Abstract
Calotropis procera and Somra (Acacia) honey are used in traditional medicine. The benefits of mixing 20% Somra honey and C. procera leaf water extract (CPLWExt) were aimed to be studied. Honey/CPLWExt were utilized to produce silver nanoparticles (AgNPs) separately. AgNPs were characterized via UV/Vis and electron microscope scanning. Bio-molecules in CPLWExt/honey were investigated utilizing FT-IR spectroscopy. Biological activities of CPLWExt and honey were tested. The outcomes showed that CPLWExt and honey have numerous functional groups and could produce AgNPs. CPLWExt, CPLWExt + AgNPs, honey and honey + AgNPs hindered the growth of rat splenocytes, while CPLWExt + honey invigorated it. Antimicrobial power was found in CPLWExt and honey, which increased in the presence of AgNPs. Honey/honey + AgNPs suppressed the proliferation of HeLa and HepG2 cells. In conclusion, honey/CPLWExt could produce AgNPs and showed immunomodulatory and antibacterial power. Somra honey/honey + AgNPs have anticancer power. Somra honey + CPLWExt reflected a good immunostimulatory powers that can be nominated as an immunostimulant.
Keywords: AgNPs, Calotropis procera, Splenocyte, Antimicrobial, Anticancer, Somra (Acacia) honey
1. Introduction
Natural compounds, especially obtained from plant origin, have a direct positive impact on the health of humans (Dias et al., 2012). In addition, some animal products, including bee products, have the same importance to humans (Kocot et al., 2018).
Calotropis procera, the wooded and evergreen shrub, is a member of the genus Calotropis, family Asclepiadaceae. The Calotropis genus is exceedingly distributed throughout the tropical and subtropical areas of both Africa and Asia. C. procera is commonly known due to the production of huge amount of the latex. The flowers, stem, roots and leaves of C. procera are used in folklore medicine (Aati et al., 2019). C. procera latex is used in wound healing and as an anti-diarrheic, anti-inflammatory and anti-rheumatic, deodorant, antifungal, antisyphilitic, spasmogenic, carminative drug, against malaria, skin illness, anticancer, proteolytic, larvicidal, antimicrobial, anti-nematodal, anti-inflammatory and against jaundice (Silva et al., 2010).
From the very long time ago, human utilized honey for nutrition and medication. Honey is a perplexing compound and there are no two honey samples alike. The variety of honey is ascribed to the differences in climatic and ecological circumstances where plants raised and thusly the honey bees gather pollens and nectars grown in. Honey is basically made out of phenolic acids, saccharides, carotenoids, enzymes, amino acids, flavonoid, organic acids, polyphenols, (glycosides and aglycones), minerals and vitamins that form the bioactive properties of honey (Beretta et al., 2005, Hermosín et al., 2003). Since honey creation is based on the plant source, it can show assorted biological characteristics. Many investigations indicated that honey types with light color as in case of acacia honey, show different values for some parameters of their biological activity than honey types that are darker (spruce, forest or chestnut) (Bertoncelj et al., 2007). Acacia honey is liked by the consumers because of its nutritive values and medicinal merits (Abeshu and Geleta, 2016) and usually found in two colors, pale and dark yellow.
Acacia tortilis, a member of the genus Acacia and the family Leguminaceae, is considered as a remarkable species of the genus. The various parts of A. tortilis plant, including exudates, leaves, gum pods and bark are known to be important for the commercial and medicinal sides. This plant can survive in dry zones due to its power to endure harsh situations leading to be helpful in preventing soil erosion (Abdallah et al., 2008).
Nanoparticles of noble metal such as silver are important and being used in biology, industry and medicine (Yokoyama and Welchons, 2007). Nanoparticles are produced via several physical and chemical procedures (Hanžić et al., 2015), but nowadays, green synthesis methods using plants (Iravani, 2011), honey (Ghramh et al., 2020b), yeast, bacteria and fungi (Singh et al., 2016) are widely used because of their cleanliness, loss toxicity, and being ecofriendly. Silver NPs are known for their anti-parasite, anticancer, insecticidal, antimicrobial and anti-inflammatory characteristics (Ghramh et al., 2020c). Preparation of NPs utilizing plant extract is an easy procedure and with low biohazardous contents.
Immunomodulation, either upregulation or downregulation of the efficacy of the immune system is well-known and studied (Sam et al., 2018). The important role of the compounds obtained from plant that are known to have immunomodulatory power need to be studied at its efficacy and safety levels (Licciardi and Underwood, 2011). Some of these experiments were done to investigate honey and Calotropis procera immunomodulatory effects (Cornara et al., 2017).
In this study, we tried to synthesize AgNPs using both the plant extract and honey. Many biological effects of both honey and the plant extract, alone and containing AgNPs were studied. In addition, synergistic effects of both honey and the plant extract were also investigated.
2. Materials and methods
2.1. Collection of the plant and extract preparation
Considerable amount of C. procera leaves were gathered in March 2020 from Abha city (Coordinates: 18°13′1″N 42°30′19″E; Elevation: 2270 m (7450 ft.)), Aseer region, KSA. C. procera leaf extract (CPLWExt) in water and stock solution (1%) were prepared the same procedures as indicated by Ibrahim et al. (2019).
