Abstract
Background
The incidence of the 2020 COVID-19 epidemic in Africa seems to be different from that of the rest of the world, however its true extent is probably underestimated. Conducting population based sero-surveys during the epidemic has moreover been extremely challenging, driving our group and others to study blood donor samples.
Methods
We collected regional epidemiological COVID-19 surveillance data, and simultaneously monitored anti-SARS-CoV-2 antibody seroprevalences monthly throughout the epidemic in 5 major Region-associated Blood Transfusion Centres of Madagascar over a period of 9 months.
Findings
Soon after attaining the first epidemic peaks between May and August 2020, both crude and population-weighted test-performance-adjusted seroprevalences of anti-SARS-CoV-2 antibodies was in Malagasy blood donors rapidly increased up to over 40% positivity.
Interpretation
These findings suggest a high cumulative incidence of infection and seroconversion, which may have contributed to the observed deceleration of infection rates, but was not sufficient to prevent the second epidemic wave that struck Madagascar in Spring 2021.
Funding
This project was funded by the United States Agency for International Development.
Keywords: Madagascar, SARS-CoV-2 Seroprevalence, Blood donors
Research in Context.
Evidence before this study
We searched PubMed for research articles published from database inception until May 10th, 2021, with no language restrictions, using the terms “SARS-CoV-2”and “seroprevalence”. Seven hundred and twenty-seven peer-reviewed publications were available. Many described serosurvey results and meta-analysis that estimate regional seroprevalence rates in humans of SARS-CoV-2 antibodies. Only 27 publications were available when adding “Africa” to the search terms. None of these articles described simultaneous epidemiological COVID-19 surveillance data, and anti-SARS-CoV-2 antibody seroprevalences monitoring throughout an entire epidemic wave (before, during and after).
SARS-CoV-2 antibodies’ seroprevalences have been reported in November 2020 to reach over 40% in one province of Northern Italy and in Manaus, Brazil. As for Africa, S. Uyoga et al. recently described that SARS-CoV-2 exposure of blood donors in Kenya was more extensive (around 10%) than indicated by case-based surveillance and closer to cumulative incidence of infection.
Added value of this study
We report for the first time the seroprevalences of anti- SARS-CoV-2 IgG antibodies amongst blood donors in Madagascar during the outbreak and how they sharply increased from 0% to 40%. This sudden increase was associated with a concomitant reduction in the virus circulation. This reproductible pattern was found in all 5 investigated regions of the country and 5 major cities amongst which its capital Antananarivo, home to 1,275,000 inhabitants. What seemed to be protective immunity however did not last and Madagascar has since undergone a second major epidemic wave.
Implications of all the available evidence
Simultaneous analysis of epidemiological COVID-19 surveillance data and anti-SARS-CoV-2 antibody seroprevalences monitoring throughout the entire epidemic remain paramount in understanding the dynamics of the pandemic. This is all the more true in countries where containment and lockdown measures could not be applied, and considering the high proportion of asymptomatic COVID-19 cases. Results of such studies will be useful for authorities to guide public health decisions.
Alt-text: Unlabelled box
1. Introduction
As of February 2021, more than one hundred million people have been infected with SARS-CoV-2 worldwide, and over 2.3M deaths have been reported, 2.7M cases and 68,487 deaths in Africa. Despite available data suggesting that the incidence of COVID-19 in Africa is different from that of the rest of the world the extent of this epidemic is probably underestimated [1].
On March 19th 2020, Madagascar identified its first case of COVID-19. Restrictions across the country were quickly adopted on March 21st in order to slow the epidemic down, simultaneously however limiting the conduct of population-based surveys. Despite these restrictions, Madagascar has experienced the epidemic in different locations, particularly in Toamasina (April-June 2020) and Antananarivo (June-August 2020). The state of health emergency was ended October 18th, and a series of relief measures was gradually implemented.
In a mixed context of aging immunity and new variants circulation, Madagascar was struck once again by an epidemic wave in Spring 2021. The Ministry of Health in Madagascar notified as of February the 15th 2021, 38,874 confirmed cases and 716 deaths linked to COVID-19 [1].
In Europe, the first epidemic wave would have affected around 5% of the population in Spain [2] and 4.4% in France and up to 10% in certain regions [3].
