Abstract
Ficus latex is rich in polyphenolic compounds and hence considered as an antioxidant and anti-proliferative. Many studies are available on Ficus carica (common fig) whereas Ficus salicifolia is less studied. F. salicifolia grows in a harsh dry environment, therefore its latex was selected in the current study along with the F. carica for their comparative anti-cancer potential and the involved molecular mechanism. Triple-negative breast cancer (TNBC) derived MDA-MB-231 cells were used in the study. MTT and morphological studies indicated that the latex of both plants has anti-proliferative effects. To know their anti-metastatic effects, a wound-healing assay was performed. Both species were able to maintain the wound size compared to the untreated cells indicating their anti-metastatic effects. Using a DNA damage assay kit, we found that both fig species have genotoxic and cytotoxic effects in MDA-MB-231 cells compared to the untreated control. To know the potential molecular mechanism involved, we used a human kinase array kit. We found that ERK2, CREB, and AKT2 were downregulated after treatment the MDA-Mb-231 cells with the latex of F. carica. We assumed that F. salicifolia will also affect the same pathways, however after confirmation through real-time (RT)-PCR, downregulations of the above mentioned pathways were confirmed in cells treated with F. carica latex, however, in cells treated with F. salicifolia the selected genes were upregulated at the transcriptional level. We conclude that latex of both species of ficus have anti-cancer effects in MDA-MB-231 cells, however differ in their level of toxicity and the mechanism of action at the molecular level.
Keywords: MDA-MB-231, Anticancer, Ficus carica, Ficus salicifolia, Fig latex
1. Introduction
Breast cancer like any other cancer is characterized with an uncontrolled cell division caused either by biological or environmental factors (Hussein and Abdullah, 2020). It is usually found in mammary glands or its ducts that deliver the milk. According to the tumor site in the breast, it can be, in situ, where the cancer cells replace the epithelial cells in the mammary gland ducts and could be expanded to lobules, however, yet not spread to the surrounding tissues. Furthermore, it can be invasive that is more common and characterized by its ability to travel beyond the ducts to the surrounding breast tissues (Kumar, 2018). Breast cancer is among the top five most commons cancers, most frequent in women and the second most common cause of death from cancers worldwide (Varghese et al., 2018, Mehanna et al., 2019). Existing therapies for breast cancer include local (surgery and radiation) and systematic (chemotherapy, hormonal therapy and other targeted therapies). The choice of therapy depends on factors like stage and type of cancer etc (Kumar, 2018).
Majority of the breast cancer cells express receptors for progesterone, estrogen and human epidermal growth factor receptor 2 (HER2). Those that do not express any of these receptors are termed as triple negative breast cancer cells (TNBC) making them exception to the hormonal therapy (Collignon et al., 2016). TNBC is the worst and most aggressive subtype and lack of receptors results in lack of targeted treatment for this type of cancer. Furthermore, it has low prognosis, more recurrence chances, regular resistance to the chemotherapy and highly proliferative with more chances to develop metastasis (Varghese et al., 2018). MDA-MB-231 cells is one of the well documented TNBC cell lines and established from pleural effusion in 1970s (Chavez et al., 2010). Besides the aggressive characteristic of any TNBC cells, MDA-MB-231 cells has mutant p53, which cause resistance to chemotherapy and make it much challenging in treatment (Huang et al., 2017).
Natural products are rich with several unique chemicals and compounds where many of these could be novel. Plants are one of the most abundant and effective natural products containing secondary metabolites and minerals. These can be flavonoid, sterols, esters, and acyl lipid etc. with reported antibacterial, anticancer and antioxidant agents (Hussein and Abdullah, 2020). Traditionally plants are used for different medicinal purposes such as garlic for heart diseases, mandrake and morphine isolated from Papaver somniferum as a pain killer, aspirin isolated from Salvia alba as an anti-inflammatory agent along with many others as anti-cancer agents (Mushtaq et al., 2018, Hassan and Ullah, 2019, Hussein and Abdullah, 2020).
Genus Ficus belongs to family Moraceae. It has 850 species distributed worldwide and considered as one of the largest genus in angiosperm plants (Badgujar et al., 2014). F. carica is known medicinally as its leaves are used as sunscreen and chemoprotective agent whereas fruits and leaves are reported as anticancer and anti-acne, and latex has effect in viral titres and roots are used in treatment of ringworm. Almost all parts of F. carica have been reported with more than one medicinal values and almost every part reported to have anticancer activity against different cancer type (Badgujar et al., 2014, Camero et al., 2014: Salma et al., 2020). F. salicifolia is a native plant in United Arab Emirates (UAE) which is used traditionally against scorpion stings, bruises, skin and chest inflammation and cough etc. (Gushash, 2006). Latex is a white secretion from laticifer cells in more than 1000 species worldwide (Ghandehari and Fatemi, 2018). It is rich in secondary metabolites such as alkaloid polyphenol and hydrolytic enzymes, in addition to their reported anticancer and antimicrobial effects. F. carica latex has shown high toxicity toward cancers such as stomach (Hashemi et al., 2011),colorectal (Soltana et al., 2019) and cervical (Ghanbari et al., 2019) cancers.
