Abstract
Background: The Naples prognostic score (NPS) is established according to nutritional or inflammatory state, which has been identified as a new prognostic score for various malignant tumors. However, its prognosis prediction effect on gastric cancer (GC) patients is still unknown so far. The present work aimed to examine the NPS function in the prediction of GC prognosis.
Methods: In this study, patients undergoing surgery with no preoperative therapy were retrospectively examined from June 2011 to August 2019. Typically, the total cholesterol level, serum albumin content, neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio were determined to calculate the NPS. Besides, the prognostic value of NPS was evaluated by survival analyses. Time-dependent receiver operating characteristic (t-ROC) curve analysis was also carried out to compare the prognostic value of the scoring systems.
Results: Altogether 1,283 cases were enrolled into the present work. NPS was markedly related to age, gender, tumor size, body mass index, vascular invasion, perineural invasion, and pTNM stage. Upon multivariate analysis, NPS was identified as an independent prognostic factor for the prediction of overall survival (OS) (P < 0.001). In subgroup analyses stratified by adjuvant chemotherapy or surgery alone, NPS was still the independent prognostic factor for OS in both groups (both P < 0.001). Furthermore, NPS exhibited higher accuracy in the prediction of OS than additional prognostic factors, as revealed by the results of t-ROC curve analysis.
Conclusions: NPS is a simple and useful scoring system that can be used to independently predict the survival of GC cases undergoing surgery.
Keywords: gastric cancer, naples prognostic score, time-dependent ROC, prognosis, prognostic factors
Introduction
Gastric cancer (GC) ranks the 5th place in terms of its morbidity, which affects 1,033,701 people annually; meanwhile, it also ranks the 3rd place among the causes of cancer-related deaths, with around 782,685 GC-related death cases being reported annually (1). Adjuvant therapy and surgical techniques have been greatly developed, but advanced GC patients still have dismal prognosis. As a result, it is of vital importance to develop a new preoperative prognostic marker to help to identify the surgery beneficial patients. Since Virchow first systematically reported the relationship of inflammation with cancer in the 19th century, more and more studies suggest the vital role of systemic inflammation in the tumor microenvironment (TME) (2, 3). Moreover, increasing studies indicate that inflammation in TME results in the proliferation, metastasis and angiogenesis of tumor cells, as well as antitumor immunity impairment and resistance to antitumor treatment (4). Accumulating studies report that, the inflammation-related prognostic scoring systems, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), are related to the prognosis of cancer including GC, hepatocellular carcinoma (HCC) and esophageal squamous cell carcinoma (ESCC) (5, 6). However, the host condition also affects the prognostic ability of a single inflammation-related marker, and a single marker may even be misleading when the cutoff value is arbitrarily determined. Additionally, simple scoring systems that indicate the nutritional or immunological status before surgery, such as the prognostic nutritional index (PNI), the systemic inflammation score (SIS), and the controlling nutritional status (CONUT), have also been extensively employed in predicting prognosis (7, 8). Recently, an increasing number of studies report that NPS, which is established based on the preoperative total cholesterol (TC) content, serum albumin (Alb) content, LMR and NLR, represents a new inflammation-related prognostic scoring system. Researchers have demonstrated that NPS shows prognostic value for pancreatic cancer, colorectal cancer (CRC), lung cancer and osteosarcoma (9–11). Besides, NPS is more accurate than other prognostic factors in predicting survival (9, 10, 12). It takes into account the effects of systemic inflammation and nutritional status on cancer prognosis. As a result, NPS outperforms other single inflammatory or nutritional markers. Nonetheless, there is little research on the role of NPS in predicting the prognosis of GC patients.
As a result, the present retrospective cohort study was carried out aiming to determine the prognostic value of NPS among the GC patients and to investigate the relationships between NPS and additional clinicopathological characteristics.
Patients and Methods
Study Population
This retrospective study assessed the patients undergoing radical surgery due to GC from June 2011 to August 2019 at the Department of Pancreatic and Gastric Surgery, the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The patient inclusion criteria were as follows, those histologically or cytologically diagnosed with GC, those who were followed up for over 12 months, those with no inflammatory disorder or infection (normal white blood cell count, with no obvious symptom or sign of infectious disease), and those with no malignant tumor at other site or multiple primary malignant tumors. Moreover, the demographic, histopathological and laboratory variables of all patients were retrospectively analyzed, and relevant data were extracted from the database and patient records at our hospital.