2.2. Collection of honey and preparation
Somra (Acacia tortilis) honey, bottled by local producers, was collected from Abha. It was clear, amber yellow without any sign of granulation or odor of fermentation. Using distilled water, collected honey samples were diluted to 20% (v/v).
2.3. Biosynthesis and characterizations of silver nanoparticles
Honey and CPLWExt were used to synthesis AgNPs as per Ghramh et al. (2020b). Analysis of active molecules owned by honey, CPLWExt and both honey and CPLWExt after the formation of AgNPs was done as per Ibrahim et al. (2019).
2.4. Antimicrobial potential test
The antimicrobial power of Acacia honey and CPLWExt, alone or containing AgNPs, was assessed against Gram positive (Staphylococcus aureus and Bacillus subtilis) and Gram negative (Proteus mirabilis and Escherichia coli) bacteria according to Al Syaad and Ibrahim (2020).
2.5. Influence of various preparations on rat splenocytes proliferation
Preparation of single-cell suspension (0.04 × 106/mL) from a healthy adult male Sprague Dawley rat spleen and cell viability test using MTT assay were done according to Ibrahim et al. (2019). All liquid preparations utilized in this experiment were filter sterilized (0.45 µm, Coaster). A 100 μL culture media containing either CPLWExt (100 μg/mL), CPLWExt containing AgNPs (100 μg/mL), honey (20%), honey containing AgNPs (20%), honey (20%) with CPLWExt (100 μg/mL) or honey (20%) with CPLWExt containing AgNPs (100 μg/mL) were mixed with 100 μL of the cell suspension (4000 cells) in cell culture plate wells separately. Untreated cell culture (base line of cell division) and phytohaemagglutinin at a final concentration of 5 μg/mL as positive control were included.
2.6. Anticancer activity test
HepG2 and HeLA cancer cell lines (both kindly donated by Prof. Ahmed Alamri, King Khalid University) were utilized to examine the anti-cancer power of honey and honey + AgNPs as per Ghramh et al. (2020a). Cells were detached from the culture flasks by the action of trypsin (2%) and a cell suspension was set to 1X105/mL. Single-cell suspension (104 cells/100 µL) was added to each well of cell culture plate (96 wells) and kept overnight in the CO2- incubator. The media in wells (each well with adhered 104 cells) were decanted and replaced with 200 µL culture media containing honey (20%) or honey + AgNPs (20%) and cell viability was assayed after 24 h.
2.7. Statistical analysis
Outcomes were articulated as means 6 values ± SD of time of researches. A Student’s t-test (GraphPad Prism-Version 7.0 for windows) for paired/unpaired values was made and a p value of 0.05 was measured statistically substantial.
3. Results
3.1. Silver nanoparticles characterization
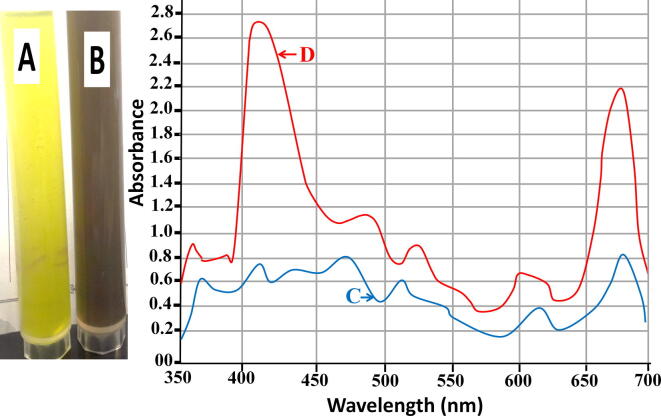
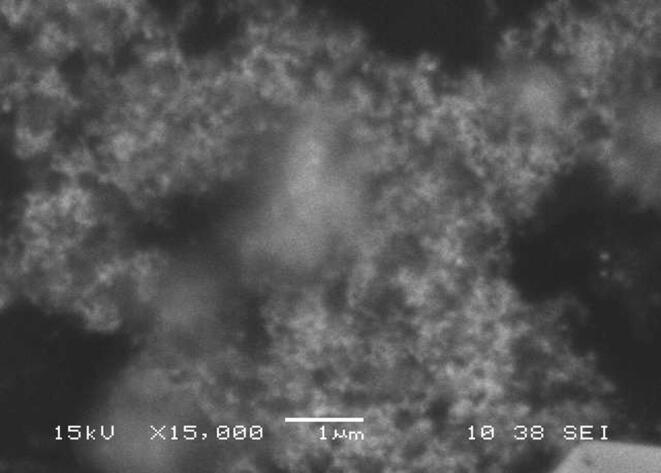
CPLWExt was blended with AgNO3 to produce silver NPs. The alteration in the blend color (from yellow to brown) was time-dependent and an indication of AgNPs formation (Fig. 1A and B). The UV–Vis absorption indicated that AgNPs were at around 410–440 nm (Fig. 1C and D). SEM image disclosed that the produced AgNPs are uniform spheres with a size of 60–85 nm (Fig. 2).
Fig. 1.
AgNPs synthesis by CPLWExt; A: extract alone; B: extract after production of AgNPs; C: light absorbance of the extract alone; D: light absorbance of the extract after production of AgNPs.
Fig. 2.
The SEM image showing the spherical silver nanoparticles produced by Calotropis procera leaf extract.