Few studies are available on anti-cancer potential of the F. carica latex, however literature is silent about latex of F. salicifolia. Therefore, the current study was designed to evaluate the comparative anticancer potential of latex from both species of ficus. Furthermore, the study explored the antimetastatic and genotoxic effects of latex from both the ficus species and the molecular mechanisms involved in their anti-cancer effects using MDA-MB-231 cells as model.
2. Materials and methods
2.1. Latex ss collection
Leaves latex was collected from two species of ficus i.e. F. carica and F. salicifolia. The earlier was collected at a local nursery and the later was collected at Jais mountain, Ras al-Khaima, UAE. Leaves were removed one by one from each plant, and the latex was collected drop by drop in 1.5 mL sterile Eppendorf tubes and filtered using a 2 mm syringe filter and stored at 4 °C till future use
2.2. Experimental design
MDA-MB-231 cells were seeded with 10 × 103 cells/well density in 96 well plate. The cells were cultured in DMEM high glucose medium (Sigma) supplemented with 10%FBS (Sigma), 5% Pen/Strip (Sigma) and incubated at 37 °C with 5% CO2 and 90% humidity. The experiments were performed three times in triplicates using the following experimental design (Fig. 1).
-
1.
Untreated control group was selected separately for each 24, 48 and 72 h treatments
-
2.
MDA-MB-231 cells were treated with four different concentrations (0.1%, 0.25%, 0.5% and 1%) of the F. carica latex. The treatments were performed at three different time points (24, 48 and 72 h)
-
3.
MDA-MB-231 cells were treated with four different concentrations (0.1%, 0.25%, 0.5% and 1%) of the F. salicifoliao latex. The treatments were performed at three different time points (24, 48 and 72 h)
Fig. 1.
Flow chart representing the experimental design of the study.
2.3. Microscopic study
The MDA-MB-231 cells were carefully observed under phase contrast inverted microscope (Optika, Italy) for their shape, size and attachment as per the experimental design mentioned in Section 2.2. Representative wells from each treated group and untreated control of the cells were photographed at 20x magnification using digital camera (Optika, Italy) and saved using OptikalSview imaging software.
2.4. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
MTT assay was performed at 24, 48 and 72 h under four different (0.1%, 0.25%, 0.5% and 1%) treatments of the two ficus plants latices (Fig. 1). To carry out MTT assay, medium was removed as per scheduled mentioned in Fig. 1 and 100 µL of working MTT solution was added in dim light to each well and incubated for 4 h at 37 °C. Then, 200 µL of DMSO was added to each well and shacked gently for 15 min at room temperature in dark. The color of the wells turned violet and was read at 570 nm wavelength using ELISA microplate reader (Thermo Scientific™ Multiskan™ GO UV/Vis microplate spectrophotometer, USA). All the experiments were performed three times in triplicate and data are expressed as Mean ± SD.
2.5. Wound healing assay
MDA-MB-231 cells were seeded at the density of 2 × 105 cells/well in 6 wells plate and incubated at 37 °C with 5% CO2 and 90% humidity for 48 h and allowed the cells to be 90% confluent. A scratch was made using 200 µL sterile micropipette tip. After scratch, the medium was removed from the wells and were gently washed with DMEM (Sigma, USA) to remove the detached cells. The cells were treated with 3 different concentrations of both F. carica (0.05%, 0.025% and 0.01%) and F. salicifolia (0.1% 0.5% 0.25%) latices and untreated cells with scratch were used as control. The concentrations of the latices were selected based on their performance in MTT assay. At 0 h, three locations of the scratch were marked in each well and photographed under the phase contrast inverted microscope (Optika, Italy) using digital camera (Optika, Italy) at 10x magnification and saved for further analysis using OptikalSview imaging software. After 24 h the same locations were photographed again. The experiment was performed three times. The area of the wound was calculated using imageJ software and the area of the wound was quantified using the following formula:
A^(t) = A(t)/A(0) × 100%, where A(t) is the area at 24 h and A(0) is the area at 0 h.
The statistical significance was calculated and the data are represented in figure as %Mean ± SD.
2.6. Genotoxicity and cytotoxicity analysis
MDA-MB-231cells were seeded at the density of 8000 cells/well in 96 wells plate and incubated at 37 °C with 5% CO2 and 90% humidity for 24 h. The cells were treated with latex of F. carica (0.1%) and F. salicifolia (1%) and incubated at 37 °C with 5% CO2 and 90% humidity for further 24 h. The DNA damage assay was performed following the manufacturer’s protocol using HCS DNA Damage kit (Invitrogen, USA). Hoechst 33342 was used for nucleus staining and Image-iT® DEAD Green™ was used for cytotoxicity. The cells were observed and then photographed under phase contrast inverted microscope (Olympus, Japan) using 40x magnification and measured the light intensity using ImageJ software. The experiment was repeated three times in triplicate and data are shown as Mean ± SD.