Anti-SARS-CoV-2 antibody detection makes it possible to define cumulative incidence of infection, confirming both symptomatic and asymptomatic infections. However due to difficulties to conduct of population based sero-surveys during the epidemic, several countries have monitored seroprevalences in blood donors [4,5]. Kenya has reported 5.2% seropositivity for antibodies to SARS-CoV-2, with the peaks up to almost 10% observed in the 3 highly urbanised regions. In the United States, the seroprevalence was monitored in dialysis patients and estimated in July at 9.3%, with peaks observed at 33.6% in New York City, an epidemic hotspot [6]. However, unlike in most European countries, blood donors in Madagascar are often family members or acquaintances of those in need of blood thus may come from different socio-economic and socio-cultural backgrounds of the general population.
As soon as the circumstances allowed us to do so (March for Antananarivo capital city, and from June for regions out of Antananarivo), serum samples were collected monthly until November 2020 in 5 of the country's Regional Blood Transfusion Centres (RBDCs) (Supplementary Figure S1) and analysed for the presence of anti-SARS-CoV-2 IgGs (anti-N) using a highly specific and sensitive ELISA kit. This serological data was then compared to COVID-19 epidemiological surveillance data (See materials and methods section) collected by the Ministry of Public Health for the corresponding regions.
2. Methods
2.1. Sample collection
Plasma or serum samples were collected in 5 out of 7 Regional Blood Transfusion Centres (RBTCs) of Madagascar for which it was possible to collect samples throughout the epidemic, and despite drastic travel restrictions. These 5 centres are localised in 5 out of 6 most populated cities of Madagascar, 36% of the Malagasy population lives in these 5 Regions (Supplementary Figure S1). The samples used come from venous blood samples intended for different quality control steps (blood counts, HIV and HCV serologies) from blood bags collected in blood banks and then for destruction.
Samples were collected twice a month from March to November at RBTC Antananarivo, and from June to November at RBTC Toamasina, Fianarantsoa, Toliara and Mahajanga. For each sample, the blood donor's gender, age, occupation and date of collection were collected.
Plasma samples from Antananarivo were aliquoted at the Joseph Ravoahangy Andrianavalona Hospital (HJRA) in 1mL cryotubes, then stored at -20°C, and analysed at the Infectious Diseases Immunology Unit of the Pasteur Institute of Madagascar.
Plasma samples from the provinces were aliquoted in RBTCs, shipped at a positive temperature between 0 and 4°C in 1mL cryotubes, then stored at -80°C until analysis at the Immunology of Infectious Diseases Unit of the Institut Pasteur in Madagascar.
Duplicates as well as samples from donors under the age of 18 were eliminated from the study. All Collected samples were analysed.
2.2. Serology
Semi-quantitative indirect ELISA for detection of anti-SARS-CoV-2 IgG – The ELISA kit used, ID Screen® SARS-CoV-2-N IgG Indirect ELISA (ID.Vet, Grabels, France) of semi-quantitative type, demonstrates the antibodies (IgG) directed against the nucleocapsid (N) of the SARS -CoV-2 in human serum or plasma.
Briefly, and following the manufacturer's instructions the samples to be tested and the controls were distributed in wells of the microplate coated with a purified recombinant antigen of the N protein. After washing, a protein G conjugate labelled with peroxidase (HRP), recognising total human IgGs (IgG1, IgG2, IgG3, IgG4), but not binding to IgA, nor to IgM and IgE, was distributed in the wells, forming an antigen-antibody-conjugate-HRP complex. After removing the excess conjugate and washing, the developing solution (TMB) was added. The reaction was then stopped and the microplate is read at a wavelength of 450 nm.
According to the manufacturer, the kit used has a specificity of 99.8% (95% CI 99.3 - 99.9) and a sensitivity of 95.2% (95% CI 95.2 - 100) (Sensitivity study performed on 41 hospitalised RT-PCR positive samples >15 days after first symptom presentation. Specificity study performed on 1247 French Blood donor samples collected before 2017. S/P threshold 40%) [7].
According to internal studies, the kit used has a specificity of 99.6% (95% CI 99.1- 99.8), and a sensitivity of 92.6% (95% CI 76.6- 98.7). The sensitivity study was performed on 27 serum samples from hospitalised patients with positive SARS-CoV-2 RT-PCR, collected more than 28 days after onset of symptoms. Specificity study was performed on 1,251 Blood donor samples collected before the COVID-19 epidemic. S/P threshold of 40% was used according to manufacturer instruction.