Routine blood test was performed at a week before surgery. The results of blood tests conducted at a week before surgery were acquired from the Laboratory Database of National Cancer Center (Beijing, China). The preoperative information was extracted from each patient, which included gender, age, body mass index (BMI), TC content, serum Alb content, tumor size, absolute monocyte count (AMC), absolute neutrophil count (ANC) and absolute lymphocyte count (ALC). According to galizia et al.'s method, the serum Alb content, TC content, NLR and LMR were determined to calculate NPS (10) (Supplementary Figure 1). For patients with serum Alb content < 40 g/L, TC content ≤ 180 mg/dL, NLR > 2.96 and LMR < 4.44, the scores were 1; while for those with serum Alb content ≥ 40 g/l, TC content > 180 mg/dL, NLR ≤ 2.96 and LMR ≥ 4.44, the scores were 0. NPS represented the total score obtained from all the scores mentioned above. All cases were classified as 3 groups according to the NPS value, including group 0 (NPS, 0), group 1 (NPS, 1 or 2) and group 2 (NPS, 3 or 4). And subgroup analysis was stratified by adjuvant chemotherapy or surgery alone.
In this study, overall survival (OS), which was defined as the time between surgery and all-cause death or the last follow-up. Deaths due to causes other than GC or survivals till the end of observation period (last alive contact date) were considered as the censored observations of OS. The last follow-up was assessed in March 2020. Survival data were extracted from medical records or through telephone interviews during follow-up visits.
Statistical Methods
Chi-square test was used to analyze categorical variables and t-tests were applied in analyzing the continuous variables. Survival curves were plotted by the Kaplan-Meier (KM) method, and log-rank test was utilized to analyze the differences. The significant variables identified from univariate analysis were incorporated in the multivariate Cox regression analysis. Concordance indices (C-indices) were calculated to evaluate the discriminatory power of the inflammation-based scores. And the time-dependent receiver operating characteristic (t-ROC) curves and the predicted values of area under the curve (AUC) were used to compare the prognostic value of NPS, SIS, CONUT and PNI (13). In addition to visually comparing the ROC curves, the AUC can be calculated. Sequential AUCs were compared between two scores using independent and identically distributed representations of AUC estimators. Each test was two-sided, and a difference of P <0.05 indicated statistical significance. The SPSS 18.0 (SPSS Inc., Chicago, IL, USA) and R ver. 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) softwares were employed for statistical analysis. Additionally, the R package “rms” was utilized to calculate the C index and the R package “timeROC” was adopted for t-ROC curve analysis. The present work gained approval from the Ethics Review Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College and all patients signed informed consents.
Results
Patient Characteristics
In total, 1,283 GC cases were enrolled into the present work (Supplementary Figure 2), including 1,023 (79.7%) males and 260 (20.3%) females. The average age at the time of surgery of these patients was 61.1 (range, 23.0– 87.3) years. According to the pTNM staging system, 326 (25.5%) patients were at stage I, 322 (25.1%) at stage II and 635 (49.4%) at stage III, respectively. Seven hundred and twenty-two (60.2%) of these 1,283 cases received adjuvant chemotherapy. According to the NPS system, 256 cases had 0 point (ratio, 19.9%), 414 had 1 point (ratio, 32.3%), 340 had 2 points (ratio, 26.5%), 183 had 3 points (ratio, 14.3%), while 90 had 4 points (ratio, 7.0%), separately. As a result, 256 patients (59.8%) were assigned into group 0 (NPS 0), 273 (60.1%) in group 1 (NPS 1 or 2), and 273 (65.1%) in group 2 (NPS 3 or 4), respectively.
Relationships of the Preoperative NPS System With Clinicopathological Characteristics
Table 1 summarizes the relationships of NPS with clinicopathological characteristics. NPS showed significant correlation with some clinicopathological characteristics. Additionally, a great NPS was related to the male sex, elder age (≥65.0 years), as well as reduced BMI (<18.5 kg/m2). With regard to tumor factors, NPS showed significant relationship with tumor size, vascular invasion, perineural invasion; however, there were no significant differences in lymphatic invasion, tumor differentiation, lauren classification, tumor location or adjuvant chemotherapy among these three NPS groups. In addition, NPS remarkably increased in patients with the serum Alb content (mg/dL) < 40 (P < 0.001), TC content (mg/dL) ≤ 180 (P < 0.001), LMR ≤ 4.44 (P < 0.001) and NLR > 2.96 (P < 0.001).
