Abstract
This study aimed to evaluate the volatile or lipophilic chemical profiling and the biological activities of avocado (Persea americana cv. Criollo sp.) seed extracts. Chemical profile of volatile compounds (GC/MS), antioxidant properties (phenolic compounds, DPPH radical scavenging activities and reducing power), and antimicrobial activity (Salmonella Typhimurium and Staphylococcus aureus) of avocado (Persea americana cv. Criollo sp.) seed extracts (ethanol and acetone) were characterized. Sixteen volatile chemical compounds were determined, including isoprenoid derivatives (estragole), esters of fatty acids (linoleic and linolenic acids), and their derivatives (9,12-Octadecadien-1-ol and 9,12,15-Octadecatrien-1-ol). Acetone was the best solvent to obtain volatile compounds from avocado seed; this extract also showed a higher reducing power (56.35 mg AAE/100 g). Maximum S. aureus and S. Typhimurium log reductions were 4.0 ± 0.3 and 1.8 ± 0.3 at the highest amount used (2000 mg/L), without significant effect (p < 0.05) of the solvent used. According to the results of the volatile chemical profiling of avocado (Persea americana cv. Criollo sp.) seed extracts, they can have potential application as antioxidant (212.75 and 183.75 mg Trolox/100 g) and antimicrobial additives.
Keywords: Avocado seed, Volatile chemical compounds, Antioxidant capacity, Antimicrobial activity
1. Introduction
Current consumer tendencies indicate that natural preservatives are preferred over synthetic compounds. Synthetic preservatives and additives are widely used in food products to maintain their quality; however, several studies have indicated that these compounds are toxic due to their constant intake (Amchova et al., 2015, Beristaín-Bauza et al., 2018). Therefore, researchers have been looking for new sources of natural compounds with antioxidant or antimicrobial activities and the evaluation of their application in food (Alañón et al., 2017). Fruits and vegetables are a good source of phytochemical compounds; however, their by-products (peel, seed core, pomace, among others) are usually discarded, although they have, in many cases, more bioactive compounds than their edible parts (Barros et al., 2017).
Avocado (Persea americana L.) is a tropical fruit belonging to the Lauraceae family. It is native of Mexico and Central America, and its varieties Hass, Bacon, Fuerte, and Criollo are the most important (Araújo et al., 2015, SAGARPA, 2011). Mexico is one of the biggest producers worldwide of avocado, with more than 2 million tons in 2018 (SIAP, 2017). Avocado is widely consumed due to its pleasant texture and flavor. However, avocado seeds (comprising about 13 to 24% of avocado total weight) are usually discarded (Alkhalaf et al., 2019, Calderón-Oliver et al., 2016). Moreover, by-products percentage, chemical composition (pulp, seed and peel) and avocado fatty acid profile will be influenced by its origin, variety, season, post-harvest, environmental, and growing conditions (Flores et al., 2019, Pedreschi et al., 2016).
Avocado seeds contain different bioactive compounds such as procyanidins (Wang et al., 2010), phenolic compounds (Saavedra et al., 2017, Segovia et al., 2016), triterpenoids (Abubakar et al., 2017), acetogenins (Rodríguez-Sánchez et al., 2013), fatty acids, amines, and weak acids (Arlene et al., 2015) that could be used as antioxidant, antimicrobial, insecticidal, hypocholesterolemic, antidiabetic, antihypertensive, lipid substitute, soap bases and other applications (Dabas et al., 2013, Ge et al., 2018, Guzmán-Rodríguez et al., 2013, Leite et al., 2009, Rodríguez-Carpena et al., 2012, Rodríguez-Carpena et al., 2011, Segovia et al., 2018) in pharmaceutical, biotechnological, chemical, and food industries (Abubakar et al., 2017, Barbosa-Martín et al., 2016, Díaz-Muñoz et al., 2016, Zhu et al., 2016).
Although there are some reports on avocado seeds chemical composition, few describe the volatile or lipophilic chemical profiling. For example, Bora et al. (2001) evaluated the volatile compounds of avocado (cv. Fuerte) seed oil. They found 32.5% of saturated fatty acids (heptadecanoic acid mainly), 20.7% of monounsaturated fatty acids (oleic acid mainly), and 46.7% of polyunsaturated fatty acids (linoleic acid mainly). The preferred solvents for antioxidant or phytochemical compounds extraction are those of intermediate polarity like ethanol (polar protic) or acetone (aprotic). Phenols are generally extracted with ethanol and methanol. While polyphenols are recovered with acetone (Bhebhe et al., 2016). Moreover, Leite et al. (2009) pointed out that hexane extract of avocado seeds from Brazil contains palmitic, palmitoleic, stearic, oleic, and linoleic acids, and also sterols and triterpenoids. On the other hand, the Criollo variety has not been studied; therefore, this study is aimed to evaluate the volatile or lipophilic chemical profiling and the antioxidant and antimicrobial activities of avocado (Persea americana cv. Criollo sp.) seed extracts.
2. Materials and methods
2.1. Plant material
Avocado (Persea americana cv. Criollo sp.) fruits were obtained from Atlixco, Puebla, Mexico. Avocados were selected in commercial ripening (No.3 according to the Florida Avocado Grades and Standard (USDA, 2015), free from physical and microbiological appearance damages. Avocado seeds were manually obtained using a stainless-steel knife, washed with distilled water and dried with absorbent paper; next, avocado seeds were cut into small pieces and dried at 50 °C until constant weight (24 h approximately). Dried seeds were ground and sieved to obtain a fine powder (450 µm) and stored covered from light until its use (Nwaokobia et al., 2018).
