Abstract
This present research investigated the anti-obesity and hepatoprotective effects of ethanolic Moringa peregrina leaf (MPLE) and bark extracts (MPBE), in the rats fed with a high-fat diet (HFD). Healthy male rats (n = 48) were randomly distributed to six groups (n = 8): control AIN-93 diet; HFD; HFD + MPBE bark extracts ((300 mg/kg); HFD + MPBE (600 mg/kg); HFD + MPLE (300 mg/kg); HFD + MPLE (600 mg/kg). HFD-fed rats in the Moringa peregrina (MP) treatment groups received orally administered MP leaf or bark extract daily for eight weeks. The results revealed that both doses of MP leaf extract significantly reduced HFD-induced increases in their food intake and the gained body weight, fat pad weights (visceral, subcutaneous, and epididymal), glucose and insulin plasma levels, and leptin and resistin serum levels in HFD-fed rats. Concomitantly, MP leaf extract improved glucose levels after oral or intraperitoneal glucose tolerance tests, reduced serum cholesterol, triglycerides, and the low-density lipoprotein LDL concentration, reduced hepatic triglycerides and cholesterol levels, and increased serum high-density lipoproteins HDL levels and triglycerides and cholesterol levels in fecal. Moreover, the administration of MPLE to HFD-fed rats improved liver architecture, reduced fat accumulation, reduced hepatic malondialdehyde, tumor necrosis factor-α, and interleukin-6 levels. Hepatic glutathione peroxidase, superoxide dismutase, and catalase activities were significantly increased. All observed effects were more pronounced in HFD-fed rats treated with a 600 mg/kg MP dose. However, neither dose of MPBE altered the measured markers in the HFD-fed rats. In conclusion, MPLE showed potential anti-obesity and hepatoprotective activity in HFD-induced obese rats, mediated by reduced lipid absorption, anti-hyperlipidemic effects, and hepatic antioxidant effects.
Keywords: High-fat diet, Hepatoprotective, Lipid profile Moringa peregrina, Obesity, Rats
1. Introduction
Obesity is a serious health problem that affects at least 13% of the global populace, numbers that are increasing dramatically in both low- and high-income countries (Yun, 2010, WHO, 2015). Obesity is a chronic metabolic disease that is the pathological result of interactions between environmental and genetic factors, leading to an energy imbalance among calorie intake and spending (Vaidya, 2006, Oussaada et al., 2019). The high consumption of energy-dense diets, such as high-fat diets (HFD), with reduced physical activities, is believed to be the leading cause of obesity in susceptible individuals (Fock and Khoo, 2013, Sasaki et al., 2014, Oussaada et al., 2019).
Obesity is associated with various phenotypic and metabolic alterations, including increased body weight, abdominal and visceral obesity, low-grade inflammation, insulin resistance (IR), hyperinsulinemia, hyperleptinemia, hyperglycemia, hyperlipidemia, systemic inflammation, and hepatic steatosis (McArdle et al., 2013, Tanti et al., 2013, Jung and Choi, 2014, Wu and Ballantyne, 2020). Therefore, obesity represents a major medical and socio-economic burden, that leads to chronic metabolic development and psychological disorders like diabetes mellitus (DM), hypertension, coronary artery disease (i.e., atherosclerosis), liver failure, cancer, rheumatoid arthritis (RA), osteoarthritis, and depression (Jarolimova et al., 2013, Apovian, 2016). Hence, management and treatment of obesity are essential to achieve a better quality of life.
Currently approved strategies to treat obesity involve lifestyle modifications and medications that reduce dietary lipid absorption and/or alter lipid mobilization and utilization (Kang and Park, 2012, Cheung et al., 2013). However, the use of anti-obesity drugs is limited by the numerous clinical side effects, including mouth dryness, insomnia, adverse cardiovascular and cerebrovascular effects, anxiety, and constipation (Yun, 2010, Kang and Park, 2012, Cheung et al., 2013). Accordingly, searching for safer plant-derived drugs that produce fewer side effects has received much attention; several plant extracts showed anti-obesity potencies (Rahman et al., 2017, Arika et al., 2019).