Table 1.
Association of NPS and clinicopathological characteristics in patients with GC.
| Clinicopathological features | All cases (n = 1,283) | Group 0 (n = 256) | Group 1 (n = 754) | Group 2 (n = 273) | P-value |
|---|---|---|---|---|---|
| Age | <0.001 | ||||
| <65.0 | 69 (5.5) | 175 (68.3) | 478 (63.3) | 136 (49.8) | |
| ≥65.0 | 1,214 (94.5) | 81 (32.7) | 276 (36.7) | 137 (50.2) | |
| Gender | <0.001 | ||||
| Male | 1,023 (79.7) | 174 (67.5) | 597 (79.2) | 252 (92.3) | |
| Female | 260 (20.3) | 82 (32.5) | 157 (20.8) | 21 (7.7) | |
| BMI (kg/m2) | <0.001 | ||||
| ≥18.5 | 1,214 (94.5) | 243 (30.9) | 745 (98.8) | 226 (82.9) | |
| <18.5 | 69 (5.5) | 13 (69.1) | 9 (1.2) | 47 (17.1) | |
| Tumor size (cm) | <0.001 | ||||
| <3.0 | 267 (20.8) | 71 (27.7) | 161 (21.3) | 35 (12.9) | |
| ≥3.0 | 1,016 (78.2) | 185 (72.3) | 593 (79.5) | 238 (87.1) | |
| Tumor differentiation | 0.104 | ||||
| Differentiated | 369 (28.8) | 79 (30.9) | 223 (29.5) | 67 (22.4) | |
| Undifferentiated | 914 (71.2) | 177 (69.1) | 531 (70.5) | 206 (75.6) | |
| Lauren Classification | 0.792 | ||||
| Intestinal-type | 479 (37.3) | 90 (35.2) | 294 (39.1) | 93 (34.1) | |
| Diffused-type | 465 (36.3) | 92 (36.0) | 276 (36.6) | 95 (35.0) | |
| Mixed | 339 (26.4) | 74 (28.8) | 184 (24.3) | 85 (30.9) | |
| Lymphatic invasion | 0.068 | ||||
| Negative | 636 (49.6) | 137 (53.4) | 379 (50.3) | 120 (44.1) | |
| Positive | 647 (50.4) | 119 (46.6) | 375 (49.7) | 153 (55.9) | |
| Vascular invasion | 0.001 | ||||
| Negative | 778 (60.7) | 171 (66.8) | 462 (62.2) | 145 (53.2) | |
| Positive | 505 (39.3) | 85 (33.2) | 292 (38.7) | 128 (46.8) | |
| Perineural invasion | 0.004 | ||||
| Negative | 628 (49.0) | 141 (55.1) | 371 (49.3) | 116 (42.5) | |
| Positive | 655 (51.0) | 115 (44.9) | 383 (50.7) | 157 (57.5) | |
| Tumor location | 0.082 | ||||
| Upper | 48 (28.6) | 58 (22.7) | 30 (27.8) | 80 (29.5) | |
| Middle /Lower | 183 (71.4) | 198 (77.3) | 120 (72.2) | 193 (70.5) | |
| pTNM stage | <0.001 | ||||
| I | 326 (25.5) | 89 (34.7) | 183 (24.4) | 54 (19.8) | |
| II | 322 (25.1) | 53 (20.7) | 214 (28.3) | 55 (20.1) | |
| III | 635 (49.4) | 114 (44.6) | 357 (47.3) | 164 (60.1) | |
| Adjuvant chemotherapy | 0.387 | ||||
| No | 511 (39.8) | 106 (41.4) | 302 (40.1) | 103 (37.7) | |
| Yes | 772 (60.2) | 150 (58.6) | 452 (59.9) | 170 (62.3) | |
| Serum albumin (mg/dL) | <0.001 | ||||
| ≥ 40 | 890 (69.4) | 256 (100.0) | 577 (76.5) | 57 (20.9) | |
| <40 | 393 (30.6) | 0 (0) | 177 (23.5) | 216 (79.1) | |
| Total cholesterol (mg/dL) | <0.001 | ||||
| >180 | 515 (40.2) | 256 (100.0) | 222 (29.5) | 37 (13.6) | |
| ≤ 180 | 768 (59.8) | 0 (0) | 532 (70.5) | 236 (86.4) | |
| Neutrophil: lymphocyte ratio | <0.001 | ||||
| ≤ 2.96 | 1,029 (80.2) | 256 (100.0) | 686 (91.0) | 87 (31.9) | |
| >2.96 | 254 (19.8) | 0 (0) | 68 (9.0) | 186 (68.1) | |
| Lymphocyte: monocyte ratio | <0.001 | ||||
| >4.44 | 845 (65.9) | 256 (100.0) | 533 (70.7) | 56 (20.5) | |
| ≤ 4.44 | 438 (34.1) | 0 (0) | 221 (29.3) | 217 (79.5) |
GC, gastric cancer; NPS, naples prognostic score; BMI, body mass index.