2.2. Solvents and reagents
All chemical reagents and solvents used in this study were obtained from Sigma-Aldrich, Inc. (Toluca, Mexico) and J.T. Baker (Mexico City, Mexico), respectively. Agars and broths were obtained from Bioxon (Mexico City, Mexico).
2.3. Avocado extracts
Avocado seed extracts were obtained following the methodology proposed by Hernández-Carranza et al. (2016) with some modifications. Extracts for chemical characterization and antioxidant assays were done by placing 1 g of avocado seed powder with 25 mL of absolute ethanol or acetone; the extraction was conducted for 24 h (230–240 rpm) at room temperature (22 ± 2 °C). All extracts were filtered using a Whatman grade four paper and centrifuged for 40 min (4 °C) at 3200 rpm; the supernatant was taken and used for further analysis. To obtain a higher concentration of avocado seed extract for microbiological assays, five grams of avocado seed was mixed with 25 mL of absolute ethanol or acetone; the extracts were completely dried and resuspended with water: ethanol (90:10 v/v) solution (preliminary tests) to reach a concentration of 10,000 mg/L. All extracts were immediately used for analysis.
2.4. Chemical characterization
Ethanol and acetone extracts were chemically characterized using a gas chromatography-mass spectrometry (GC/MS) Perkin Elmer Turbo Mass Gold MS-Auto system XLTM (Perkin-Elmer, Norwalk, Conn., USA), with a splitless injector and 70 eV electronic fragmentation detector, fragmentation mass within the molecular weight range of 35–400 m/z, and equipped with a 5-MS column (60 m * 0.25 mm I.D. * 0.25 µm film thickness) using helium as the carrier gas. The following conditions were set for the analysis: injector temperature of 230 °C, initial oven temperature of 120 °C held for 1 min, followed by a ramp-up of 28 °C/min up to 225 °C held for 5 min with a helium flow of 1 mL/min. One µL of each extract was injected by triplicate. Chemical characterization was conducted by parent ions obtained. The spectra obtained were compared with the mass profile of the US National Institute of Standard Technology (NIST) library. A correlation index higher than 98% was obtained for each of the chemical compounds of the avocado seed.
2.5. Antioxidant assays
Total phenolic compounds (TPC) were evaluated following the methodology proposed by Hernández-Carranza et al. (2016). Briefly, one mL of extract was mixed with 1 mL of Folin-Ciocalteau reagent (0.1 M) and three minutes later 1 mL of Na2CO3 (0.5% w/v) solution was added. The mixture was incubated (30 min) at room temperature under a dark environment. TPC were read at 765 nm using a Jenway UV–Vis spectrophotometer (model 6405, Staffordshire, UK). TPC quantification was done using a standard curve of gallic acid. Results were expressed as mg of gallic acid equivalents (GAE) per 100 g of dried sample.
Antioxidant capacity (AC) was evaluated using both the inhibition of the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical and the iron (III) to iron (II) reduction (FRAP) assay. The inhibition of DPPH radical was done following the methodology proposed by Hernández-Carranza et al. (2016). One mL of extract was placed in an amber glass tube and mixed with 1 mL of DPPH (0.004% w/v) solution. The mixture was incubated for 30 min at room temperature under a dark environment. Absorbance was read at 517 nm using a UV–Vis spectrophotometer. Results were expressed as mg of Trolox per 100 g of sample using a standard curve of Trolox. The iron reduction assay was conducted following the methodology proposed by Dorman et al. (2004). First, one mL of extract was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 7.0) and 2.5 mL of potassium hexacyanoferrate solution (1% w/v) and then the mixture was incubated for 30 min at 50 °C, next, 2.5 mL of trichloroacetic acid solution (10% w/v) was added and it was centrifuged for 10 min at 1000 rpm. Finally, 2.5 mL of the supernatant were mixed with 2.5 mL of water and 0.5 mL of FeCl3 solution (0.1% w/v). Absorbance was recorded at 700 nm using a UV–Vis spectrophotometer. Iron reduction was expressed as mg of ascorbic acid (AA) per 100 g of dried sample.
2.6. Microbiological assay
Avocado seed extracts antimicrobial activity was evaluated using Salmonella enterica subsp. enterica serovar Typhimurium (ATCC 14028) and Staphylococcus aureus (ATCC 2913). Both microorganisms were obtained from Departamento de Bioquímica-Alimentos, BUAP. Microorganisms were grown at 37 °C (24 h approximately) in soy-trypticase broth until a microbial load of 1.5 × 108 CFU/mL was obtained. Microbiological assay was conducted following the methodology reported by Idris et al. (2009) with some modifications. First, 100 µL of S. Typhimurium or S. aureus inoculum were added to a mixture of avocado seed extract and soy-trypticase broth prepared in the following ratios 0:9.9, 0.5:9.4, 1.0:8.9, and 2.0:7.9 (v/v); next, 1 mL of each dilution was added to Salmonella-Shigella and mannitol salt agars for S. Typhimurium or S. aureus growth, respectively. A control with the same mixtures with solvent instead of the extract were used. Petri dishes were incubated at 37 ± 2 °C and counted after 24 h. The microbial assay was performed by triplicate.
2.7. Statistical analysis
Results were analyzed by analysis of variance (ANOVA) was performed by Minitab 15 software (Minitab Inc., State College, PA, USA, 2008). A p-value of 0.05 was used for deciding significant differences between samples (Tukey’s test).