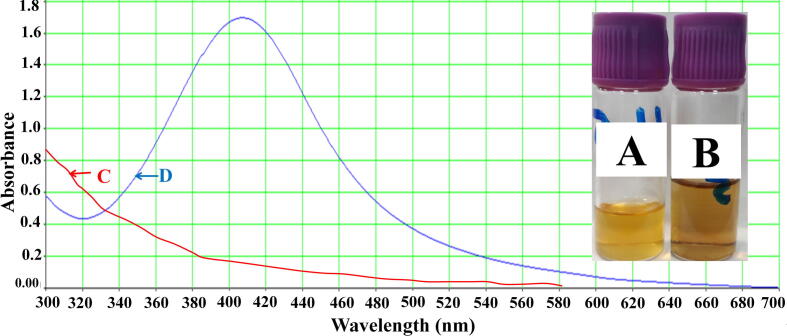
Honey samples (diluted) were blended with AgNO3 to synthesize silver NPs. The alteration in the blend color (from pale yellow to brown) was a sign of silver NPs formation (Fig. 3A and B). The alteration in the blend color continued to dark brown. The degree of the alteration in the blend color was pH-dependent that made the visual monitoring easy. Results reflected the production of AgNPs after adding of AgNO3 showing a peak at 415 nm (Fig. 3C and D).
Fig. 3.
AgNPs production by 20% honey; A: honey alone; B: honey after production of AgNPs; C: light absorbance of honey alone; D: light absorbance of honey after production of AgNPs.
SEM image disclosed that the honey-produced silver NPs are spheres having a size ranging from 70 to 80 nm (Fig. 4).
Fig. 4.
The SEM image showing the spherical AgNPs formed by honey.
3.2. Functional groups characterization
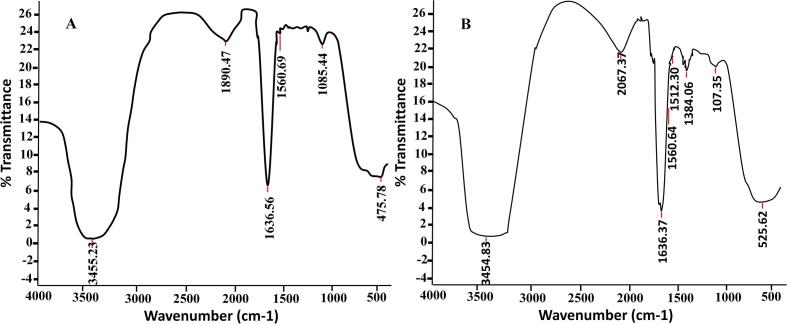
The results of FT-IR spectroscopy test (Fig. 5) showed that there is no substantial difference between the FT-IR spectra of CPLWExt and CPLWExt + AgNPs. CPLWExt showed peaks within the region 3500–500 cm−1. Broad U-shaped peak appeared at 3455.23 cm−1 is characteristic to O—H stretching of alcohol. Weakened band at 1890. 47 cm−1may be because of C—H bending identical to aromatic compound. A strong band at 1636 cm−1 may be attributed to C C stretching corresponding to alkene. Strong peak at 1560.69 and 1085.44 cm−1 could be due to stretching vibration of N—O and C—O due to nitro compound and primary alcohol, respectively. Weak band observed at 500 cm−1 may be due to C-I of alkyl halide. However, in the FT-IR spectra of leaf extract of Calotropis procera containing synthesized AgNPs, some peaks shifted such as peak at 1085.44 cm−1 to 1107.35 cm−1 and others disappeared like that at 1890.47 and 1560.69 cm−1 while new peak appeared at 2067.37 and 1384.06 cm−1 may be attributed to N C S stretching C—H bending ascribed to isothiocyanate and alkane. This is crucial evidence about the responsibility of isothiocyanate compound and alkane in the process of reduction/capping of silver NPs (Umoren et al., 2014).
Fig. 5.
FT-IR spectra of CPLWExt where (A) before and (B) after the addition of AgNO3.
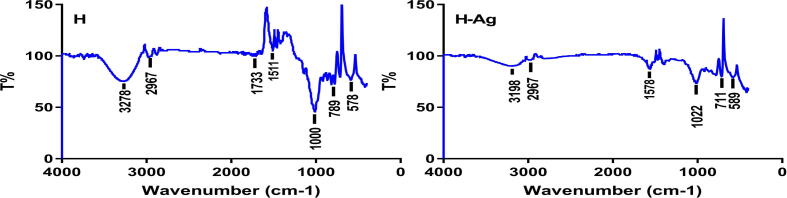
The biomolecules present in honey and honey + AgNPs detected by FT-IR spectroscopy are presented in Fig. 6. Honey/honey + AgNPs presented peaks within the area of 3500–500 cm−1. The wide peak at 3278 cm−1 in the spectrum demonstrates the presence of O-H group of alcohol. Weakened bands around 2967 and 1511 cm−1 are specific for the C—H/N—O expansion, vibration demonstrates the existence of alkane and nitro structures, respectively. The powerful peaks at 1000, 789 and 578 cm−1 match with C—O, C C and C—Br expansion vibrations explaining the existence of vinyl ether, alkene and bromocompounds. When nanoparticles were formed, the bands at 3278, 1511, 1000, 789 cm−1 578 and shifted to 3197, 1578, 1022, 711 and 589 cm−1 respectively. Besides, the strength of the former peaks turned lesser which could be presupposed that they were utilized in the reduction/ capping process of AgNPs.