Results interpretation – For each sample, the “S / P” “Sample / Positive control” ratio, expressed as a percentage (S/P%) was calculated with the formula:
S/P=DOsample-DOCTRL-/DOCTRL+-DOCTRL-
The negative control used contains a matrix based on bovine serum albumin, while the positive control of human origin, contains anti-SARS-Cov-2 IgG in a standardised quantity.
Samples showing an S/P:
-
-
Below 40% are considered negative.
-
-
Greater than or equal to 40% were considered positive.
Relative levels of anti-SARS-Cov-2 IgGs from samples considered positive are S/P values of samples greater than or equal to 40%.
2.3. Epidemiological surveillance data
The monthly seroprevalence rates observed in each of the RBTC were compared with the epidemic dynamics described, in each administrative region to which the RBTC belongs, by the number of cases confirmed by SARS-CoV-2 RT-PCR and the positivity rate (number of confirmed cases by SARS-CoV-2 RT-PCR / numbers of SARS-CoV-2 RT-PCRs performed).
All the surveillance data for the Analamanga (Antananarivo), Haute Matsiatra (Fianarantsoa), Atsinanana (Toamasina), Boeny (Mahajanga) and Atsimo Andrefana (Toliara) regions were obtained from the Department of Health Watch, Epidemiological and Response Surveillance (DVSSER): number of SARS-CoV-2 RT-PCRs performed by four laboratories for the whole country, number of confirmed cases by SARS-CoV-2 RT-PCR and RT-PCR positive rate (Case confirmation rate).
2.4. Statistical analysis and modeling
To allow direct standardisation of estimated seroprevalences in RBTC donors, we tabulated seropositive results by gender, age and region, because the study was a non-random sample of the population. We then calculated standardised prevalence estimates [8] using population data from the 2018 Malagasy Population and Housing Census [9]. We used direct standardisation on the observed seroprevalence and population weights in 250 gender-age-month-CRTS strata: gender (n=2), age (n=4), month and CRTS (n=5). Some age groups or genders may not have investigated at certain months in specific RBTCs due to low sample size. Only investigated age and gender groups were considered for direct standardisation, Bayesian multilevel regression modeling, and post-stratification.
Monthly prevalence estimates calculated using multilevel regression and post-stratification (MLRP) were used to account for differences in the sample and the regional populations [4]. To predict stratum (age-gender) prevalence for MLRP we fitted a Bayesian logistic regression model by month using the rjags package (version 4.3.0) in R, that included gender as a fixed effect, and age as a random effect; except for Mahajanga in September when only the 18-24 age group was represented. A fixed effect model on gender was then fitted. We used vague or weakly informative priors for all parameters [4,10].
Analysis was conducted in R (R Core Team, 2014) URL http://www.R-project.org/ or using GraphPad Prism version 8.0. Statistical tests are mentioned, confidence intervals calculated using Wilson/Brown method.
2.5. Ethics
This study was authorised by the Ministry of Health of Madagascar and by the BioMedical Research Ethics Committee (CERBM) that informed the investigators it required neither the approval of the committee nor specific donor consent (CERBM: IOR0000851) (Authorisation N°205-MSANP/SG/AGMED/CERBM). Blood donors are advised their blood may be tested for surveillance purposes.
2.6. Role of funders
This project was funded by the United States Agency for International Development (USAID) cooperation agreement 72068719CA00001 and supported by the Institut Pasteur de Madagascar, and by the French Ministry for Europe and Foreign Affairs through the REPAIR Covid-19-Africa project coordinated by the Pasteur International Network association. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID and the United States Government.