3. Results and discussion
3.1. Chemical characterization
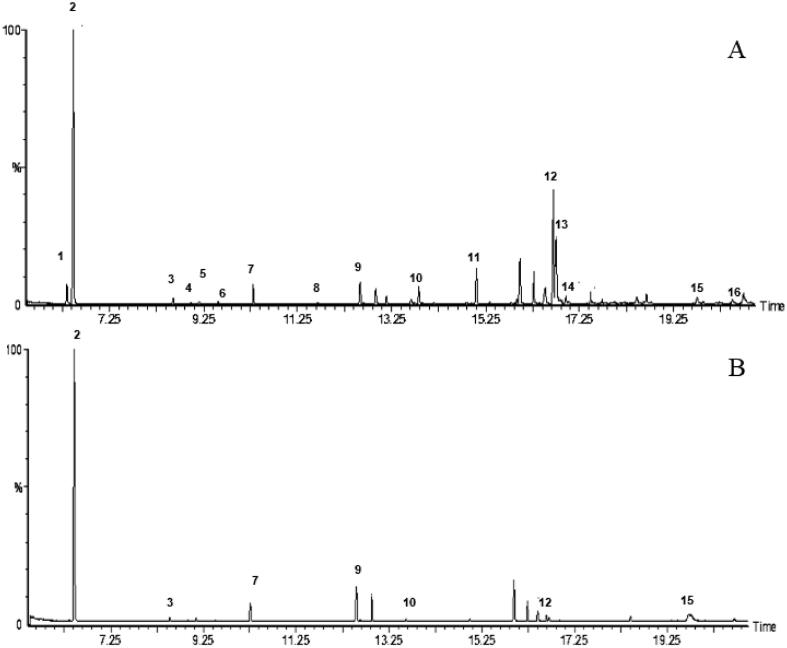
Avocado criollo variety (Persea americana cv. Criollo sp.) is a small fruit (85–100 g), whose thin skin during its ripening changes from green tones (immature) to a dark brown color. The seed is near 22.93 ± 1.98% (w/w) of the fruit total weight (56.80 ± 2.59% of moisture). Fig. 1 shows the chemical profiling of avocado seed extracts. Acetone chromatogram (Fig. 1A) presented more compounds than the ethanolic extract (Fig. 1B). The major compounds identified in acetone extract were estragole (peak 2), linoleic acid, methyl ester (peak 12), linolenic acid, methyl ester (peak 13), and tridecanoic acid, methyl ester (peak 11). On the other hand, the main compounds identified in the ethanolic extract include the estragole (peak 2), 11-dodecen-2-one (peak 9), and 9,12-octadecadien-1-ol, (peak 15). Terpenes and fatty acid derivatives (major compounds in this research) have shown effects as phytochemical agents in various plants. Although many studies have been conducted on avocado mesocarp, few of them have studied avocado seed; in this aspect, phenolic compounds such as catechin, chlorogenic acid, caffeic acid, and ferulic acid were detected by Saavedra et al. (2017) using Hass avocado byproducts (peels and seeds). López-Cobo et al. (2016) found 11 compounds in avocado seed (Hass variety), being hydroxytyrosol glucoside, tyrosol glucoside, vanillic acid glucoside, and (1′S, 6′R)-8′-hydroxyabscisic acid beta-D-glucoside the major compounds obtained. Moreover, Figueroa et al. (2018) made a full characterization of avocado (cv. Hass) polar compounds from seeds and seed coat. They found 83 compounds in both by-products, being organic acids and flavonoids higher in the seed coat, hydroxycinnamic acids, phenolic alcohol derivatives, catechins, and condensed tannins higher in the seed, and procyanidins type A, with potential health benefits, was found in both samples. The volatile or lipophilic compounds have not been described thoroughly, even when avocado has a large non-hydrophilic fraction.
Fig. 1.
Chromatogram of acetone (A) and ethanolic (B) extracts of avocado seed (Persea americana cv. Criollo sp.).
As observed, avocado seed showed compounds such as isoprenoids derivates, esters of fatty acids and their derivatives (Table 1). In this regard, seven compounds of terpenoid family and phenylpropanoid were identified (isoestragole, estragole, α-cubebene, cubebene, α-caryophyllene, α-farnesene, and germacrene). Similar results were reported by Canto-Pereira et al. (2013) in the avocado pulp, where they reported the presence of sesquiterpenoids such as caryophyllene and farnesene. Moreover, four esters of fatty acids (tridecanoic acid, methyl ester, linoleic acid, methyl ester, linolenic acid, methyl ester, and linolelaidic acid, methyl ester) and two alcoholic derivatives of linoleic and linolenic acid (9,12-Octadecadien-1-ol, 9,12,15-Octadecatrien-1-ol) were found. The relationship between fatty acids and volatile compounds is related to the biosynthesis and concentration of aromatic compounds in Hass avocado (García-Rojas et al., 2016). Rodríguez-Carpena et al. (2012) showed that a better profile of healthy fatty acids, gives oxidative stability and important notes of spicy and fruity flavor.
Table 1.
Chemical compounds, retention time, and structure of acetone and ethanolic extracts of avocado seed (Persea americana cv. Criollo sp.).