Fig. 6.
FT-IR spectra of honey. Where (H) before and (H-Ag) after addition of AgNO3.
3.3. Antimicrobial vulnerability
The outcome of examining the antimicrobial power of CPLWExt and CPLWExt + AgNPs toward Gram-positive/negative bacteria and the fungus are demonstrated in Table 1. CPLWExt and CPLWExt + AgNPs inhibited the bacterial/fungal growth, but Candida albicans did not affect by CPLWExt. The average inhibition zone diameters formed by CPLWExt + AgNPs toward Gram negative/positive bacteria were bigger than that produced by CPLWExt alone.
Table 1.
Antimicrobial potentials of CPLWExt and CPLWExt containing AgNPs.
| Test organisms | Inhibition Zone (mm) |
||
|---|---|---|---|
| CPLWExt | CPLWExt + AgNPs | Penicillin (10 µg) | |
| Micrococcus luteus | 5.0 ± 0.01 | 19 ± 0.036 | 8 ± 0.011 |
| Bacillus subtilis | 8 ± 0.021 | 29 ± 0.040 | 10 ± 0.012 |
| Proteus mirabilis | 11 ± 0.033 | 28 ± 0.043 | 9 ± 0.015 |
| Klebsiella pneumoniae | 10 ± 0.030 | 19 ± 0.037 | 10 ± 0.010 |
| Candida albicans | NI | 22 ± 0.041 | 10 ± 0.015 |
(NI) stands for no inhibition.
The antimicrobial activity of Acacia honey against different bacteria and fungus are presented in Table 2. The average inhibition zone diameters formed by honey + AgNPs against Gram negative and Gram positive bacteria were greater than that formed by honey.
Table 2.
Antimicrobial potentials of honey and honey containing AgNPs.
| Test organisms | Inhibition Zone (mm) |
||
|---|---|---|---|
| Honey | Honey + AgNPs | Penicillin (10 µg) | |
| Micrococcus luteus | 7.0 ± 0.02 | 17 ± 0.041 | 8 ± 0.011 |
| Bacillus subtilis | 7.1 ± 0.25 | 10 ± 0.017 | 10 ± 0.012 |
| Proteus mirabilis | 7.1 ± 0.28 | 11 ± 0.021 | 9 ± 0.015 |
| Klebsiella pneumoniae | 12.1 ± 0.28 | 14 ± 0.029 | 10 ± 0.010 |
| Candida albicans | 7.1 ± 0.28 | 7 ± 0.039 | 10 ± 0.015 |
3.4. Anticancer activity
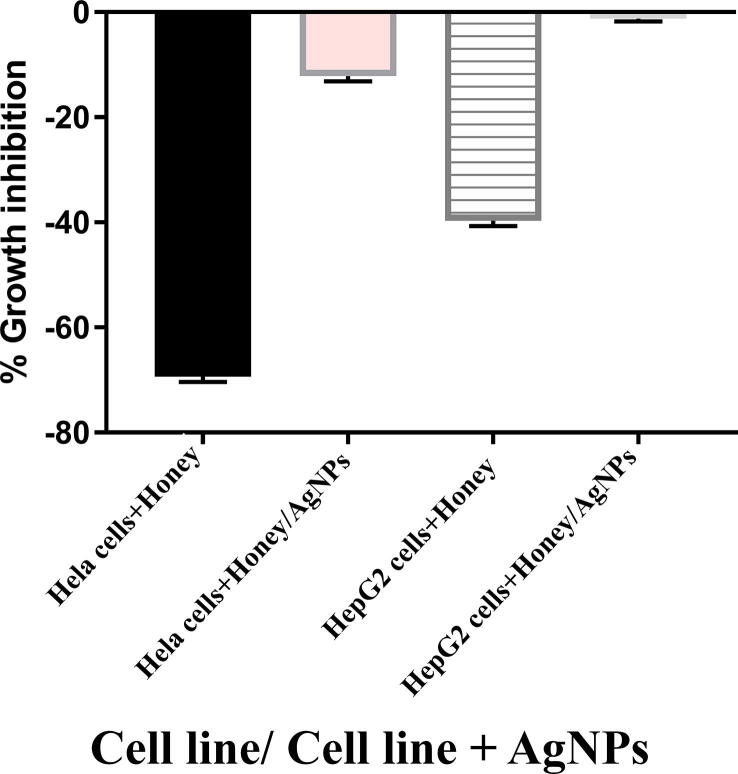
Somra honey showed anticancer power on both HeLA and HepG2 cells (Fig. 7) and it showed significantly (p > 0.0001) stronger cell growth suppressive effects on HeLA cancer cells than that on HepG2 cancer cells. The growth suppressive effects of honey significantly decreased (p > 001) when adding AgNPs to honey.
Fig. 7.
Effects of 20% honey and honey with AgNPs on HeLA and GepG2 cancer cell line growth.