OS Examined Based on NPS
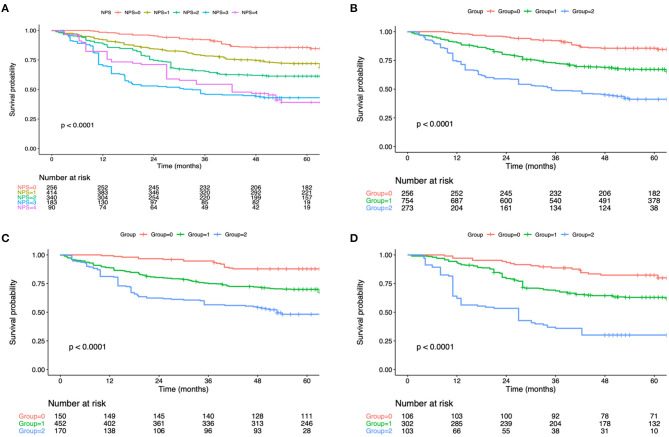
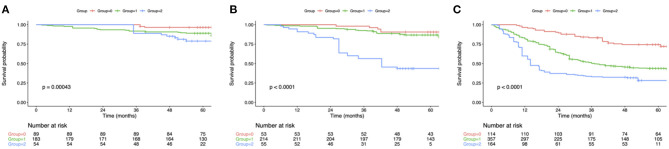
The OS curve was statistically analyzed, as shown in Figure 1. For all the enrolled patients, their 1-, 3- and 5-year OS rates were 89.1, 70.6, and 46.7%, separately, and the median OS was 51.9 months. With regard to OS, the median OS was 67.7, 52.5 and 35.7 months for groups 0, 1 and 2, respectively. According to KM survival analysis, NPS score 0–4 was tightly related to OS, and the elevation of preoperative NPS by 1 point was markedly related to the poor OS (Log-rank P <0.001; statistic power >80%; Figure 1A). Also, KM survival analyses indicated that OS was markedly shortened with the elevation of NPS group in a step-wise manner (Log-rank P < 0.001; statistic power >80%; Figure 1B). Furthermore, significant difference was observed in OS based on NPS in single surgery group as well as postoperative adjuvant chemotherapy group (Figures 1C,D). When stratified by pTNM stage, the most significant differences in OS and RFS were observed in the stage III subgroup based on NPS system (Figure 2).
Figure 1.
(A) Kaplan–Meier survival analyses of OS according to the NPS score. (B) Kaplan–Meier survival analyses of OS according to the NPS group. OS, overall survival; NPS, naples prognostic score. (C) Association of the NPS with the OS in the adjuvant chemotherapy group. (D) Association of the NPS with the OS in the surgery alone group. OS, overall survival; NPS, naples prognostic score.
Figure 2.
Kaplan-Meier analysis of OS of GC patients at each pTNM stage according to the NPS. (A) Association of the NPS with OS of patients with stage I GC. (B) Association of the NPS with OS of patients with stage II GC. (C) Association of the NPS with OS of patients with stage III GC. OS, overall survival; NPS, naples prognostic score; GC, gastric cancer.