2.7. Phospho-protein array analysis
MDA-MB-231 cells were seeded at the density of 2 × 105 cells/well in 6 wells plate and they were incubated at 37 °C with 5% CO2 and 90% humidity for 24 h to attached and re-gain their normal shape. After 24 h, the cells were treated with latex of F. carica (0.1%) in triplicates and the untreated cells were used as control and incubated for further 24 h in the same conditions mentioned above. After 24 h, the cells were washed with PBS (Sigma, USA) and trypsinized for 2 min at 37 °C and 1 mL of DMEM high glucose medium (Sigma, USA) was added to each well. The cells for each treated and control group were collected in separate 15 mL blue cap tubes and centrifuged at 1200 g at 4 °C for 10 min. The supernatant was discarded carefully, and the pellet was lysed in lysate buffer (R and D systems, Minnesota) and mixed well every 2 min for 5 times on ice. The suspension was centrifuged at 14,000g for 7 min and each supernatant was collected in new tube and kept in ice for further processing.
Proteins were quantified using DC Protein assay (BIO-RAD, USA) kit. The manufacturer’s protocol was followed with minor modifications.
Protein profile was studied using Human Phospho-kinase array kit (R and D System, Cat: ARY003B, Minnesota). In brief, the membranes were blocked with 1 mL of array buffer 1 per well and rocked for 1 h at room temperature. The blocking buffer was removed and 1 mL of the lysates were added to both membranes A and B and incubated at 4 °C overnight. The membranes were washed three times with washing buffer and 1 mL of diluted detection antibody cocktail A and B were added to the respective membranes A and cocktail B and incubated for 2 h at room temperature on rocking platform. Both the membranes were washed three time rigorously and 1 mL of Strepdavidin-HRP was added to each membrane and incubated for 30 min at room temperature on rocking platform. Both the membranes were washed three time for 10 min and then Chemi Reagent Mix was applied and read using ChemiDoc MP Imaging system (BIO-RAD, USA). The intensity of the dots were quantified using imageJ software.
2.8. Real time - polymerase chain reaction (RT-PCR)
In order to perform quantitative real time -polymerase chain reaction (RT-PCR), MDA-MB-231 cells were seeded in 6 well plates with the density of 2 × 105 cells/well and incubated for 24 h at 37 °C with 5% CO2 and 90% humidity to attach and re-gain their normal shape. The cells were divided into three groups i.e. untreated control, cells treated with leaves latex of F. carica (0.1%) and cells treated with F. salicifolia latex (1%). After treatment, the cells were incubated for 24 h and total RNA was extracted using RNeasy Mini kit (Qiagen, Germany) following manufacturer’s protocol. Complementary DNA (cDNA) was synthesized using Quantitect Reverse Transcription Kit (Qiagen, Germany) following manufacturer’s instructions. Real time PCR reaction was performed using Maxima SYBR Green/ROX using Rotor-Gene Q (Qiagen, Germany). The relative expression of the genes of interest was measured using 2–ΔΔCt method and the studied genes were normalized with housekeeping gene GAPDH. The list of the primers is given in Table 1.
Table 1.
Forward and reverse primers of studied genes.
| Gene Name | Forward | Reverse |
|---|---|---|
| GAPDH | ACGGATTTGGTCGTATTGGG | TGATTTTGGAGGGATCTCGC |
| ERK-2 | CATTATTCGAGCACCAACC | CTCTGAGGATCTGGTAGAG |
| CREB | CTGTAACGGTGCCAACTCC | GAATGGTAGTACCCGGCTG |
| AKT-2 | TGAAAACCTTCTGTGGGACC | TGGTCCTGGTTGTAGAAGGG |
2.9. Statistical analysis
For the cell viability assay, wound healing and genotoxicity ANOVA was used whereas for RT-PCR, students-test were used for calculation of statistical difference. The statistical difference was accepted at <0.05. The data are expressed as Mean ± SD.
3. Results
3.1. Latex of F. carica and F. salicifolia affect morphology of MDA-MB-231 cells
MDA-MB-231 cells are fibroblast-like shape. They have elongated spindle shaped morphology with cell–cell adhesion in addition to cellular crowding. The treated MDA-MB-231 cells with minimum concentration of F. carica treatment (0.1%) showed stress and apoptosis after 24 h. The cells began to lose their fibroblast morphology and their microvilli shape, in addition to cytoplasmic vacuolation, cell blebbing, round and uneven shape indicating apoptosis. At 0.25% concentration, the sphere shape of the cells was dominant, with small percentage of shrunk spindle shape. The dominancy of sphere shape continued at higher concentrations of 0.5% and 1% with more toxicity at 1% that exhibited almost no spindle shape. At 48 and 72 h the results were similar, yet spindle shape was absent from 0.1% in both time lines and cells shrunk and reduced in the plate (Fig. 2A, C).