| Peak | RT | Component | % area acetone | % area ethanol | CAS* |
|---|---|---|---|---|---|
| 1 | 6.34A | Isoestragole | 3.070 | ND | 104-46-1 |
| 2 | 6.48A 6.48B |
Estragole | 42.420 | 62.830 | 140-67-0 |
| 3 | 8.61A 8.53B |
α-Cubebene | 0.558 | 0.540 | 17699-14-8 |
| 4 | 9.16A | Cubebene | 0.093 | ND | 17669-14-8 |
| 5 | 9.54A | α-Caryophyllene | 0.186 | ND | 6753-98-6 |
| 6 | 10.06A | α-Farnesene | 0.186 | ND | 502-61-4 |
| 7 | 10.31A 10.26B |
Germacrened D | 2.791 | 4.459 | 23986-74-5 |
| 8 | 11.68A | Palmitaldehyde | 0.074 | ND | 629-80-1 |
| 9 | 12.57A 12.55B |
11-Dodecen-2 one | 1.860 | 5.945 | 5009-33-6 |
| 10 | 13.67A 13.62B |
9,12-Octadienal | 2.605 | 0.202 | 26537-70-2 |
| 11 | 15.06A | Tridecanoic acid, methyl ester | 5.470 | ND | 1731-88-0 |
| 12 | 16.69A 16.65B |
Linoleic acid, methyl ester | 12.058 | 1.451 | 112-63-0 |
| 13 | 16.75A | Linolenic acid, methyl ester | 5.917 | ND | 301-00-8 |
| 14 | 17.03A | Linolelaidic acid, methyl ester | 0.402 | ND | 2566-97-4 |
| 15 | 19.75A 19.69B |
9,12-Octadecadien-1-ol, | 0.322 | 3.243 | 506-43-4 |
| 16 | 20.73A | 9,12,15-Octadecatrien-1-ol | 0.167 | ND | 506-44-5 |
RT: Retention time; A: Acetone extract; B: Ethanolic extract.
*Chemical Abstracts Service Registry Number from US National Institute of Standard Technology (NIST) library.
In this study, peaks 10, 12, 13, 14, 15, and 16 are important chemical compounds associated with polyunsaturated fatty acids (linoleic and linolenic acids). Furthermore, only one saturated fatty acid derivatives was obtained (tridecanoic acid, methyl ester), while in avocado seed oil cultivated in Brazil, Rodrigues and Meirelles (2008) identified palmitic, oleic, and linoleic acids as the main compounds and small traces of lauric, myristic, stearic linolenic, arachidonic and gadoleic acids. Otherwise, Flores et al. (2019) determined the composition of avocado seed oil from two varieties harvested in Chile (Negra de la Cruz and Hass) and described linoleic and oleic acid as the predominant fatty acids, respectively. The authors suggest that the essential fatty acids and beneficial for health, (ω-6 and ω-3), are precursors of fatty acids with larger side chain and more unsaturated.
3.2. Antioxidant capacity
Total phenolic compounds and antioxidant capacity evaluated by DPPH inhibition and iron reduction of acetone and ethanolic extracts of avocado seed are shown in Table 2. Acetone extract exhibited a higher power reduction than ethanol extract (p < 0.05), but no significant difference was found in DPPH inhibition and phenolic compounds content (p > 0.05). The selection of solvent extraction needs to consider food composition, since it will affect the compounds extracted; a less polar solvent such as acetone should be more useful for the extraction of bioactive compounds and antioxidant capacity from avocado seed. A similar result was reported by Vega-Arroy et al. (2017), where acetone was a better solvent for extraction than ethanol for compounds with high antioxidant capacity. The use of solvents in food industry is regulated (Meneses et al., 2013). Ethanol is recognized as safe (GRAS) since it can be employed directly without a subsequent process, toxicological and medical studies have shown it does not produce adverse effects on human health (Molino et al., 2018). In contrast, acetone is classified as an unsafe solvent (Bhebhe et al., 2016); however it is naturally present in fruits and vegetables (grapes, onions, beans). According to the European Directive 2009/32/CE, acetone is GRAS. It is present in beverages, baked goods and desserts in concentrations of 5 to 8 mg/L (Molino et al., 2018). Ethanol as solvent can be suitable to extract compounds with antioxidant activity from food or by-products matrices, due to its low toxicity and high extractive efficiency (Melgar et al., 2018, Segovia-Goméz et al., 2014). On the other hand, phenolic compounds content is lower (155.30 mg GAE/ 100 g) than those reported by Kosinska et al. (2012), although the antioxidant activity evaluated by the inhibition of the DPPH radical is similar to those results reported by Figueroa et al. (2018).
Table 2.
Phenolic compounds and antioxidant capacity of acetone and ethanolic extracts of avocado seed (Persea americana cv. Criollo sp.).a
| Extract | Phenolic compounds (mg GAE/100 g) | Antioxidant capacity (mg Trolox/100 g) | Reduction power (mg AAE/100 g) | |
|---|---|---|---|---|
| Ethanolic | 30.25 ± 0.21a | 183.75 ± 7.14a | 45.05 ± 1.20a | |
| Acetone | 30.80 ± 0.84a | 212.75 ± 12.37a | 56.35 ± 1.90b |
Mean ± standard deviation. Different letters within the same column are statistically different (p < 0.05).
Villa-Rodríguez et al. (2011) determined the antioxidant activity of 2 fractions (hydrophilic and lipophilic) of avocado pulp. The results showed that lipophilic extracts contributed to a greater extent to the total antioxidant capacity, with the three methods used. Similar results were described by Rodríguez-Sánchez et al. (2013), who determined that the antioxidant capacity of the lipophilic fraction of a crude extract of avocado pulp was significantly higher than the hydrophilic fraction. In both investigations, they suggest fatty acids or acetogenins as the main components of antioxidant activity. Moreover, they described that this type of antioxidants could penetrate cell membranes and reach higher levels of bioavailability (hydrophilic antioxidants are excreted in urine). Previously, Richard et al. (2008), had reported that unsaturated fatty acids could act as antioxidants depending on their degree of unsaturation.
According to the results reported by different authors, linoleic acid, linolenic acid, estragole and the mixture of sesquiterpenoids, some of them found in this study possess antioxidant properties evaluated by different antioxidant assays (Bozin et al., 2006, Villa-Rodríguez et al., 2011). Aliphatic chain’s unsaturation is important since they have been shown to be useful for stabilizing radicals in vitro assays (DPPH and TEAC) whose mechanism is based on electron transfer (Figueroa et al., 2018, Villa-Rodríguez et al., 2011). Bioactive compounds extraction and antioxidant capacity from fruits, vegetables, and their by-products may be affected by the fruit variety, the drying procedure, the extraction technique, the crop variety, and agronomic conditions, as well as post-harvest handling and fruit ripening stage (Villa-Rodríguez et al., 2011).