Univariate and Multivariate Analyses on the Prognostic Predictors in GC Cases
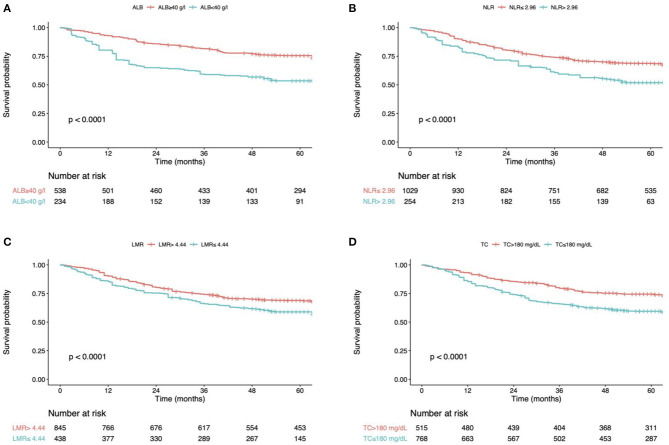
In univariate analysis, patients in group 0 (low NPS) showed markedly extended OS compared with that in groups 1 and 2 (both P < 0.001, respectively; Table 2). As for patient characteristics, the elder age (≥ 65.0 years) was markedly associated with dismal OS (HR = 1.78, P < 0.001). Additionally, the decreased BMI (<18.5 kg/m2) was related to dismal OS (HR = 1.69, P < 0.001). In terms of the tumor characteristics, the undifferentiated (HR = 1.58, P < 0.001), diffused-type (HR = 1.23, P = 0.001), vascular invasion (HR = 1.70, P < 0.001), lymphatic invasion (HR = 1.93, P < 0.001) and perineural invasion (HR = 1.83, P < 0.001) showed marked correlation with dismal prognostic outcomes. NPS was identified as the independent factor to predict the OS upon multivariate analysis (Figure 1B). In addition, OS was markedly impaired among cases with serum Alb content (mg/dL) < 40 (HR = 0.72, P = 0.001), LMR ≤ 4.44 (HR = 1.71, P < 0.001) and NLR> 2.96 (HR = 1.59, P < 0.001) (Figure 3; Table 2). In addition, more independent factors for prognosis prediction included an old age, female sex, decreased BMI, pTNM stage, undifferentiated, diffused-type and the presence of vascular invasion, lymphatic invasion and perineural invasion (Table 2).
Table 2.
Univariate and multivariate analysis of clinicopathologic variables in relation to OS in patients with GC.
| Clinicopathological features | Univariate analysis | P-value | Multivariate analysis | P-value |
|---|---|---|---|---|
| Age | ||||
| <65.0 | Reference | Reference | <0.001 | |
| ≥65.0 | 1.78 (1.41, 2.25) | <0.001 | 1.60 (1.44, 1.79) | |
| Gender | ||||
| Male | Reference | |||
| Female | 0.85 (0.46, 1.62) | 0.182 | ||
| BMI (kg/m2) | ||||
| ≥18.5 | Reference | Reference | ||
| <18.5 | 1.69 (1.27, 2.13) | <0.001 | 1.54 (1.21, 1.80) | <0.001 |
| Tumor size (cm) | ||||
| <3.0 | Reference | Reference | ||
| ≥3.0 | 2.03 (1.50, 2.52) | <0.001 | 1.27 (0.76, 1.63) | 0.438 |
| Tumor differentiation | ||||
| Differentiated | Reference | Reference | ||
| Undifferentiated | 1.58 (1.32, 2.15) | <0.001 | 1.47 (1.13, 1.92) | 0.008 |
| Lauren Classification | ||||
| Intestinal-type | Reference | Reference | ||
| Diffused-type | 1.23 (1.05, 1.78) | 0.001 | 1.16 (1.01, 1.45) | 0.009 |
| Mixed | 1.04 (0.67, 1.51) | 0.871 | ||
| Lymphatic invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 1.93 (1.42, 2.88) | <0.001 | 1.60 (1.32, 2.12) | 0.004 |
| Vascular invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 1.70 (1.28, 2.21) | <0.001 | 1.55 (1.32, 1.83) | <0.001 |
| Perineural invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 1.83 (1.49, 2.65) | <0.001 | 1.62 (1.43, 1.90) | 0.011 |
| Tumor location | ||||
| Upper | Reference | |||
| Middle /Lower | 0.85 (0.39, 1.61) | 0.230 | ||
| pTNM stage | ||||
| I | Reference | Reference | ||
| II | 2.14 (1.45, 2.67) | 0.001 | 1.83 (1.32, 2.28) | <0.001 |
| III | 5.47 (2.31, 8.41) | 0.007 | 3.22 (2.10, 4.19) | <0.001 |
| Adjuvant chemotherapy | ||||
| No | Reference | |||
| Yes | 0.79 (0.45, 1.48) | 0.173 | ||
| Serum albumin (mg/dL) | ||||
| <40 | Reference | Reference | ||
| ≥40 | 0.65 (0.52, 0.94) | 0.001 | 0.72 (0.56, 0.90) | <0.001 |
| Total cholesterol (mg/dL) | ||||
| >180 | Reference | Reference | ||
| ≤ 180 | 1.52 (1.14, 2.10) | 0.001 | 1.46 (1.10, 1.87) | <0.001 |
| Neutrophil: lymphocyte ratio | ||||
| ≤ 2.96 | Reference | Reference | ||
| >2.96 | 1.69 (1.28, 2.26) | 0.003 | 1.59 (1.23, 1.92) | <0.001 |
| Lymphocyte: monocyte ratio | ||||
| >4.44 | Reference | Reference | ||
| ≤ 4.44 | 1.78 (1.33, 2.32) | <0.001 | 1.71 (1.22, 2.01) | <0.001 |
| NPS | ||||
| 0 | Reference | Reference | ||
| 1 | 2.68 (1.45, 4.01) | 0.002 | 2.21 (1.27, 3.31) | <0.001 |
| 2 | 3.82 (1.89, 6.80) | <0.001 | 3.45 (1.43, 5.17) | <0.001 |
OS, overall survival; GC, gastric cancer; BMI, body mass index; NPS, naples prognostic score.