Fig. 2.
The figure represents effects of (A) F. carica and (B) F. salicifolia latex on morphology of MDA-MB-231 cells after treatment with four different concentrations (0.1%, 0.25%, 0.5, 1%) and three time intervals (24 h, 48 h, 72 h). (C) Represents a zoom in for morphological changes caused by latex cell blebbing (c1, c5, c6), uneven cell shape (c2, c3), cytoplasmic vacuolation (c4).
F. salicifolia also exhibited similar effects on MDA-MB-231 cells morphology. At the lower concentration of 0.1% and lesser time point of 24 h, the cells presented a range of abnormal morphologies like cytoplasmic vacuolation, shrunk in spindle shape, sphere shrunk shape, cell blebbing and uneven cell shape, however the round cell shape was dominant at the higher doses of 0.5 and 1.0% at longer time points of 48 and 72 h (Fig. 2B, C).
3.2. Latex of F. carica and F. salicifolia inhibit proliferation of MDA-MB-231 cells
Treatment of MDA-MB-231 cells with different concentrations (0.1%, 0.25%, 0.5% and 1%) of leaves latex of F. carica showed reduction in cell proliferation after 24 h of treatment. The treated cells showed significant (p < 0.05) decrease in cell viability at all concentration of 0.1%, 0.25%, 0.5% and 1% at the three time points (24, 48 and 72 h) compared to untreated control. However, the trends in viability are dose and time dependent (Fig. 3A).
Fig. 3.
The figure represents the cytotoxic effects of F. carica and F. salicifolia latex on viability in MDA-MB-231 cancer cells. The MDA-MB-231 cells were treated with latex of F. carica and F. salicifolia at four different concentrations (0.1, 0.25, 0.5, 1%) and three time points (24 h, 48 h, 72 h). A and B represent dose and time dependency for each treatment separately. C, D and E represent comparative cytotoxic effects of the two species at the different doses at 24 h, 48 h and 72 h respectively. The data are presented as Mean ± SD and statistical significance is accepted at p < 0.05. The symbols (a) represent control, (b) represents 0.1% dose, (c) represents 0.25% dose, (d) represents 0.5% dose and (e) represents 1.0% dose.
Treatment of MDA-MB-231 cells with F. salicifolia also showed significantly (p < 0.05) reduced viability compared to the untreated control at all four doses and three time points (Fig. 3B). The trends showed time and dose dependency with few exceptions, which might be due to experimental errors. Furthermore, we compared the toxicity of both latices at the similar concentration and we found that F. carica exhibit significantly (p < 0.05) higher toxicity level compared to F. salicifolia at all doses and time points (Fig. 3C–E).
3.3. Latex of F. carica and F. salicifolia reduce metastasis in MDA-MB-231 cells
Metastasis is one of the vital characteristics of cancer cells. Using the scratch healing experiment, we found that both F. carica and F. salicifoliac exhibit significant (p < 0.05) reduction in cell migration. Treatment of cells with three concentrations (0.01%, 0.025%, 0.05%) of leaves latex of F. carica for 24 h showed 188%, 301% and 446% wound size respectively compared to untreated control. It shows that migration rate was dose dependently reduced by the latex of F. carica compared to untretaed control (100%). Treatment with F. salicifolia also inhibit metastatis. We found, 247%, 243% and 265% wound size compared to untreated control (100%) after 24 h at 0.25%, 0.5%, 1% respectively. The wound size is indirectly indicating the containing of the cells in their relevant areas. Our results clearly indicate a dose dependent anti-metastatic effect of treatment in both species (Fig. 4A–D).
Fig. 4.
The figure represents effect of F. carica and F. salicifolia latex on on the MDA-MB-231 cancer cells migration. MDA-MB-231 cells treated with (0.01%, 0.025%, 0.05%) of F. carica and with (0.25%, 0.5%, 1%) of F. salicifolia for 24 h. The representative photographs of wound size at 0 h and 24 h for both F. carica (A) and F. salicifolia (C) are given. Figure B and D represents the quantified relative wound area after treatments with F. carica and F. salicifolia latex treatments.
3.4. Latex of F. carica and F. salicifolia cause cytotoxicity and genotoxicity in MDA-MB-231 cells
FITCI and Hoechst 33342 stains are used to estimate levels of cytotoxicity and genotoxicity respectively by HCS DNA damage kit. We treated MDA-MB-231 cells with 0.1% leaves latex of F. carica and 1% leaves latex of F. salicifolia for 24 h. We found higher light intensity of FITCI stain in both treated cells compared to the untreated control (Fig. 5A). The light intensity was quantified using imageJ software and we found approximately two folds’ increase in cytotoxicity for both plants compared to the untreated controls (Fig. 5B). After analysing results of hoechst 33342 stains, we found healthier and homogamous nuclei in untreated control cells compared to cells treated with F. carica and F. salicifolia leaves latices (Fig. 5C). The untreated cells exhibit dividing morphology with metaphase and anaphase as shown with white arrows (Fig. 5C) whereas the treated cells exhibit a range of unhealthy morphological changes like nucleus blebbing, shrinkage, crescent shape (more abundant in F. carica). These abnormal morphologies indicate different apoptotic stages. The stain intensity of Hoechst 33342 was quantified using imageJ software and we found increase in both treated cells groups compared to the untreated controls (Fig. 5B).