3.5. Effect on splenocytes
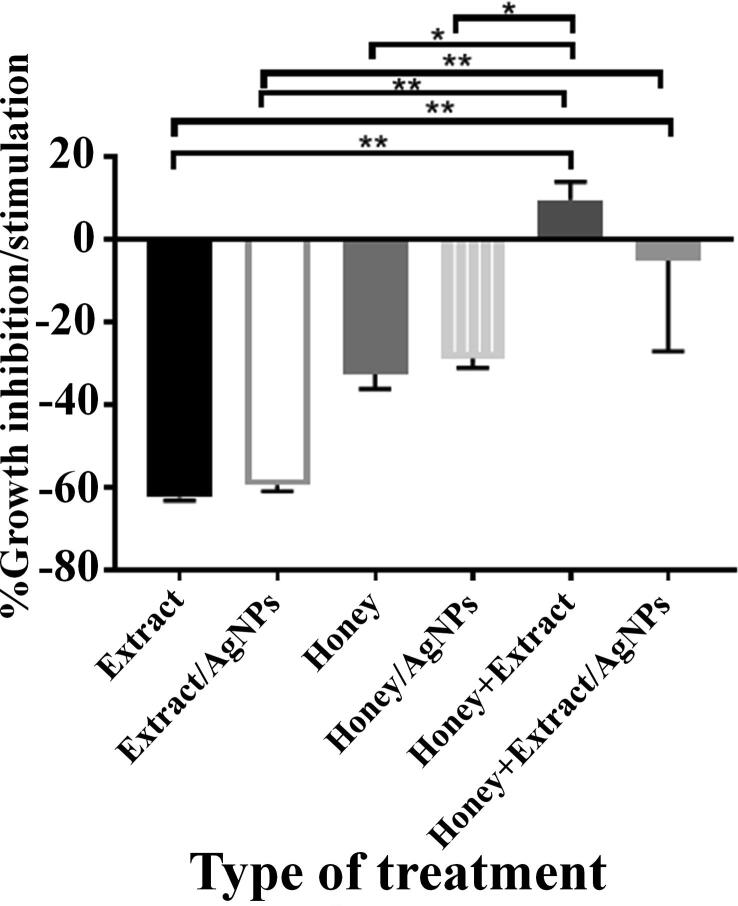
Normal rat splenocytes were mixed with honey and CPLWExt and their NPs (Fig. 8). Adding of CPLWExt or CPLWExt + AgNPs to splenocytes showed cell growth suppressive impact. CPLWExt + AgNPs showed non-significantly less inhibitory than that shown by the CPLWExt alone.
Fig. 8.
Effects of 20% honey, honey + AgNPs, CPLWExt, CPLWExt + AgNPs and mixes of them on normal rat splenic cells. NB: *= p < 0.05; ** = p > 0.001.
4. Discussion
To produce silver NPs, CPLWExt was added to AgNO3. The change in color is attributed to the excitation of AgNPs Surface Plasmon Resonance (Ranganathan et al., 2012). Color alteration of the liquids may be attributed to the some chemical structures such as steroids, alkaloids, saponins, flavonoids, etc. present in CPLWExt that could drive the reduction action that lead to the reduction of Ag+ to an atom form (Ag0). This phenomenon was discussed by Ranganathan et al. (2012) and explained that several biologically active molecules contained in the milieu may possess the strength to convert ions of silver to the nanoparticles state and other biomolecules act as capping agents.
Silver nitrate was blended with the dilute form of honey to produce silver NPs. The alteration in the blend color was a prove of the AgNPs formation. There is a likelihood that sugars (e.g. sucrose and glucose) and proteins (possibly enzymes) have an impact in the Ag+ reduction operation. Adding of NaOH to increase the pH of the medium made a direct effect on the expected volume of the obtained NPs. The increase in the medium pH may result in the increase of gluconic acid formation from glucose.
FT-IR spectroscopy test revealed the presence of many functional groups in CPLWExt and honey. These biomolecules are the source of reducing and capping agents.
CPLWExt + AgNPs showed antibacterial and antifungal power. Our results are in agreement with other’s results (Kareem et al., 2008, Shobowale et al., 2013). Nenaah (2013) showed that the powerful antimicrobial properties of CPLWExt are a result of some flavonoid glycosides namely kaempferol, 7-dimethoxyflavone-4′-O-β-glucopyranoside, isorhamnetin, 3-O-rutinosides of quercetin, and 5-hydroxy-3, as bioactive constituents. Shobowale et al. (2013) demonstrated that the qualitative phytochemical study of C. procera indicated the presence of alkaloids, flavonoids, saponins and tannins. It was previously reported (Mungole et al., 2010) that phytochemicals inhibit bacteria growth. Others (Sodipo et al., 1991) indicated tannins do not hinder the progressive growth of microorganisms by the prohibition of beneficial nutrients from being obtained by the organism resulting in the microbial protein deposition.
Acacia honey showed antimicrobial activity against bacteria and fungus. As to the consequences of antimicrobial, obviously honey (20%), indicated restraint the growth of the tested microbes. The restraint of microbial this outgrowth might be because of numerous variables as the osmotic impact of honey, hydrogen peroxide existence, substances other than peroxide and volatile substances with antibacterial impact (Kwakman and Zaat, 2012). Other researchers showed that at 40% or above, honey could assessed the development of different Gram positive/negative bacteria (Jeddar et al., 1985).
In our study, honey suppressed HeLA and HepG2 cells growth, which is in agreement with work done by Fauzi et al., 2011, Hassan et al., 2012 respectively.