3.3. Antimicrobial activity
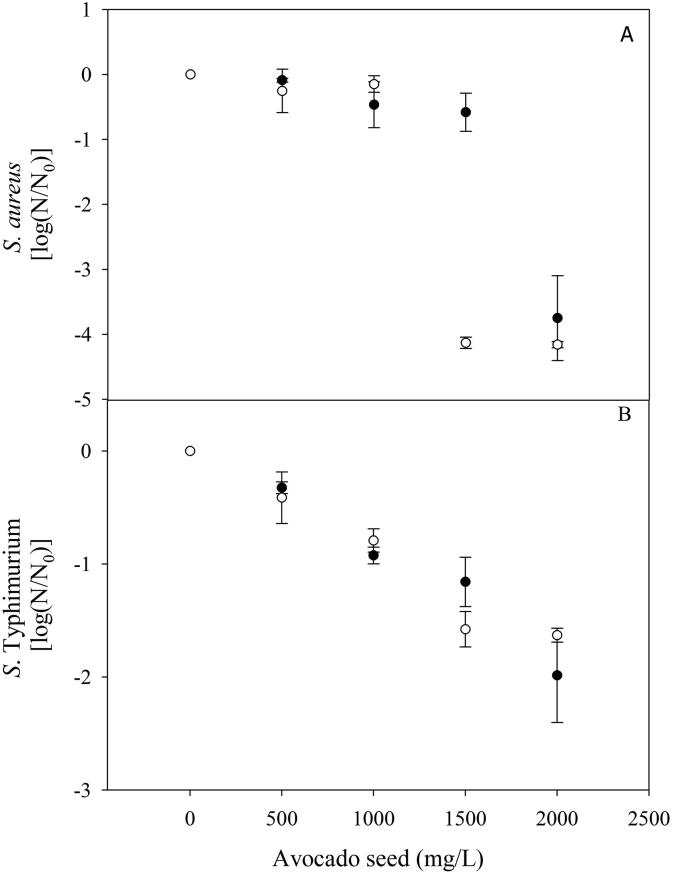
Fig. 2 shows the antimicrobial activity of acetone and ethanolic extracts of avocado seed. As observed, by increasing the concentration of avocado seed extracts also increased the microbial inhibition, determined by the Log reduction in microbial growth in the presence of the extract for both microorganisms. In this aspect, oxygenated isoprene such as isoestragole and estragole display antimicrobial activity by the increasing of the membrane fluidity, with the consequent proton and potassium ions leakage, with membrane potential collapsing the inhibition of ATP synthesis (Fisher and Phillips, 2008). The unsaturated fatty acids found in this investigation, have also shown antimicrobial capacity. These compounds are inserted into the membrane due to their double bonds in cis configuration and alter the membrane functionality due to a disorder in the phospholipid chains (acyl group), which leads to fluidity, disorganization and disintegration of the cell membrane. The alterations in membrane fluidity also increase membrane oxidative stress, through autoxidation (formation of peroxides and radicals) that inhibit cell growth (Salinas-Salazar et al., 2016). Moreover, Zheng et al. (2005) indicated that unsaturated fatty acids such as linolenic also possess antimicrobial activity; inhibiting a non-acyl carrier protein reductase (rate-limiting protein of the bacterial fatty acid chain elongation process); due to it can inhibit the fatty acids biosynthesis of microorganisms.
Fig. 2.
Effect of acetone (○) and ethanolic (●) extracts of avocado seed (Persea americana cv. Criollo sp.) against Staphylococcus aureus (A) and Salmonella Typhimurium (B).
Maximum microbial reductions were obtained with the highest concentration of avocado seed extract (4.0 ± 0.3 and 1.8 ± 0.3 log cycles for S. aureus and S. Typhimurium, respectively), regardless of the solvent used (p > 0.05). The minimum microbial reduction was obtained against S. Typhimurium, which may be attributed to the composition and structure of its cell wall. In this aspect, Gram-negative bacteria present a lipid bilayer, which provides more protection against antimicrobial compounds (Beristain-Bauza et al., 2019). Antimicrobial effect of avocado extracts against S. aureus displays different behaviors. Acetone extract showed its maximum antimicrobial activity at 1000 mg/L, and then a non-significant reduction (p > 0.05) was observed. On the other hand, ethanolic extract significantly reduced (p < 0.05) S. aureus at 2000 mg/L. Villarreal-Lara et al. (2019) determined the antimicrobial activity of Gram-positive and Gram-negative bacteria from an avocado seed extract added with acetogenins. The results showed that Gram-positive spore-forming bacteria were inhibited, whereas no inhibition was observed for non-spore-forming species. Furthermore, they did not observe growth inhibition of Gram-negative bacteria; these researchers associate lipophilic compounds with antimicrobial capacity. Moreover, Melgar et al., 2018, Rodríguez-Carpena et al., 2012 show that the same concentration of avocado seed extract is needed to inhibit Gram-positive and Gram-negative bacteria growth regardless of their membrane composition. The bactericidal activity is associated with phenolic compounds (catechins, procyanidins, and hydroxycinnamic acids) present in the extract. In this report, a larger inhibition of Gram-positive bacteria was observed, and their microbiological activity is related to low molecular weight volatile and lipophilic compounds. The activity is related to low molecular weight of volatile and lipophilic compounds (terpenes). Since their hydrophobicity damages cell integrity (expansion, permeability, increased fluidity, alteration of membrane proteins). In addition, the inhibition of metabolic processes and alteration of ion transport processes forms membrane pores and therefore the constituents pass easily and cause bacteria collapse and change of functions (França Orlanda and Nascimento, 2015, Zengin and Baysal, 2014).