Fig. 5.
The figure represents effects of F. carica and F. salicifolia latex on cytotoxicity and genotoxicity in MDA-MB-231 cells. The cells were treated with F. carica (0.1%) and F. salicifolia (1%) for 24 h. (A) represents cytotoxicity level in cells treated with F. carica and F. salicifolia latex and untreated cells using florescent microscopy and FTCI filter. (B) Represents quantified light intensity for FITC filter in all three groups. (C) Represents the genotoxicity of F. carica and F. salicifolia latex using florescent microscopy and DAPI staining. Red circles represent mitotic stages like metaphase and anaphase and white arrows represent stress morphologies like crescent shape, nucleus blebbing, uneven nucleus shape and chromatin condensation in addition to general nucleus shrinkage.
3.5. Effect of F. carica leaf latex on phospho-proteomic signature in MDA-MB-231 cells
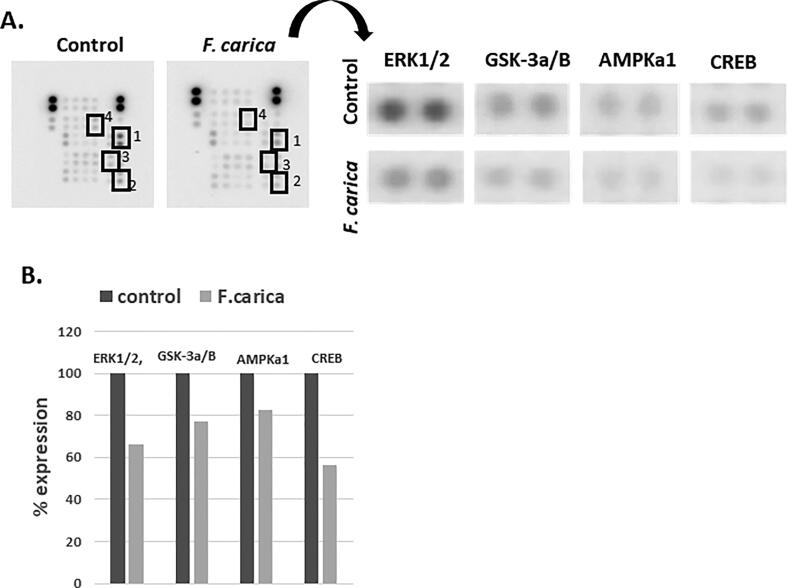
MDA-MB-231 cells were treated with 0.1% of F. carica leaf latex for 24 h and regulation of phosphoproteins and their associated signaling cascades mediation was studied using human phospho-kinase array kit. We found decreased expression of ERK (66%), CREB (56%), GSK-3α/β (77%) and AMPKa (82%) in cells treated with F. carica leaf latex compared to the untreated control (Fig. 6).
Fig. 6.
The figure shows effects of F. carica latex treatment on key proteins expression in MDA-MB-231 cells. (A) Represents difference in proteins expression and the proteins having difference in expression under ficus latex treatment compared to untreated controls are shown bound in squares. (B) Represents quantification of the proteins bound in square in A. The quantity was normalized with standard proteins expression.
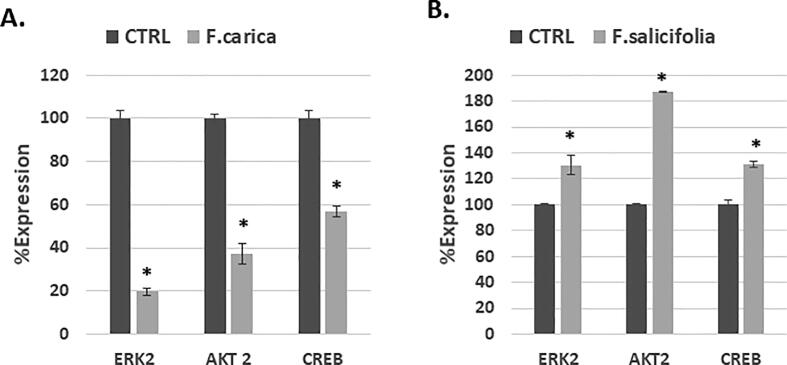
3.6. F. carica leaf latex treatment decreases whereas F. salicifolia leaf latex increases expression of ERK, CREB and AKT at transcriptional level
At transcriptional level, F. carica leaf latex (0.1%) caused decreased expression of ERK, CREB and AKT in MDA-MB-231 cells after treatment for 24 h confirming results of protein expression. However, F. salicifolia showed an increased expression in all of the mentioned genes by 0.4 fold for CREB, 0.25 for AKT2 and 0.3 fold for ERK2 at 1% of treatment. ERK2, CREB and AKT all play a critical role in both cell proliferation and cells attachment which result in affecting cell viability (Fig. 7).
