline susceptibility and stability of insecticide resistance against Plutella xylostella (Lepidoptera: Plutellidae) in the absence of selection pressure. Saudi J. Biol. Sci. 2020;27(1):1–5. doi: 10.1016/j.sjbs.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal Z.A., Abou-Shaara H.F., Qamer S., Alotaibi M.A., Khan K.A., Khan M.F. Future expansion of small hive beetles, Aethina tumida, towards North Africa and South Europe based on temperature factors using maximum entropy algorithm. J. King Saud University-Sci. 2021;33(1):101242. [Google Scholar]
- Lau P.W., Nieh J.C. Salt preferences of honey bee water foragers. J. Exp. Biol. 2016;219(6):790–796. doi: 10.1242/jeb.132019. [DOI] [PubMed] [Google Scholar]
- Lawal O., Banjo A. Appraising the beekeepers knowledge and perception of pests problem in beekeeping business at different ecological zones in south western Nigeria. World J. Zool. 2010;5(2):137–142. [Google Scholar]
- Lihoreau M., Buhl J., Charleston M.A., Sword G.A., Raubenheimer D., Simpson S.J. Nutritional ecology beyond the individual: a conceptual framework for integrating nutrition and social interactions. Ecol. Lett. 2015;18(3):273–286. doi: 10.1111/ele.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayntz D. Nutrition, ecology and nutritional ecology: toward an integrated framwork. Funct. Ecol. 2009;23(1):4–16. [Google Scholar]
- McLellan A. Growth and decline of honeybee colonies and inter-relationships of adult bees, brood, honey and pollen. J. Appl. Ecol. 1978:155–161. [Google Scholar]
- Michener C.D. Johns Hopkins University Press; 2007. The bees of the world. [Google Scholar]
- Nation J.L. Insect Diets: Science and Technology. Fla. Entomol. 2004;87(3):423. [Google Scholar]
- Nawaz A., Ali H., Sufyan M., Gogi M.D., Arif M.J., Ali A. In-vitro assessment of food consumption, utilization indices and losses promises of leafworm, Spodoptera litura (Fab.), on okra crop. J. Asia-Pac. Entomol. 2020;23(1):60–66. [Google Scholar]
- Olejnícek, J.J.E.J.o.E., 2004. Insect diets: science and technology. Eur. J. Entomol. 101(4), 512.
- Paoli P.P., Donley D., Stabler D., Saseendranath A., Nicolson S.W., Simpson S.J. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids. 2014;46(6):1449–1458. doi: 10.1007/s00726-014-1706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C., Dickson B. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 2010;20(11):1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Ricigliano V. Microalgae as a promising and sustainable nutrition source for managed honey bees. Arch. Insect Biochem. Physiol. 2020;104(1):e21658. doi: 10.1002/arch.21658. [DOI] [PubMed] [Google Scholar]
- Ricigliano V.A., Mott B.M., Floyd A.S., Copeland D.C., Carroll M.J., Anderson K.E. Honey bees overwintering in a southern climate: longitudinal effects of nutrition and queen age on colony-level molecular physiology and performance. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-28732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp, R., 2015. Why micronutrient deficiency is a macroproblem. National Geographic: The Plate . URL http://theplate. nationalgeographic. com//09/23/why-micronutrientdeficiency-is-a-macro-problem/.
- Saeed S., Jaleel W., Naqqash M.N., Saeed Q., Zaka S.M., Sarwar Z.M. Fitness parameters of Plutella xylostella (L.)(Lepidoptera; Plutellidae) at four constant temperatures by using age-stage, two-sex life tables. Saudi J. Biol. Sci. 2019;26(7):1661–1667. doi: 10.1016/j.sjbs.2018.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel M., Ahmad S., Ali H., Al-Kahtani S.N., Ghramh H.A., Khan K.A. Seasonal impact and comparative analysis of hypopharyngeal glands in worker and forager honey bees of two different species: Apis mellifera and A. cerana. Fresenius Environ. Bull. 2020;29(10):9024–9030. [Google Scholar]
- Simpson, C., Simpson, S., Abisgold, J., 1990. The role of various amino acids in the protein compensatory response of Locusta migratoria. In: Symp. Biol. Hungarica, pp. 39-46.
- Simpson S.J., Raubenheimer D. The nature of nutrition: a unifying framework. Aust. J. Zool. 2012;59(6):350–368. [Google Scholar]
- Simpson S.J., Raubenheimer D. Princeton University Press; 2012. The nature of nutrition: a unifying framework from animal adaptation to human obesity. [Google Scholar]
- Somerville, D., 2005. Fat bees skinny bees. A manual on honey bee nutrition for beekeepers. Australian Government Rural Industries Research Development Corporation, Goulburn, 1-142.
- T’ai, H.R., Cane, J.H., 2000. Pollen nutritional content and digestibility for animals. Pollen pollination, 187-209.
- Toshima N., Tanimura T. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J. Exp. Biol. 2012;215(16):2827–2832. doi: 10.1242/jeb.069146. [DOI] [PubMed] [Google Scholar]
- Wang T.-H., Jian C.-H., Hsieh Y.-K., Wang F.-N., Wang C.-F. Spatial distributions of inorganic elements in honeybees (Apis mellifera L.) and possible relationships to dietary habits and surrounding environmental pollutants. J. Agric. Food Chem. 2013;61(21):5009–5015. doi: 10.1021/jf400695w. [DOI] [PubMed] [Google Scholar]
- Wright G.A., Nicolson S.W., Shafir S. Nutritional physiology and ecology of honey bees. Annu. Rev. Entomol. 2018;63:327–344. doi: 10.1146/annurev-ento-020117-043423. [DOI] [PubMed] [Google Scholar]