Somra honey showed anticancer activity on both HeLA and HepG2 cells. Malignant growth chemoprevention, by the utilization of natural food and plant extracts, particularly flavonoids that can reverse, stifle or frustrate cancer progression, has become an engaging procedure to battle the dogma related with the increasing cases of cancers worldwide (Surh, 2003). Honey has the ability to potentiate the antitumor activity of some chemotherapeutic drugs (Attia et al., 2008). Studies showing anticancer impacts of honey include in vitro (cell cultures) and in vivo (animals) leading to human clinical trials. Honey contains polyphenols, which are deemed to be the main agent accountable for the honey anticancer capability (Abubakar et al., 2012). The presence of nanoparticles significantly lowered the cell growth suppressive actions of honey on both HeLA and HepG2 cell lines. This may be due to the use of these inhibitory materials in silver ion reduction and capping.
Normal rat splenocytes were treated with honey and CPLWExt, with and without NPs. The preliminary phytochemical screening of CPLWExt revealed presence of steroids, sugars, tannins, phenols, cardenolides, terpenoids, flavonoids, saponins and alkaloids in the leaves (Mossa et al., 1991). The leaves likewise included bitter molecules (e.g. mudarine) and others (calactin, calotropin, glycosides, uscharin and calotoxin). Some biomolecules found in C. procera like cardenolides were indicated to possess growth suppression power on in vitro cultured cells (Mathur et al., 2009). Steroids e.g. methylprednisolone and fluorohydrocortisone), have the power to in vitro repress T, NK, and K immune cells (Langhoff et al., 1985). The facts mentioned above may explain the growth suppressive effects of CPLWExt and CPLWExt + AgNPs on in vitro cultured cells.
Some distinct proteins direct and check all of the cell cycle events. The Ki-67 nuclear protein marker probes the “growth fraction” of cell division. Ki-67 protein marker is missing in the resting phase (G0) but its expression is activated during the cell cycle phases (G1, S, G2, and M) (Lee et al., 2003). Honey was demonstrated to influence cell cycle arrest. Blending the honey with some plant preparations (e.g. Aloe vera) decreased the expression of Ki-67-LI marker in rat cancer cells (Tomasin and Cintra Gomes-Marcondes, 2011). This may propose that honey application had the power to prompt bringing down cell division via cell cycle arresting (Tomasin and Cintra Gomes-Marcondes, 2011). Honey, through the components included in it (e.g. phenolics and flavonoids) is stated to hinder the cell cycle of some cancer cell lines (e.g. colon, glioma and melanoma) (Lee et al., 2003, Mandal and Jaganathan, 2009, Pichichero et al., 2010), at the phase G0/G1. This cell growth suppressive action is preceded by cellular pathways down-regulation (e.g. kinase, tyrosine cyclooxygenase and ornithine decarboxylase) (Mandal and Jaganathan, 2009, Pichichero et al., 2010). The results of MTT assay proved that the cell growth suppressive power of honey is a dose- and time-dependent way (Pichichero et al., 2010).
Honey showed a growth suppressive power on rat splenocytes in vitro. Duddukuri and his co-workers (Duddukuri et al., 2002) proposed that the growth suppressive power of honey might be because of the inhibitory impact of honey on the division of T cells. FT-IR analysis results in the current work revealed that some biomolecules are wasted to manufacture AgNPs. The growth suppressive power of honey non-significantly reduced when AgNPs were included, indicating that the utilization of some suppressive agents in NPs production. When adding honey to CPLWExt, the blend was growth stimulatory to the rat splenocytes. The inhibitory biomolecules found in both the honey and CPLWExt complexed together resulting in their neutralization. It displayed an increase in cell growth promotion than CPLWExt and the honey did. When CPLWExt + AgNPs were blended with the honey, a growth inhibitory behavior was obtained. This might be attributed to the action of AgNPs on the cells. Some researcher mixed honey with C. procera and used for wound healing study (Aderounmu et al., 2013) and found that the mix is more effective than using each product alone and better than the positive control product.
5. Conclusions
The synergistic effects of honey and C. procera plant extract were not studied before. Both Somra honey and C. procera gathered from Abha, have immunomodulatory and antimicrobial power and the capability to manufacture AgNPs. Somra honey, alone or in combination with AgNPs demonstrated anticancer potentials. Blending of Somra honey and CPLWExt demonstrated a good immunostimulatory effects reflecting their potential as an immunostimulant.