Fig. 7.
Expression of ERK2, AKT2, CREB at transcriptional level after treatment with F. carica and F. salicifolia for 24 h RT-PCR was used to study the expression level of ERK2, AKT2 and CREB after they treated with (A) F. carica and (B) F. salicifolia.
4. Discussion
In this study we reported effects of F. carica and F. salicifolia latex on MDA-MB-231 cells. Effects of F. carica latex against stomach cancer (Hashimi et al,. 2011), colorectal cancer (Soltana et al., 2019) and cervical cancers (Ghanbari et al., 2019) are reported in earlier studies. However, lesser information is available about the involved molecular mechanism. F. salicifolia, a native plant to UAE and close relative of the F. carica, is unexplored regarding its anti-cancer potential. Therefore, we conducted a comparative study on anticancer potential of the two ficus species.
Both F. carica and F. salicifolia latex were screened for their anti-proliferative potential in MDA-MB-231 cells using MTT assay at different time points and concentrations. We found significant decrease in the cellular proliferation with latex treatment from both species. Earlier studies have also reported the antiproliferative effects of F. carica latex against cells derived from esophageal cancer and stomach cancer separately using MTT assay (Hashemi et al., 2011, Hashemi and Abediankenari, 2013). Furthermore, seeds, fruits, and leaves extract of F. carica have also been reported to be cytotoxic against HeLa, A549, BT549, MCF-7 and MDA-MB-231 cell lines (Felimban, 2016, Zhang et al., 2018). We report the anti-cancer potential of F. salicifolia for the first time as per our expectations due to its close relation with F. carica. As this plant is native to UAE and is adopted to extreme climatic conditions making it a good candidate for further studies. Anticancer effects were expected due to presence of phytochemicals like 6-O-acyl-b-d-glucosyl-b-sitosterols in F. carica latex (Mawa et al., 2013).
We found consistent changes in cell shaped after treatment with the latex of both ficus species. The microscopic analysis supported the MTT results, where the cells showed morphological changes such as round shape and shrinkage, which indicated stoppage of proliferation and turning on apoptosis (Khorsandi et al., 2020). Although, round shape was the dominant morphology, however, other cell shapes like cell blebbing, cytoplasmic vacuolation, uneven cell structure and distribution were also found, confirming apoptosis (Häcker, 2000, Povea-Cabello et al., 2017). Latex of other ficus species like F. bengalensis, F. exasperate and F. pseudopalma showed similar findings in breast cancer MDA-MB-231 cells, ovarian cancer A2780 cells and human prostate cancer PRST2 cells respectively (De Las Llagas et al., 2014, Bafor et al., 2017, Tulasi et al., 2018).
Metastasis is one of vital characteristics of triple negative breast cancer cells. We found anti-metastatic effects in MDA-MB-231 cells after treatment of latex from both species compared to untreated control using wound healing scratch assay. We found that both F. salicifolia and F. carica have antimetastatic effects. Other studies found similar effects of bark and leaves extracts from F. umbellate vahl in MDA-MB-231 cells and F. hispida Linn in HT-29 cells respectively (Silihe et al., 2017, Zhang et al., 2018). It seems difficult to conclude from this comparison any molecular clue, however it shows that the molecules involve in anti-metastatic effects might be shared in latex, leaves and bark of different species of ficus.
The above antiproliferative and cytotoxic effects were further confirmed by DNA damage assay. We found that both treatments were supporting the MTT assay findings and microscopic analysis. DNA damage is previously reported under latex treatment in glioblastoma hepatocellular cells (Wang et al., 2008).
In order to know about any clue to the molecular signatures, we treated MDA-MB-231 cells with F. carica latex and human kinase array kit was used to know the effects of Ficus latex on a list of kinases or their substrates involved in cancer. We found reduced expression of ERK2, CREB, GSK-3α/β and AMPKa with F. carica latex treatment. ERK2 and CREB are actively involved in cellular proliferation processes and were further confirmed using RTPCR. Though Akt expression in the kinase array kit was reduced only up to 13%, however being a key player in cell attachment (Li et al., 2016), we studied its expression at transcriptional level. Literature states that downregulation of both ERK and Akt pathways together indicates aggressive toxicity (Li et al., 2016). This might explain the intense toxicity of F. carica in MDA-MB-231 cells even at very low dose of 0.1%. Furthermore, Akt pathway is well known for its roles in cell attachment and movement, therefore the cells with downregulated Akt will lose their attachment and ability to move. This support our above results in wound healing and morphological studies. Studies show that both ERK and AKT pathways seems to be involved in anoikis, a form of program cell death due to detachment in anchorage dependent cells (Paoli et al., 2013). Usually cancer cells avoid this by overexpression of some genes such as ERK and AKT and can result in metastasis in cells like MDA-MB-231 cells (Yu et al., 2009, Palorini et al., 2016). This also support the idea that F. carica might cause anoikis and reduce metastasis after it downregulated both ERK and Akt pathways. Unexpectedly, the cells treated with latex of F. salicifolia showed increased expression of ERK, CREB and Akt at transcriptional level. This is confirming that although many of the anti-cancer effects of both ficus species are similar, however both use different molecular mechanisms to show their effects.