3. Results
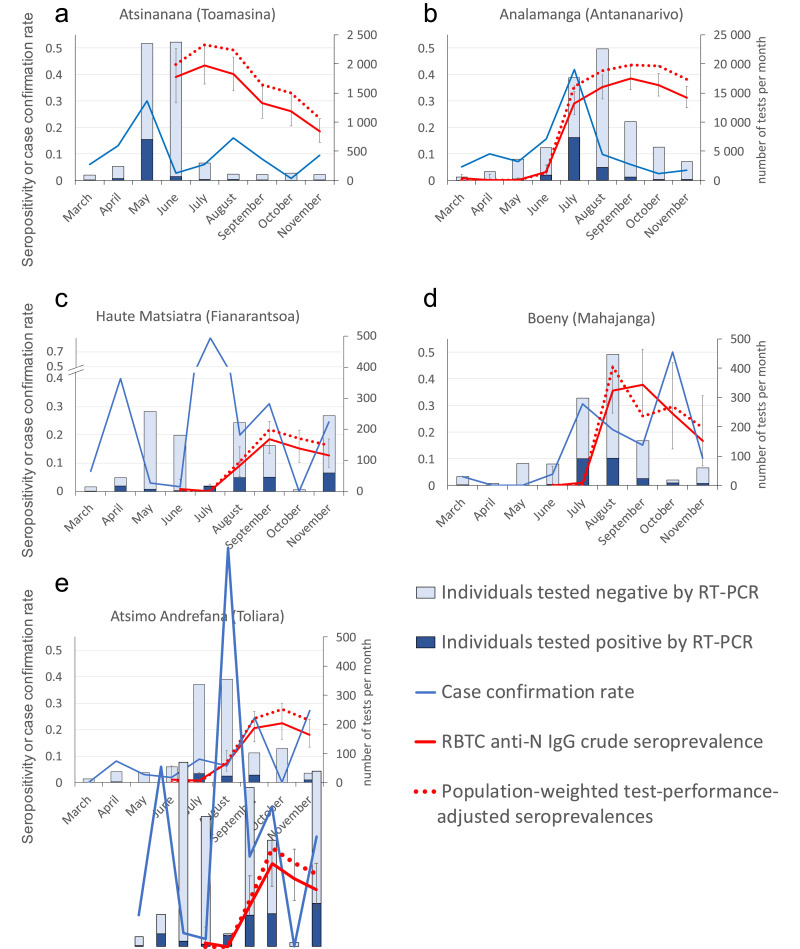
The first epidemic peak took place in May in the Atsinanana region, case confirmation rates reaching 30%. The Analamanga region (including Antananarivo) subsequently reached its peak in July. Eighty two percent of cases were confirmed between July and August (n=9,570) and case confirmation rate reached 41.8% in July. The Boeny region was also reached in July (case confirmation rate 30.5%) and Haute Matsiatra region and Atsimo Andrefana were reached early August (respectively 60 cases and 52 cases diagnosed in July and August). (Fig. 1 Table S1).
Fig. 1.
Monthly anti-SARS-CoV-2 IgG seropositivity in blood donors (crude seroprevalences and Bayesian model population-weighted and manufacture test-performance-adjusted) and epidemiological indicators in regions of Madagascar; a – Atsinanana, b – Analamanga, c – Haute Matsiatra, d – Boeny and e – Atsimo Andrefana. Light blue bars: COVID-19 suspected individuals tested negative for SARS-CoV-2 by RT-PCR; Dark blue bars: COVID-19 suspected individuals tested positive for SARS-CoV-2 by RT-PCR; Blue line: SARS-coV-2 case confirmation rate; Red line: anti-N IgG crude seroprevalence among RBTC collected samples (95% confidence interval); Dotted red line: population-weighted test-performance adjusted seroprevalences (95% confidence interval). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Toamasina RBTC sample collection was initiated in June 2020. Samples were 39.0% positive for anti-SARS-CoV-2 antibodies, then increased to 43.4% in July and stabilised around 40% in August, before decreasing in September to 29.1%, 26.1% in October and 18.4% in November (Fig. 1, Table S2 Panel a).
Antananarivo samples had very low seroprevalences (mean 0.4%) from March to May (n=1,251), then started to increase reaching 38.5% in September (n=483) (Fig. 1 Table S2 Panel a). As among Toamasina blood donors, a seroprevalence slight decrease was observed in October and November (down to 31.3%).
In Mahajanga, Fianarantsoa and Toliara RBTCs, the first samples collected in June and July showed no to low seropositivity then sharply increased in Mahajanga to 35.5% (n=107) in August (stabilising at 37.7% in September). Toliara and Fianarantsoa however showed a milder increase stabilising around 20% in September. Seroprevalences then decreased in Mahajanga and Fianarantsoa and seemed to stabilise just around 20% in Toliara, suggesting residual virus circulation in later months (Fig. 1 Table S2 Panel a).
Compared with the 2018 Malagasy Population and Housing Census [9], our participants were older (mean age 32 yo versus 22 yo in the census) and more commonly males (80.2% males in our study versus 49.3% in the census) (Supplementary Table S3). Only Mahajanga showed mild differences when performing bivariate seropositivity analysis according to the demographic characteristics of the population and the month of sample collection in the 5 RBTCs from March to November 2020 (Supplementary Table S4).