Author contributions
All authors contributed equally in this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the Scientific Research Deanship at King Khalid University and the Ministry of Education in KSA for funding this research work through the project number IFP-KKU-2020/5.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aati H., El-Gamal A., Shaheen H., Kayser O. Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed. 2019;15:2–9. doi: 10.1186/s13002-018-0263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah F., Noumi Z., Touzard B., Belgacem A.O., Neffati M., Chaieb M. The influence of Acacia tortilis (Forssk.) Subsp. raddiana (Savi) and livestock grazing on grass species composition, yield and soil nutrients in arid environments of South Tunisia. Flora Morphol. Distrib. Funct. Ecol. Plants. 2008;203:116–125. doi: 10.1016/j.flora.2007.02.002. [DOI] [Google Scholar]
- Abeshu M.A., Geleta B. Medicinal uses of honey. Biol. Med. 2016;8:1–7. doi: 10.4172/0974-8369.1000276. [DOI] [Google Scholar]
- Abubakar M.B., Abdullah W.Z., Sulaiman S.A., Suen A.B. A review of molecular mechanisms of the anti-leukemic effects of phenolic compounds in honey. Int. J. Mol. Sci. 2012;13:15054–15073. doi: 10.3390/ijms131115054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderounmu A.O., Omonisi A.E., Akingbasote J.A., Makanjuola M., Bejide R.A., Orafidiya L.O., Adelusola K.A. Wound-healing and potential anti-keloidal properties of the latex of Calotropis procera (Aiton) Asclepiadaceae in rabbits. African J. Tradit. Complement. Altern. Med. AJTCAM. 2013;10:574–579. doi: 10.4314/ajtcam.v10i3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Syaad K.M., Ibrahim E.H. Study of the antioxidant, immunomodulatory and antibacterial properties of Origanum majorana leaf acetone extract. Pak. J. Pharm. Sci. 2020;33:2209–2218. doi: 10.36721/PJPS.2020.33.5.SUP.2209-2218.1. [DOI] [PubMed] [Google Scholar]
- Attia W.Y., Gabry M.S., El-Shaikh K.A., Othman G.A. The anti-tumor effect of bee honey in Ehrlich ascite tumor model of mice is coincided with stimulation of the immune cells. Egypt. J. Immunol. 2008;15:169–183. [PubMed] [Google Scholar]
- Beretta G., Granata P., Ferrero M., Orioli M., Facino R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta. 2005;533:185–191. doi: 10.1016/j.aca.2004.11.010. [DOI] [Google Scholar]
- Bertoncelj J., Doberšek U., Jamnik M., Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007;105:822–828. doi: 10.1016/j.foodchem.2007.01.060. [DOI] [Google Scholar]
- Cornara L., Biagi M., Xiao J., Burlando B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017;8:412. doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias D.A., Urban S., Roessner U. A Historical overview of natural products in drug discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddukuri G.R., Rao D.N., Athota R.R. Suppressive effect of honey on antigen/mitogen stimulated murine T cell proliferation. Pharm. Biol. 2002;40:39–44. doi: 10.1076/phbi.40.1.39.5851. [DOI] [Google Scholar]
- Fauzi A.N., Norazmi M.N., Yaacob N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011;49:871–878. doi: 10.1016/j.fct.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Ghramh H.A., Ibrahim E.H., Kilany M. Study of anticancer, antimicrobial, immunomodulatory, and silver nanoparticles production by Sidr honey from three different sources. Food Sci. Nutr. 2020;8:445–455. doi: 10.1002/fsn3.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghramh H.A., Ibrahim E.H., Kilnay M. Majra honey abrogated the normal and cancer cells proliferation inhibition by juniperus procera extract and extract/honey generated AgNPs. Anticancer. Agents Med. Chem. 2020;20:970–981. doi: 10.2174/1871520620666200213104224. [DOI] [PubMed] [Google Scholar]
- Ghramh H.A., Ibrahim E.H., Kilnay M., Ahmad Z., Alhag S.K., Khan K.A., Taha R., Asiri F.M. Silver nanoparticle production by ruta graveolens and testing its safety, bioactivity, immune modulation, anticancer, and insecticidal potentials. Bioinorg. Chem. Appl. 2020;2020 doi: 10.1155/2020/5626382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanžić N., Jurkin T., Maksimović A., Gotić M. The synthesis of gold nanoparticles by a citrate-radiolytical method. Radiat. Phys. Chem. 2015;106:77–82. doi: 10.1016/j.radphyschem.2014.07.006. [DOI] [Google Scholar]
- Hassan M.I., Mabrouk G.M., Shehata H.H., Aboelhussein M.M. Antineoplastic effects of bee honey and nigella sativa on hepatocellular carcinoma cells. Integr. Cancer Ther. 2012;11:354–363. doi: 10.1177/1534735410387422. [DOI] [PubMed] [Google Scholar]
- Hermosín I., Chicón R.M., Cabezudo M.D. Free amino acid composition and botanical origin of honey. Food Chem. 2003;83:263–268. doi: 10.1016/S0308-8146(03)00089-X. [DOI] [Google Scholar]
- Ibrahim E.H., Kilany M., Ghramh H.A., Khan K.A., ul Islam S. Cellular proliferation/cytotoxicity and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J. Biol. Sci. 2019;26:1689–1694. doi: 10.1016/j.sjbs.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. doi: 10.1039/c1gc15386b. [DOI] [Google Scholar]