Figure 3.
(A) overall survival curves according to the preoperative ALB. (B) overall survival curves according to the preoperative NLR. (C) overall survival curves according to the preoperative LMR. (D) overall survival curves according to the preoperative TC. ALB, albumin; TC, total cholesterol; NLR, neutrophil-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio.
Prognostic Value of NPS
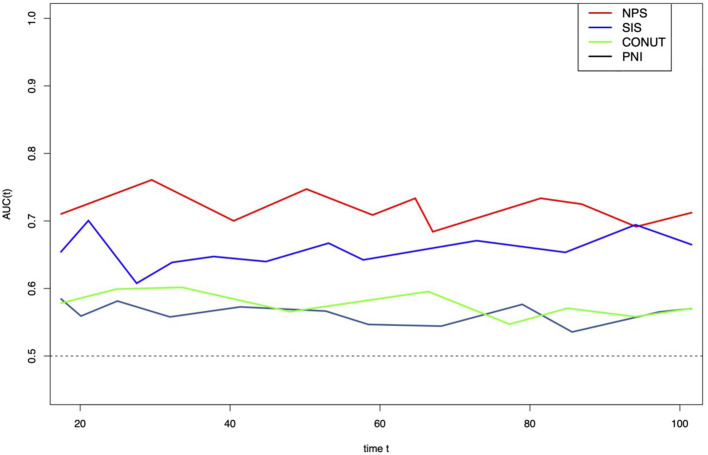
In this study, the prognostic value of NPS was compared with that of more prognostic factors (PNI, CONUT and SIS). As suggested by results of t-ROC curve analysis to predict OS by different scoring systems, and the AUC value was high for NPS compared with those for other scoring systems (Figure 4). Typically, the AUC values in the prediction of 5-year OS were 0.708, 0.549, 0.625, and 0.580 for NPS, PNI, SIS and CONUT, respectively (Figure 4).
Figure 4.
Time-dependent ROC curves of NPS, SIS, CONUT, and PNI for prediction of overall survival. The horizontal axis represents year after surgery, and the vertical axis represents the estimated AUC for survival at the time of interest. NPS, naples prognostic score; SIS, systemic inflammation score; CONUT, controlling nutritional status; PNI, prognostic nutritional index.
Discussion
As suggested by this work, the preoperative NPS was related to gender, age, BMI, tumor size, perineural invasion and vascular invasion in GC patients. NPS might serve as an independent factor to predict the OS for GC cases, and a large NPS was possibly related to the dismal prognosis. Additionally, NPS was more accurate than other prognostic scoring systems developed in previous studies (PNI, SIS and CONUT) in predicting OS.