Nevertheless, we adjusted the prevalence estimates for the demographics of the sample using poststratification, and for the sensitivity and specificity of the test as described by Uyoga S. et al. [4]. Population-weighted and test-performance-adjusted seroprevalence in Antananarivo was highest in September (43.5% vs crude 38.5%), reached 51.2% in Toamasina in July (vs crude 43.4), 21.9% in Fianarantsoa in September (vs crude 18.5%), 27.7% in Toliara in October (vs crude 22.3%) and 44.5% in August in Mahajanga, whereas the crude peak only happened in September (37.7%). It is noteworthy however that only the 18-24 age group was represented in Mahajanga in September, and that a fixed effect model on gender was thus fitted (Supplementary Table S2 Panel a, b, c).
Test-performance-adjusting using in-house calculated specificity (99.6%, 95% CI 99.1- 99.8) and sensitivity (92.6%, 95% CI 76.6- 98.7), had very little effect when compared to manufacture values: Mean values of peak seroprevalences in the 5 RBTCs were 32.1% (crude seroprevalence), 35.7% (Standardised, population-weighted, manufacture test-performance-adjusted), 36.6% (Standardised, population-weighted, in-house test-performance-adjusted) and 37.8% using the Bayesian model (population-weighted and manufacture test-performance-adjusted) (Supplementary Table S2 Panels a, b, data not shown and c).
4. Discussion
Blood donors represent a simple, accessible and inexpensive means of collecting samples from an adult (over 18 yo) population. SARS-CoV-2 hospitalised patients do not commonly require blood transfusion. Blood donor exposure is thus not particularly biased in this respect. Young male samples are however over-represented, most importantly in Tulear (92.2%) and Toamasina (90.1%) due to the conditions associated with donating blood (blood donation is only allowed after a non-donation period of 3 months for males and 4 months for females. The donor must have a weight greater than 50 kg, be non -pregnant, -breastfeeding, -menstruation -use of implant or injectable contraceptive).
Several factors may affect the accuracy of our seroprevalence estimates. Half of the population of Madagascar is outside the age range sampled in this study (Age and gender distribution of RBTC sampled population in comparison with the overall age-sex distribution of the regions’ adult population is represented in Supplementary Fig. S2), and the seroprevalence in children, that represent 50% of the Malagasy population [9], and older adults is thought to be low [2]. Moreover, the more prudent fraction of the population avoiding exposure may not be properly represented in our sampled population. However, our sampling may as well be underestimating seroprevalences as potential donors are excluded from giving blood if they have not been healthy for at least a few weeks. Last, antibodies specific to the nucleocapsid antigen are known to disappear early [11,12], resulting in low seroprevalences only a few month after exposure. Our analysis is consequently not entirely representative, even after population-weighting and test-performance-adjusting.
An important indicator during an epidemic is the number of confirmed cases. This is not absolutely relevant in Madagascar, where the number of tests performed is not large enough to reflect the true incidence of the disease. Indeed, the strategy in Madagascar was at first to detect early virus circulation. It then evolved with the epidemic to test predominantly people coming to hospitals to be diagnosed and obtain medical care consequently testing distinct populations. Case confirmation rates thus appears to be the best indicator of the epidemic dynamics, provided that the number of tests carried out monthly is sufficiently large, as it is the case in Antananarivo and Toamasina. Regions such as Haute Matsiatra, Boeny and Atsimo Andrafana did not show stable testing capacity resulting in surprisingly variable case confirmation rates from one month to the other (Fig. 1 Panels c, d and e). Whatever the setting however, seroprevalences increase after the first notified cases and reach a plateau, providing good indicators for both cumulative incidence and progression during the epidemic.
Correcting seroprevalences using either population-weighted, test-performance direct standardisation or the Bayesian model generated seroprevalences extremely close to crude values. The maximal seroprevalence of anti-SARS-CoV-2 antibodies fluctuates around 40% among blood donors from Toamasina, Antananarivo and Mahajanga, which is at least 4-fold higher than the post-epidemic seroprevalences described elsewhere in particular in Kenya, whose blood donors are as in Madagascar predominantly males aged less than 45 years. This high rate suggests a greater spread of the virus in Madagascar and is likely linked to an incomplete observance by the population of restrictions imposed by the health authorities due to difficult economic conditions in certain populational categories. Assuming seroprevalences in the whole population of the 5 regions (9.5M inhabitants, 37% of the country's population) were identical to those from their paired blood donors, which is certainly not the case, as only 20-25 % of regions’ population live in these 5 regional capital cities, we estimated 3.6M individuals (900 000 if considering the Capital cities only) would have been infected by SARS-CoV-2, in comparison to 12,930 notified cases reported during the study period in these regions.