- Jeddar A., Kharsany A., Ramsaroop U.G., Bhamjee A., Haffejee I.E., Moosa A. The antibacterial action of honey. An in vitro study. South African Med. J. 1985;67:257–258. [PubMed] [Google Scholar]
- Kareem S.O., Akpan I., Ojo O.P. Antimicrobial activities of Calotropis procera on selected pathogenic microorganisms. African J. Biomed. Res. 2008;11:105–110. doi: 10.4314/ajbr.v11i1.50674. [DOI] [Google Scholar]
- Kocot J., Kiełczykowska M., Luchowska-Kocot D., Kurzepa J., Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxid. Med. Cell. Longev. 2018;2018:1–29. doi: 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakman P.H.S., Zaat S.A.J. Antibacterial components of honey. IUBMB Life. 2012;64:48–55. doi: 10.1002/iub.578. [DOI] [PubMed] [Google Scholar]
- Langhoff E., Ladefoged J., Dickmeiss E. The immunosuppressive potency of various steroids on peripheral blood lymphocytes, T cells, NK and K cells. Int. J. Immunopharmacol. 1985;7:483–489. doi: 10.1016/0192-0561(85)90067-0. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Kuo H.C., Chu C.Y., Wang C.J., Lin W.C., Tseng T.H. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem. Pharmacol. 2003;66:2281–2289. doi: 10.1016/j.bcp.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Licciardi P.V., Underwood J.R. Plant-derived medicines: a novel class of immunological adjuvants. Int. Immunopharmacol. 2011;11:390–398. doi: 10.1016/j.intimp.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Mandal M., Jaganathan S.K. Antiproliferative effects of honey and of its polyphenols: a review. J. Biomed. Biotechnol. 2009;2009:1–13. doi: 10.1155/2009/830616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur R., Gupta S.K., Mathur S.R., Velpandian T. Anti-tumor studies with extracts of Calotropis procera (Ait.) R. Br. Root employing Hep2 cells and their possible mechanism of action. Indian J. Exp. Biol. 2009;47:343–348. [PubMed] [Google Scholar]
- Mossa J.S., Tariq M., Mohsin A., Ageel A.M., al-Yahya M.A., al-Said M.S., Rafatullah S. Pharmacological studies on aerial parts of Calotropis procera. Am. J. Chin. Med. 1991;19:223–231. doi: 10.1142/s0192415x91000302. [DOI] [PubMed] [Google Scholar]
- Mungole A., Day S., Kamble R., Kanfade H., Chaturvedi A., Zanwar P. Active phytochemical and antibacterial potentiality of in-vitro regenerated plantlets of Canscora decurrens (Dalzell) Indian J. Sci. Technol. 2010;3:679–683. doi: 10.17485/ijst/2010/v3i6/29782. [DOI] [Google Scholar]
- Nenaah G. Antimicrobial activity of Calotropis procera Ait. (Asclepiadaceae) and isolation of four flavonoid glycosides as the active constituents. World J. Microbiol. Biotechnol. 2013;29:1255–1262. doi: 10.1007/s11274-013-1288-2. [DOI] [PubMed] [Google Scholar]
- Pichichero E., Cicconi R., Mattei M., Muzi M.G., Canini A. Acacia honey and chrysin reduce proliferation of melanoma cells through alterations in cell cycle progression. Int. J. Oncol. 2010;37:973–981. doi: 10.3892/ijo-00000748. [DOI] [PubMed] [Google Scholar]
- Ranganathan R., Madanmohan S., Kesavan A., Baskar G., Krishnamoorthy Y.R., Santosham R., Ponraju D., Rayala S.K., Venkatraman G. Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. Int. J. Nanomed. 2012;7:1043–1060. doi: 10.2147/IJN.S25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam Q.H., Yew W.S., Seneviratne C.J., Chang M.W., Chai L.Y.A. Immunomodulation as therapy for fungal infection: are we closer? Front. Microbiol. 2018;9:1612. doi: 10.3389/fmicb.2018.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobowale O.O., Ogbulie N.J., Itoandon E.E., Oresegun M.O., Olatope S.O.A. Phytochemical and antimicrobial evaluation of aqueous and organic extracts of calotropis procera ait leaf and latex. Niger. Food J. 2013;31:77–82. doi: 10.1016/s0189-7241(15)30059-x. [DOI] [Google Scholar]
- Silva M.C.C., da Silva A.B., Teixeira F.M., de Sousa P.C.P., Rondon R.M.M., Honrio J.E.R., Sampaio L.R.L., Oliveira S.L., Holonda A.N.M., de Vasconcelos S.M.M. Therapeutic and biological activities of Calotropis procera (Ait.) R. Br. Asian Pac. J. Trop. Med. 2010;3:332–336. doi: 10.1016/S1995-7645(10)60081-8. [DOI] [Google Scholar]
- Singh P., Kim Y.J., Zhang D., Yang D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–599. doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Sodipo O.A., Akanji M.A., Kolawole F.B., Odutuga A.A. Saponin is the active antifungal principle in Garcinia kola, heckle seed. Biosci. Res. Commun. 1991;3:171. [Google Scholar]
- Surh Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Tomasin R., Cintra Gomes-Marcondes M.C. Oral administration of Aloe vera and honey reduces walker tumour growth by decreasing cell proliferation and increasing apoptosis in tumour tissue. Phyther. Res. 2011;25:619–623. doi: 10.1002/ptr.3293. [DOI] [PubMed] [Google Scholar]
- Umoren S.A., Obot I.B., Gasem Z.M. Green synthesis and characterization of silver nanoparticles using red apple (malus domestica) fruit extract at room temperature. J. Mater. Environ. Sci. 2014;5:907–914. [Google Scholar]
- Yokoyama K., Welchons D.R. The conjugation of amyloid beta protein on the gold colloidal nanoparticles’ surfaces. Nanotechnology. 2007;18 doi: 10.1088/0957-4484/18/10/105101. [DOI] [PubMed] [Google Scholar]