5. Conclusions
In conclusion, leaves latex of F. carica and F. salicifolia share anticancer potential in MDA-MB-231 cells showing antiproliferative and anti-metastatic effects along with significant effects on cell shape. However, both plants latex uses different molecular mechanism of action. Further studies are needed to unravel the details of the involved molecular mechanisms.
Funding
This research is funded partially by Office of Research and graduate studies, University of Sharjah through grant number 1702145049-P and partially by college of graduate studies, University of Sharjah.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Fatma Mousa AlGhalban, Email: u16101515@sharjah.ac.ae.
Amir Ali Khan, Email: amkhan@sharjah.ac.ae.
Muhammad Nasir Khan Khattak, Email: mnasir@sharjah.ac.ae.
References
- Badgujar S.B., Patel V.V., Bandivdekar A.H., Mahajan R.T. Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharm. Biol. 2014;52(11):1487–1503. doi: 10.3109/13880209.2014.892515. [DOI] [PubMed] [Google Scholar]
- Bafor E.E., McKenna J., Rowan E.G., Edrada-Ebel R. Characterisation of the antiproliferative constituents and activity of Ficus exasperata (Vahl) on ovarian cancer cells–a preliminary investigation. Nat. Prod. Res. 2017;31(18):2164–2168. doi: 10.1080/14786419.2016.1277348. [DOI] [PubMed] [Google Scholar]
- Camero M., Marinaro M., Lovero A., Elia G., Losurdo M., Buonavoglia C., Tempesta M. In vitro antiviral activity of Ficus carica latex against caprine herpesvirus-1. Nat. Prod. Res. 2014;28(22):2031–2035. doi: 10.1080/14786419.2014.918120. [DOI] [PubMed] [Google Scholar]
- Chavez K.J., Garimella S.V., Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32(1–2):35. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collignon J., Lousberg L., Schroeder H., Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer: Targets Therapy. 2016;8:93. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Llagas M.C., Santiago L., Ramos J.D. Cytotoxicity and apoptotic activity of Ficus pseudopalma blanco leaf extracts against human prostate cancer cell lines. Trop. J. Pharm. Res. 2014;13(1):93–100. [Google Scholar]
- Felimban A.I. Tennessee State University; 2016. Cytotoxic activity of Lepidium sativum and Ficus carica extracts on four tumor cell lines Doctoral dissertation. [Google Scholar]
- Ghanbari A., Le Gresley A., Naughton D., Kuhnert N., Sirbu D., Ashrafi G.H. Biological activities of Ficus carica latex for potential therapeutics in human papillomavirus (HPV) related cervical cancers. Sci. Rep. 2019;9(1):1–11. doi: 10.1038/s41598-018-37665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandehari F., Fatemi M. The effect of Ficus carica latex on 7, 12-dimethylbenz (a) anthracene-induced breast cancer in rats. Avicenna J. Phytomed. 2018;8(4):286. [PMC free article] [PubMed] [Google Scholar]
- Hashemi S.A., Abediankenari S., Ghasemi M., Azadbakht M., Yousefzadeh Y., Dehpour A.A. The effect of fig tree latex (Ficus carica) on stomach cancer line. Iran. Red Crescent Med. J. 2011;13(4):272. [PMC free article] [PubMed] [Google Scholar]
- Gushash A.S. first ed. Sarawat Designer and Printers; Saudi Arabia: 2006. Plants in the Mountains of Sarat and Hejaz. Madinah. [Google Scholar]
- Häcker G. The morphology of apoptosis. Cell Tissue Res. 2000;301(1):5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- Hashemi S.A., Abediankenari S. Suppressive effect of fig (Ficus carica) latex on esophageal cancer cell proliferation. Acta Facultatis Medicae Naissensis. 2013;30(2):93–96. [Google Scholar]
- Hashemi S.A., Abediankenari S., Ghasemi M., Azadbakht M., Yousefzadeh Y., Dehpour A.A. The effect of fig tree latex (Ficus carica) on stomach cancer line. Iran. Red Crescent Med. J. 2011;13(4):272. [PMC free article] [PubMed] [Google Scholar]
- Hassan A., Ullah H. Antibacterial and antifungal activities of the medicinal plant veronica biloba. J. Chem. 2019;2019 [Google Scholar]
- Huang L., Li A., Liao G., Yang F., Yang J., Chen X., Jiang X. Curcumol triggers apoptosis of p53 mutant triple-negative human breast cancer MDA-MB 231 cells via activation of p73 and PUMA. Oncol. Lett. 2017;14(1):1080–1088. doi: 10.3892/ol.2017.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein H.A., Abdullah M.A. Anticancer compounds derived from marine diatoms. Mar. Drugs. 2020;18(7):356. doi: 10.3390/md18070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorsandi K., Kianmehr Z., Hosseinzadeh R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020;20(1):1–14. doi: 10.1186/s12935-020-1100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A., 2018. A review article on breast cancer. Ijppr.humanjournals.com. Available at: <http://ijppr.humanjournals.com/wp-content/uploads/2018/02/25.Biplob-Dey-Arun-Kumar.pdf> [Accessed 4 November 2020].