The COVID-19 epidemic occurred sequentially in the 5 different regions of our study. We observed a slowdown in the spread of the virus concurrent with 40% population seropositivity. Decreased infection rates were seen as early as June in Toamasina, by the end of July in Antananarivo, began in Mahajanga at the end of August. Finally, the low seroprevalences in blood donors of Toliara and Fianarantsoa at the end of September could suggest a slower progression of the pandemic in these provinces. Seroprevalence monitoring of collected blood donor samples and epidemiological indicators obtained until the end of November do not indicate a recirculation of the virus 5-7 months after the first epidemic peak.
Seroprevalences drop from September to November 2020, and most importantly in Toamasina (from 29.1% to 18.4%), after 3 months of seroprevalences of anti-SARS-CoV-2 antibodies in over 40% of donors. This drop has already been described elsewhere [11,[13], [14], [15]], in particular as in our case when the antigenic target is the Nucleocapsid (N) protein of SARS-CoV-2. Indeed, the anti-N antibody levels of seropositive donors from Toamasina drop significantly from August to September and October to November 2020, as more and more seropositive individuals approach the seropositivity threshold (Supplementary Fig. S3).
Each component of SARS-CoV-2 immune memory seems to exhibit distinct kinetics: Sero-neutralizing ability decrease has been documented in the first 2 months after infection, particularly after mild COVID-19 [15], others have shown that neutralizing antibodies are stably found for at least 5-7 months after infection with SARS-CoV-2 [14]. Spike-specific memory B cells however have been found more abundant at 6 months than at 1 month, and with no apparent half-life at 5+ months post-infection, unlike SARS-CoV-2-specific CD4+ T cells and CD8+ T cells that seem to decline with half-lives of 3 to 5 months [16].
Natural reinfections, which occur when humoral and cellular immunity are insufficient, seem to be a common feature for all human seasonal coronaviruses after the 12th month post-infection [17], suggesting a non-permanent protective immunity. This could also be the case with SARS-CoV-2 infections, even though currently circulating strains of SARS-CoV-2 are not very variable, unless the Spike-specific memory B cells developed during COVID-19 turn out to be long-lived or very long-lived, as observed after other infections as multi-decade old smallpox vaccination memory B cells [18] or influenza memory B cells [19]. However, correlates of protection against SARS-CoV-2 and the longevity of the immune response are still not well understood.
This study however has limitations, as longitudinal data was not accessible in Madagascar during this epidemic, resulting in a multi-cross-sectional young population overrepresenting males. Drops in infection rates could be due to factors unrelated to seropositivity, such as seasonality [20], social interventions, or awareness of danger leading to behaviour changes [21].
The pandemic however has evolved with a resurgence in peaks happening throughout the world, and new variants seemingly affecting its course as in Manaus, Brazil. High seroprevalences and attack rates as high as 75% were described after the first epidemic peak [22], but this city was devastated by a second peak in December. Preliminary data suggesting 42% of samples collected mid-December contained a new viral lineage called P.1 [23]. One possibility is that in some people, P.1 could elude the human immune response triggered by the lineage that ravaged the city earlier in 2020. As in Brazil, high seroprevalence rates were not sufficient to prevent a second epidemic wave from happening. Aging immunity and new variants circulation have led Madagascar to be struck by a second major peak in Spring 2021.
Our data describes the dynamics of the first peak in SARS-CoV-2 in Madagascar, in which we observed a drop in circulation of the virus concurrent with a peak of 40% anti-SARS-CoV-2 seropositivity. This seemingly high natural immunisation rate was however clearly not enough to provide permanent immunity, a substantial proportion of the population having remained susceptible or regained susceptibility to infection. Population vaccination should be intensified, and early detection of new variants having the potential to affect the course of the epidemic continuously monitored, for immediate public health decision-making.