4. Conclusion
Volatile chemical profiling indicates that avocado seed (Persea americana cv. Criollo sp.) contains sesquiterpenoids, unsaturated fatty acids esters, and polyunsaturated fatty acids. Moreover, ethanolic and acetone extracts from avocado seed presented phenolic compounds with antioxidant and antimicrobial (Gram positive and negative bacteria) capacities. Although avocado seed is considered a by-product; it can be suggested that fatty acids esters and unsaturated fatty acids determined in this research are essential and beneficial for health; therefore, our results obtained in this study indicate that solvent-extracted compounds can be used for application in food products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors would like to thank the Benemérita Universidad Autónoma de Puebla for the support provided in this research.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abubakar A.N.F., Achmadi S.S., Suparto I.H. Triterpenoid of avocado (Persea americana) seed and its cytotoxic activity toward breast MCF-7 and liver HepG2 cancer cells. Asian Pac. J. Trop. Biomed. 2017;7:397–400. doi: 10.1016/j.apjtb.2017.01.010. [DOI] [Google Scholar]
- Alañón M.E., Alarcón M., Marchante L., Díaz-Maroto M.C., Pérez-Coello M.S. Extraction of natural flavorings with antioxidant capacity from cooperage by-products by green extraction procedure with subcritical fluids. Ind. Crops Prod. 2017;103:222–232. doi: 10.1016/j.indcrop.2017.03.050. [DOI] [Google Scholar]
- Alkhalaf, M.I., Alansari, W.S., Ibrahim, E.A., ELhalwagy, M.E.A., 2019. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. - Sci. 31, 1358–1362. https://doi.org/10.1016/j.jksus.2018.10.010.
- Amchova P., Kotolova H., Ruda-Kucerova J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015;73:914–922. doi: 10.1016/j.yrtph.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Araújo M.M., Marchioni E., Villavicencio A.L.C.H., Zhao M., di Pascoli T., Kuntz F., Bergaentzle M. Mechanism of folic acid radiolysis in aqueous solution. LWT - Food Sci. Technol. 2015;63:599–603. doi: 10.1016/j.lwt.2015.03.038. [DOI] [Google Scholar]
- Arlene A.A., Prima K.A., Utama L., Anggraini S.A. The Preliminary Study of the Dye Extraction from the Avocado Seed Using Ultrasonic Assisted Extraction. Procedia Chem. 2015;16:334–340. doi: 10.1016/j.proche.2015.12.061. [DOI] [Google Scholar]
- Barbosa-Martín E., Chel-Guerrero L., González-Mondragón E., Betancur-Ancona D. Chemical and technological properties of avocado (Persea americana Mill.) seed fibrous residues. Food Bioprod. Process. 2016;100:457–463. doi: 10.1016/j.fbp.2016.09.006. [DOI] [Google Scholar]
- Barros H.D.F.Q., Grimaldi R., Cabral F.A. Lycopene-rich avocado oil obtained by simultaneous supercritical extraction from avocado pulp and tomato pomace. J. Supercrit. Fluids. 2017;120:1–6. doi: 10.1016/j.supflu.2016.09.021. [DOI] [Google Scholar]
- Beristaín-Bauza S., Martínez-Niño A., Ramírez-González A.P., Ávila-Sosa R., Ruíz-Espinosa H., Ruiz-López I.I., Ochoa-Velasco C.E. Inhibition of Salmonella Typhimurium growth in coconut (Cocos nucifera L.) water by hurdle technology. Food Control. 2018;45:615–621. doi: 10.1016/j.foodcont.2018.02.042. [DOI] [Google Scholar]
- Beristain-Bauza S.D.C., Hernández-Carranza P., Cid-Pérez T.S., Ávila-Sosa R., Ruiz-López I.I., Ochoa-Velasco C.E. Antimicrobial Activity of Ginger (Zingiber Officinale) and Its Application in Food Products. Food Reviews International. 2019;35(5):407–426. doi: 10.1080/87559129.2019.1573829. [DOI] [Google Scholar]
- Bhebhe M., Füller T.N., Chipurura B., Muchuweti M. Effect of Solvent Type on Total Phenolic Content and Free Radical Scavenging Activity of Black Tea and Herbal Infusions. Food Anal. Methods. 2016;9:1060–1067. doi: 10.1007/s12161-015-0270-z. [DOI] [Google Scholar]
- Bora P.S., Narain N., Rocha R.V.M., Queiroz Paulo M. Characterization of the oils from the pulp and seeds of avocado (cultivar: Fuerte) fruits. Grasas y Aceites. 2001;52:171–174. doi: 10.3989/gya.2001.v52.i3-4.353. [DOI] [Google Scholar]
- Bozin B., Mimica-Dukic N., Simin N., Anackov G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- Calderón-Oliver M., Escalona-Buendía H.B., Medina-Campos O.N., Pedraza-Chaverri J., Pedroza-Islas R., Ponce-Alquicira E. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT - Food Sci. Technol. 2016;65:46–52. doi: 10.1016/j.lwt.2015.07.048. [DOI] [Google Scholar]
- Canto-Pereira M.E., Tieman D.M., Sargent S.A., Klee H.J., Huber D.J. Volatile profiles of ripening West Indian and Guatemalan-West Indian avocado cultivars as affected by aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013;80:37–46. doi: 10.1016/j.postharvbio.2013.01.011. [DOI] [Google Scholar]
- Dabas D., Shegog R., Ziegler G., Lambert J. Avocado (Persea americana) Seed as a Source of Bioactive Phytochemicals. Curr. Pharm. Des. 2013;19:6133–6140. doi: 10.2174/1381612811319340007. [DOI] [PubMed] [Google Scholar]