The biological mechanism by which NPS affects patient prognosis is possibly dependent on the serum Alb content, serum TC content, neutrophil count, monocyte count and lymphocyte count, which are easily measured before surgery. Hypoalbuminemia suggests the low nutritional status along with the elevated inflammatory level, and it may negatively affect the surivval of GC patients. In addition, hypoalbuminemia can reduce the transport of substances like fatty acid and cholesterol as well as the scavenging of free oxygen radicals, and these show adverse effects on OS. The TC content is identified to be related to patient survival and tumor development in a variety of cancer types, such as GC, since the plasma TC content and calorie intake are reduced in tumor tissues0 (14). LMR is determined by lymphocytes and monocytes, whereas NLR is related to neutrophils and lymphocytes. Biologically, LMR and NLR were possibly revealed by the lymphocyte, monocyte and neutrophil functions. And lymphocytosis indicates the immunological status, and it enhances the anticancer response against the proliferation, new vessel formation and migration of cancer cells (15). In this paper, an example is the clinical significance of tumor-infiltrating lymphocytes (TILs), which are related to the superior prognosis for various cancer types, and this may be related to the anticancer effects induced by TILs and the suppression of angiogenesis (15, 16). Therefore, cancer patients with lymphopenia had dismal survival (17). Neutrophils can invade primary tumor, release the pro-angiogenic factors and promotes tumor cell migration along the new blood vessels, thus facilitating tumor metastasis. And neutrophils are reported to enhance the adhesion of circulating cancer cells with end organs, which thereby increases the risk of metastatic seeding. The circulating monocytes facilitate cancer growth and decrease the immune monitoring (18). Further, studies have shown that monocytes may promote tumor cell metastasis via the tumor-monocyte-endothelial interaction (19). Thus, monocytes and neutrophils enhanced the proliferation of cancer cells and regulated the TME, thus facilitating tumor metastasis, invasion and new vessel formation. Therefore, the high neutrophil count while low monocyte count in cancer patients indicate dismal OS.
As for the SIS scoring system that developed on the basis of preoperative LMR and Alb contents, it is identified to be related to survival of a variety of cancers and can serve as a creditable inflammatory-based scoring system (8, 20). It was discovered by Melling and colleague that, GPS might also independently predict the long-term prognosis for GC patients receiving surgery (21). CONUT is determined based on the total lymphocyte count, serum Alb content and TC content, is recognized as a useful approach to assess nutritional status (22). Moreover, it was discovered by Kuroda and colleagues that, CONUT was an efficient approach to estimate the the nutritional status and to predict the long-term OS for GC cases who underwent radical surgery (7). PNI, which predicts the nutritional and immune statuses, is a scoring system used to evaluate patient general condition and an efficient factor to predict the long-term survival for GC case (23). In addition, Galizia et al. revealed that NPS possibly outperformed the other scoring systems (like PNI, SIS and CONUT) in predicting the CRC survival (10). Nakagawa et al. indicated that NPS was more sensitive than CONUT in predicting the OS in pancreatic cancer (9). As found in this work, NPS outperformed PNI, SIS and CONUT in predicting the prognosis for GC cases who underwent radical surgery.
Compared with the existing tools to target immunonutritional interventions, our system is superior in that, by combining the oncological, nutritional, and immunological parameters, it outperforms the other existing nutritional indices in predicting the postoperative adverse events. And it targets the immunonutritional intervention to patients who may benefit the most. The results of our study indicated that early inflammation control and nutritional support might improve the prognosis for cancer patients. Preoperative identification of patient status could have several uses in clinical practice, including prognostic stratification and treatment. Early detection and improvement of malnutrition and inflammation may result in better patient outcomes (24).
Certain limitations should be noted in this study. Firstly, selection bias was inevitable due to the retrospective nature, even though the samples were strictly selected according to the inclusion and exclusion criteria. And the significance of NPS needs to be validated using other cohorts. Secondly, patients who received NACT were eliminated from this study, but it was difficult to guarantee the identical patient status prior to blood sample collection, and our findings did not apply to GC cases after NACT.
Conclusions
To sum up, this work suggests that preoperative NPS can serve as a simple and useful predictor to predict the prognosis of GC. Besides, NPS is also utilized as one part of the preoperative prognosis stratification as well as postoperative follow-up for the development of individual treatment for GC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Review Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JX conceived the study and wrote the manuscript. WK, HH, and FM searched the database, reviewed the studies, and collected the data. HL and SM performed the statistical analyses. YL and PJ performed revision of the manuscript. YT arranged for and provided the funding for this work and is the guarantor for this study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- GC
Gastric cancer
- HCC
hepatocellular carcinoma
- ESCC
esophageal squamous cell carcinoma
- OS
overall survival
- NPS
naples prognostic score
- SIS
systemic inflammation score
- CONUT
controlling nutritional status
- PNI
prognostic nutritional index
- ALB
albumin
- TC
total cholesterol
- NLR
neutrophil-to-lymphocyte ratio
- PLR
platelet-to-lymphocyte ratio
- LMR
lymphocyte-to-monocyte ratio
- BMI
body mass index
- AUC
area under the curve
- ROC
receiver operating characteristic
- GC
Gastric cancer
- HCC
hepatocellular carcinoma
- ESCC
esophageal squamous cell carcinoma.