Contributors
Conceptualisation and methodology: MS, ZAR, AS. Investigation: NR, MS. Formal analysis: MS, JMR, RR, MCAV. (MS, NR and JMR have verified the underlying data). Resources and funding acquisition: MS, VR, ZAR, AS. Supervision: MS, ZAR, AS. Writing – Original draft preparation: MS, PD, RR, AS. Writing – Review and editing: all authors.
Data sharing
Data will be made available with publication (ELISA data and deidentified participant age and gender) upon request to the corresponding author.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
We thank the blood donors and RBTC staff who supported this work, importantly Drs Jocelyne Nirina ANDRIAMBELO (RBTC Toamasina), Léa Isabelle RAMANDIMBISOA (RBTC Toliara), Michael Marie Osé HARIOLY NIRINA (NRTC Antananarivo), Dr Danielle FITAHIANA (RBTC Fianarantsoa) and Pr Tahiry RABENANDRIANINA (RBTC Mahajanga).
We thank the Immunology of Infectious Diseases unit staff for performing assays, as well as the 4 laboratories in Madagascar where RT-PCRs were run particularly the IPM virology unit.
We express our gratitude to the National Institute of Statistics of Madagascar and its Director General Isaora Zefania ROMALAHY, for sharing with us the 2020 demographics of Madagascar [9].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103419.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. https://covid19.who.int.
- 2.Pollán M, Pérez-Gómez B, Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salje H, Kiem CT, Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science (80-) 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uyoga S, Adetifa IMO, Karanja HK. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science (80-) 2020 doi: 10.1126/science.abe1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filho LA, Szwarcwald CL, Mateos S de OG. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Rev Saude Publica. 2020;54:1–10. doi: 10.11606/s1518-8787.2020054002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand S, Montez-Rath M, Han J. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;6736:1–10. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ID.VET. ID Screen SARS-CoV-2-N IgG Indirect. SARSCOV2S version 0419FR DOC_107142020.
- 8.Breslow NE, Day NE, Cancer IA for R on. Statistical methods in cancer research Volume II-the design and analysis of cohort studies (IARC Scientific Publications). 1986. [PubMed]
- 9.Institut National de la Statistique (INSTAT) de Madagascar Résultats définitifs du RGPH-3. Résultats Définitifs du RGPH-3 Tome 1 2. 2020 https://www.instat.mg/recensement-general-de-la-population-et-de-lhabitat-rgph/resultats-definitifs-rgph-3/ [Google Scholar]
- 10.Gelman A, Hill J. Cambridge Univ Press; 2006. Data Analysis Using Regression and Multilevel/Hierar(Analytical Methods for Social researchchical) [Google Scholar]
- 11.Ripperger TJ, Uhrlaub JL, Watanabe M. Orthogonal SARS-CoV-2 serological assays enable surveillance of low prevalence communities and reveal durable humoral immunity. Immunity. 2020 doi: 10.1016/j.immuni.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudbjartsson DF, Norddahl GL, Melsted P. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 doi: 10.1056/nejmoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kutsuna S, Asai Y, Matsunaga A. Loss of Anti-SARS-CoV-2 Antibodies in Mild Covid-19. N Engl J Med. 2020;383:1–4. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 14.Ibarrondo FJ, Fulcher JA, Goodman-Meza D. Rapid decay of Anti–SARS-CoV-2 antibodies in persons with mild covid-19. N Engl J Med. 2020;1:10–12. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long QX, Tang XJ, Shi QL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 16.Dan JM, Mateus J, Kato Y. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. bioRxiv. 2020 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edridge AW, Kaczorowska JM, Hoste AC. Coronavirus protective immunity is short-lasting. medRxiv. 2020 doi: 10.1038/s41591-020-1083-1. 2020.05.11.20086439. [DOI] [PubMed] [Google Scholar]
- 18.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 19.Yu X, Tsibane T, McGraw PA. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature. 2008 doi: 10.1038/nature07231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merow C, Urban MC. Seasonality and uncertainty in global COVID-19 growth rates. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2008590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercker M, Betzin U, Wilken D. 2020. What influences COVID-19 infection rates: a statistical approach to identify promising factors applied to infection data from Germany. medRxiv. [DOI] [Google Scholar]
- 22.Buss LF, Prete CA, Abrahim CMM. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science (80-) 2020:eabe9728. doi: 10.1126/science.abe9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faria NR, Claro IM, Candido D. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. Virological. 2021 https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.