- Díaz-Muñoz L.L., Bonilla-Petriciolet A., Reynel-Ávila H.E., Mendoza-Castillo D.I. Sorption of heavy metal ions from aqueous solution using acid-treated avocado kernel seeds and its FTIR spectroscopy characterization. J. Mol. Liq. 2016;215:555–564. doi: 10.1016/j.molliq.2016.01.022. [DOI] [Google Scholar]
- Dorman H.J.D., Bachmayer O., Kosar M., Hiltunen R. Antioxidant Properties of Aqueous Extracts from Selected Lamiaceae Species Grown in Turkey. J. Agric. Food Chem. 2004;52:762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- Figueroa J.G., Borrás-Linares I., Lozano-Sánchez J., Segura-Carretero A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018;105:752–763. doi: 10.1016/j.foodres.2017.11.082. [DOI] [PubMed] [Google Scholar]
- Fisher K., Phillips C. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends Food Sci. Technol. 2008;19:156–164. [Google Scholar]
- Flores M., Ortiz-Viedma J., Curaqueo A., Rodriguez A., Dovale-Rosabal G., Magaña F., Vega C., Toro M., López L., Ferreyra R., Defilippi B.G. Preliminary Studies of Chemical and Physical Properties of Two Varieties of Avocado Seeds Grown in Chile. J. Food Qual. 2019:1–11. [Google Scholar]
- França Orlanda J.F., Nascimento A.R. Chemical composition and antibacterial activity of Ruta graveolens L. (Rutaceae) volatile oils, from São Luís, Maranhão. Brazil. South African J. Bot. 2015;99:103–106. doi: 10.1016/j.sajb.2015.03.198. [DOI] [Google Scholar]
- García-Rojas M., Morgan A., Gudenschwager O., Zamudio S., Campos-Vargas R., González-Agüero M., Defilippi B.G. Biosynthesis of fatty acids-derived volatiles in ‘Hass’ avocado is modulated by ethylene and storage conditions during ripening. Sci. Hortic. (Amsterdam) 2016;202:91–98. [Google Scholar]
- Ge Y., Si X., Wu B., Dong X., Xu Z., Ma W. Oil Content and Fatty Acid Composition of the Seeds of 16 Avocado (Persea americana) Accessions Collected From Southern China and Their Application in a Soap Bar. J. Agric. Sci. 2018;10:69–78. [Google Scholar]
- Guzmán-Rodríguez J.J., Ibarra-Laclette E., Herrera-Estrella L., Ochoa-Zarzosa A., Suárez-Rodríguez L.M., Rodríguez-Zapata L.C., Salgado-Garciglia R., Jimenez-Moraila B., López-Meza J.E., López-Gómez R. Analysis of expressed sequence tags (ESTs) from avocado seed (Persea americana var. drymifolia) reveals abundant expression of the gene encoding the antimicrobial peptide snaking. Plant Physiol. Biochem. 2013;70:318–324. doi: 10.1016/j.plaphy.2013.05.045. [DOI] [PubMed] [Google Scholar]
- Hernández-Carranza P., Ávila-Sosa R., Guerrero-Beltrán J.A., Navarro-Cruz A.R., Corona-Jiménez E., Ochoa-Velasco C.E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. J. Food Process. Preserv. 2016;40 doi: 10.1111/jfpp.12588. [DOI] [Google Scholar]
- Idris S., Ndukwe G.I., Gimba C.E. Preliminay phytochemical screening antimicrobial activity of seed extracts of Persea americana (avocado pear) Bayero J. Pure Appl. Sci. 2009;2:173–176. [Google Scholar]
- Kosinska A., Karamac M., Estrella I., Hernández T., Bartolomé B., Dykes G.A. Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varieties. J. Agric. Food Chem. 2012;60:4613–4619. doi: 10.1021/jf300090p. [DOI] [PubMed] [Google Scholar]
- Leite J.J., Brito E.H., Cordeiro R.A., Brilhante R.S., Sidrim J.J., Bertini L.M., Morais S.M., Rocha M.F. Chemical composition, toxicity and larvicidal and antifungal activities of Persea americana (avocado) seed extracts. Rev. Soc. Bras. Med. Trop. 2009;42:110–113. doi: 10.1590/s0037-86822009000200003. [DOI] [PubMed] [Google Scholar]
- López-Cobo A., Gómez-Caravaca A.M., Pasini F., Caboni M.F., Segura-Carreto A., Fernández-Gutiérrez A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. Food Sci. Technol. 2016;73:505–513. [Google Scholar]
- Melgar B., Dias M.I., Ciric A., Sokovic M., Garcia-Castello E.M., Rodriguez-Lopez A.D., Barrosa L., Ferreira I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crops Prod. 2018;111:212–218. [Google Scholar]
- Meneses N.G.T., Martins S., Teixeira J.A., Mussatto S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013;108:152–158. doi: 10.1016/j.seppur.2013.02.015. [DOI] [Google Scholar]
- Molino A., Rimauro J., Casella P., Cerbone A., Larocca V., Chianese S., Karatza D., Mehariya S., Ferraro A., Hristoforou E., Musmarra D. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018;283:51–61. doi: 10.1016/j.jbiotec.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Nwaokobia K., Oguntokun M.O., Okolie P.L., Ogboru R.O., Idugboe O.D. Evaluation of the chemical composition of Persea americana (Mill) pulp and seed. J. Biosci. Biotechnol. Discov. 2018;3:83–89. 10.31248/JBBD2018.071. [Google Scholar]
- Pedreschi R., Hollak S., Harkema O., Otma E., Robledo P., Westra E., Somhorst D., Ferreyra R., Defilippi B.G. Impact of postharvest ripening strategies on ‘Hass’ avocado fatty acid profiles. South African J. Bot. 2016;103:32–35. [Google Scholar]