- Li L., Zhao G.D., Shi Z., Qi L.L., Zhou L.Y., Fu Z.X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016;12(5):3045–3050. doi: 10.3892/ol.2016.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawa, S., Husain, K., & Jantan, I. (2013). Ficus carica L. (Moraceae): phytochemistry, traditional uses and biological activities. Evid.-Based Complement. Alternative Med. 2013. [DOI] [PMC free article] [PubMed]
- Mehanna J., Haddad F.G., Eid R., Lambertini M., Kourie H.R. Triple-negative breast cancer: current perspective on the evolving therapeutic landscape. Int. J. Women's Health. 2019;11:431. doi: 10.2147/IJWH.S178349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq S., Abbasi B.H., Uzair B., Abbasi R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018;17:420. doi: 10.17179/excli2018-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palorini R., Votta G., Pirola Y., De Vitto H., De Palma S., Airoldi C., Rizzi R. Protein kinase A activation promotes cancer cell resistance to glucose starvation and anoikis. PLoS Genet. 2016;12(3) doi: 10.1371/journal.pgen.1005931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli, P., Giannoni, E., Chiarugi, P., 2013. Anoikis molecular pathways and its role in cancer progression. Biochimica et Biophysica Acta (BBA)-Mol. Cell. [DOI] [PubMed]
- Povea-Cabello S., Oropesa-Ávila M., la Cruz-Ojeda D., Villanueva-Paz M., De la Mata M., Suárez-Rivero J.M., Sánchez-Alcázar J.A. Dynamic reorganization of the cytoskeleton during apoptosis: the two coffins hypothesis. Int. J. Mol. Sci. 2017;18(11):2393. doi: 10.3390/ijms18112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salma S., Shamsi Y., Ansari S., Nikhat S. Ficus Carica L.: a panacea of nutritional and medicinal benefits. Cellmed. 2020;10(1) 1 1. [Google Scholar]
- Silihe K.K., Zingue S., Winter E., Awounfack C.F., Bishayee A., Desai N.N., Honorine Riwom S. Ficus umbellata vahl. (moraceae) stem bark extracts exert antitumor activities in vitro and in vivo. Int. J. Mol. Sci. 2017;18(6):1073. doi: 10.3390/ijms18061073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltana H., Pinon A., Limami Y., Zaid Y., Khalki L., Zaid N., Hammami M. Antitumoral activity of Ficus carica L. on colorectal cancer cell lines. Cell. Mol. Biol. 2019;65(6):6–11. [PubMed] [Google Scholar]
- Tulasi C.D.S.L.N., Narasu M.L., Saida L. Cytotoxic effect of Ficus religiosa and Ficus benghalensis latex extracts on MCF-7 cell line. Int. J. Sci. Res. Biol. Sci. 2018;5:6. [Google Scholar]
- Varghese E., Samuel S.M., Abotaleb M., Cheema S., Mamtani R., Büsselberg D. The “yin and yang” of natural compounds in anticancer therapy of triple-negative breast cancers. Cancers. 2018;10(10):346. doi: 10.3390/cancers10100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang X., Jiang S., Lin P., Zhang J., Lu Y., Yang H. Cytotoxicity of fig fruit latex against human cancer cells. Food Chem. Toxicol. 2008;46(3):1025–1033. doi: 10.1016/j.fct.2007.10.042. [DOI] [PubMed] [Google Scholar]
- Yu, J., Han, W., Kim, J., Lee, J., Ko, E., Kim, E., Noh, D., 2009. Anoikis-resistant MDA-MB-231 cells: characteristics and pathway analysis.
- Zhang Y., Wan Y., Huo B., Li B., Jin Y., Hu X. Extracts and components of Ficus carica leaves suppress survival, cell cycle, and migration of triple-negative breast cancer MDA-MB-231 cells. OncoTargets Therapy. 2018;11:4377. doi: 10.2147/OTT.S171601. [DOI] [PMC free article] [PubMed] [Google Scholar]