Footnotes
Funding. This work was supported by grants from the National Natural Science Foundation of China (81772642) and the Beijing Municipal Science & Technology Commission (Z161100000116045). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.617744/full#supplementary-material
Calculation of the Naples prognostic score.
Study design. GC, gastric cancer.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 3.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. (2009) 30:1073–81. 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- 4.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. (2006) 441:431–6. 10.1038/nature04870 [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. (2018) 44:607–12. 10.1016/j.ejso.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. (2019) 234:1794–802. 10.1002/jcp.27052 [DOI] [PubMed] [Google Scholar]
- 7.Kuroda D, Sawayama H, Kurashige J, Iwatsuki M, Eto T, Tokunaga R, et al. Controlling nutritional status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer. (2018) 21:204–12. 10.1007/s10120-017-0744-3 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kondo T, et al. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Ann Surg. (2018) 267:527–31. 10.1097/SLA.0000000000002115 [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa N, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, et al. Clinical implications of naples prognostic score in patients with resected pancreatic cancer. Ann Surg Oncol. (2020) 27:887–95. 10.1245/s10434-019-08047-7 [DOI] [PubMed] [Google Scholar]
- 10.Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Podzemny V, et al. Naples prognostic score, based on nutritional and inflammatory status, is an independent predictor of long-term outcome in patients undergoing surgery for colorectal cancer. Dis Colon Rectum. (2017) 60:1273–84. 10.1097/DCR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Chen T, Yao Z, Zhang X. Prognostic value of pre-treatment naples prognostic score (NPS) in patients with osteosarcoma. World J Surg Oncol. (2020) 18:24. 10.1186/s12957-020-1789-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto Y, Hiyoshi Y, Daitoku N, Okadome K, Sakamoto Y, Yamashita K, et al. Naples prognostic score is a useful prognostic marker in patients with metastatic colorectal cancer. Dis Colon Rectum. (2019) 62:1485–93. 10.1097/DCR.0000000000001484 [DOI] [PubMed] [Google Scholar]
- 13.Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. (2017) 17:53. 10.1186/s12874-017-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang R, Li P, Wang T, Li X, Wei Z, Zhang Z, et al. Apolipoprotein E epsilon 2 allele and low serum cholesterol as risk factors for gastric cancer in a chinese han population. Sci Rep. (2016) 6:19930. 10.1038/srep19930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. (2012) 30:2678–83. 10.1200/JCO.2011.37.8539 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. (2003) 348:203–13. 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 17.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. (2009) 69:5383–91. 10.1158/0008-5472.CAN-08-3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Augier S, Ciucci T, Luci C, Carle GF, Blin-Wakkach C, Wakkach A. Inflammatory blood monocytes contribute to tumor development and represent a privileged target to improve host immunosurveillance. J Immunol. (2010) 185:7165–73. 10.4049/jimmunol.0902583 [DOI] [PubMed] [Google Scholar]
- 19.Evani SJ, Prabhu RG, Gnanaruban V, Finol EA, Ramasubramanian AK. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. Faseb J. (2013) 27:3017–29. 10.1096/fj.12-224824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, An H, Xu L, Zhu Y, Yang Y, Lin Z, et al. Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma. Br J Cancer. (2015) 113:626–33. 10.1038/bjc.2015.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melling N, Grüning A, Tachezy M, Nentwich M, Reeh M, Uzunoglu FG, et al. Glasgow prognostic score may be a prognostic index for overall and perioperative survival in gastric cancer without perioperative treatment. Surgery. (2016) 159:1548–56. 10.1016/j.surg.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 22.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. (2005)20:38–45. [PubMed] [Google Scholar]
- 23.Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. (2016) 42:1176–82. 10.1016/j.ejso.2016.05.029 [DOI] [PubMed] [Google Scholar]
- 24.Tokunaga R, Sakamoto Y, Nakagawa S, Ohuchi M, Izumi D, Kosumi K, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis. (2017) 32:99–106. 10.1007/s00384-016-2668-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculation of the Naples prognostic score.
Study design. GC, gastric cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.