- Richard D., Kefi K., Bausero P., Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008;57:451–455. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Rodrigues C.E.C., Meirelles A.J.A. Extraction of free fatty acids from peanut oil and avocado seed oil: liquid-liquid equilibrium data at 298.2 K. J. Chem. Eng. Data. 2008;53:1698–1704. [Google Scholar]
- Rodríguez-Carpena J.G., Morcuende D., Andrade M.-J., Kylli P., Estévez M. Avocado (Persea americana Mill.) Phenolics, In Vitro Antioxidant and Antimicrobial Activities, and Inhibition of Lipid and Protein Oxidation in Porcine Patties. J. Agric. Food Chem. 2011;59:5625–5635. doi: 10.1021/jf1048832. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carpena J.G., Morcuende D., Estévez M. Avocado, sunflower and olive oils as replacers of pork back-fat in burger patties: Effect on lipid composition, oxidative stability and quality traits. Meat Sci. 2012;90:106–115. doi: 10.1016/j.meatsci.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Sánchez D.G., Pacheco A., García-Cruz M.I., Gutiérrez-Uribe J.A., Benavides-Lozano J.A., Hernández-Brenes C. Isolation and structure elucidation of avocado seed (Persea americana) lipid derivatives that inhibit Clostridium sporogenes endospore germination. J. Agric. Food Chem. 2013;61:7403–7411. doi: 10.1021/jf401407s. [DOI] [PubMed] [Google Scholar]
- Saavedra J., Córdova A., Navarro R., Díaz-Calderón P., Fuentealba C., Astudillo-Castro C., Toledo L., Enrione J., Galvez L. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 2017;198:81–90. [Google Scholar]
- SAGARPA, 2011. Monografía de Cultivos. Subsecretaría de Fomento a los Agronegocios. Aguacate [WWW Document]. URL http://www.sagarpa.mx/agronegocios/Documents/pablo/Documentos/Monografias/Monografía del aguacate.pdf (accessed 2.6.19).
- Salinas-Salazar C., Hernández-Brenes C., Rodríguez-Sánchez D.G., Castillo E.C., Navarro-Silva J.M., Pacheco A. Inhibitory Activity of Avocado Seed Fatty Acid Derivatives (Acetogenins) Against Listeria Monocytogenes. J. Food Sci. 2016;82:134–144. doi: 10.1111/1750-3841.13553. [DOI] [PubMed] [Google Scholar]
- Segovia-Goméz F., Peiró-Sánchez S., Gallego-Iradí M.G., Mohd-Azman N.A., Almajano P. Avocado Seeds: Extraction Optimization and Possible Use as Antioxidant in Food. Antioxidants. 2014;3:459–464. doi: 10.3390/antiox3020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia F.J., Corral-Pérez J.J., Almajano M.P. Avocado seed: Modeling extraction of bioactive compounds. Ind. Crops Prod. 2016;85:213–220. [Google Scholar]
- Segovia, F.J., Hidlago, G., Villasante, J., Ramis, X., Almajano, M.P., 2018. Avocado seed: A comparative study of antioxidant content and capacity in protecting oil models from oxidation. Molecules 23, 2421–2429. [DOI] [PMC free article] [PubMed]
- SIAP, 2017. Analisis Estadístico de la Producción [WWW Document]. Serv. Inf. Agroaliment. y Pesq. URL https://nube.siap.gob.mx/cierreagricola/ (accessed 2.6.19).
- USDA, 2015. United States Standards for Grades of Florida Avocados [WWW Document]. United States Dep. Agric. URL https://www.ams.usda.gov/sites/default/files/media/Avocado_Florida_Grade_Standard%5B1%5D.pdf (accessed 2.1.19).
- Vega-Arroy J.D., Ruiz-Espinosa H., Luna-Guevara J.J., Luna-Guevara M.L., Hernández-Carranza P., Ávila-Sosa R., Ochoa-Velasco C.E. Effect of solvents and extraction methods on total anthocyanins, phenolic compounds and antioxidant capacity of Renealmia alpinia (Rottb.) maas peel. Czech J. Food Sci. 2017;35:1–10. [Google Scholar]
- Villa-Rodríguez J.A., Molina-Corral F.J., Ayala-Zavala J.F., Olivas G.I., González-Aguilar G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of ‘Hass’ avocado. Food Res. Int. 2011;44:1231–1237. [Google Scholar]
- Villarreal-Lara R., Rodríguez-Sánchez D.G., Díaz De La Garza R.I., García-Cruz M.I., Castillo A., Pacheco A., Hernández-Brenes C. Purified avocado seed acetogenins: Antimicrobial spectrum and complete inhibition of Listeria monocytogenes in a refrigerated food matrix. CYTA - J. Food. 2019;17:228–239. [Google Scholar]
- Wang W., Bostic T.R., Gu L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010;122:1193–1198. [Google Scholar]
- Zengin H., Baysal A. Antibacterial and Antioxidant Activity of Essential Oil Terpenes against Pathogenic and Spoilage-Forming Bacteria and Cell Structure-Activity Relationships Evaluated by SEM Microscopy. Molecules. 2014;19:17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C.-J., Yoo J.-S., Lee T.-G., Cho H.-Y., Kim Y.-H., Kim W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579:5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Kolar P., Shan S.B., Cheng J.J., Lim P.K. Avocado seed-derived activated carbon for mitigation of aqueous ammonium. Ind. Crops Prod. 2016;92:34–41. [Google Scholar]