Moringa peregrina (MP) is a member belong the Moringaceae family, which includes 13 species; MP is the most common Moringa species in the Arabian Peninsula. Compared to Moringa oleifera (MO), the health benefits of MP have not been investigated (Azim et al., 2017). MO has been extensively investigated as a natural drug with several therapeutic benefits. Experimental and clinical studies have demonstrated that extracts from different parts of MO possess anti-metabolic properties that can prevent or treat hyperlipidemia and hyperglycemia, DM, and NAFLD, those potent anti-obesity properties (Bais et al., 2014, Al-Malki and El Rabey, 2015, Metwally et al., 2017). Although the studies are limited, MP has also been revealed to exert potent hypoglycemic, antioxidant, and anti-inflammatory effects (El-Alfy et al., 2011, Azim et al., 2017). Despite the potential therapeutic value of MP, information on the anti-obesity effects of MP is scarce. Therefore, we examined the anti-obesity in addition to hepatoprotective effects of MP using an HFD-fed rat model and investigated the possible mechanisms of action.
2. Materials and methods
2.1. Plant collection and authentication
Moringa peregrina whole plant (leaves and bark) was obtained from a local farm in the Riyadh region of Saudi Arabia. The voucher specimen (15,642) was placed in Pharmacognosy Department herbarium, Pharmacy College, King Saud University, Riyadh, Saudi Arabia. The leaves and bark were manually separated, washed with water, slice into small chops, and sun-dried. The dried specimens were homogenized using a grinder and stored in brown glass jars at 25 °C until extraction.
2.2. Ethanol extract preparation
The ethanolic extraction was carried out executed according to the previously described method by Hasan et al. (2007). Briefly, the leaf and bark samples (500 g) were extracted using 1.0 L of ethanol for 30 h ambient temperature with continuous shaking succussion. The extracts were filtered by Whatman filter paper No. 4, then centrifuged. The resulting solvent containing the extracted phytochemicals was evaporated using a rotatory evaporator. The extracts were lyophilized and kept in brown containers at − 20 °C until use in the animal feeding trial. The extracts then were dissolved in distilled H2O (saline solution) to the selected final concentrations before oral administration.
2.3. Animals
Forty-eight adult male Wister albino rats (200 ± 20 g, 10 weeks old) were gotten from the Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. The rats were kept in ambient conditions (22 ± 2 °C, 50% humidity, 12/12 h light/dark) under control, they were allowed to access freely their designated diets and drinking water. The experimental procedures have been approved through the KSU Research Ethics Committee (Ethics Reference No: SE-19–114) and accomplished per the animal center guidelines.
2.3.1. Diet preparation
All ingredients used to prepare the standard and HF diet were processed according to the (AIN)-93 M method of the American Institute for Nutrition (Reeves, 1997; Woods et al., 2003), as shown in Table 1. The HFD was previously used in our laboratory to establish hyperlipidemia and obesity over 6–8 weeks. The rats (n = 48) were divided randomly into 6 rat groups 8 in each (n = 8) as follows: 1) Control: standard diet (AIN-93 M); 2) HFD; 3) HFD + 300 mg/kg MP leaf extract; 4) HFD + 600 mg/kg MP leaf extract; 5) HFD + 300 mg/kg MP bark extract; 6) HFD + 600 mg/kg MP bark extract. The leaf and bark extracts were orally administered daily for eight weeks. The food intake and body weight were measured weekly.
Table 1.
The formulation of both standard and high-fat diet for rats.
| Ingredients | Standard diet (grams) | High-fat diet (grams) |
|---|---|---|
| Casein, Lactic, 30 Mesh | 200.00 | 200.00 |
| Cystine, L | 3.00 | 3.00 |
| Starch, Corn | 550.00 | 72.80 |
| Lodex 10 | 150.00 | 100.00 |
| Sucrose | 4.00 | 176.80 |
| Solka Floc, FCC200 | 50.00 | 50.00 |
| Soybean Oil, USP | 25.00 | 25.00 |
| Fat (Butter oil) | 20.00 | 177.5 |
| Minerals (S10026B) | 50.00 | 50.00 |
| Choline Bitartrate | 2.00 | 2.00 |
| Vitamins (V10001C) | 1.00 | 1.00 |
| Dye | 0.05 | 0.05 |
2.3.2. Dose selection
The MP dose used in this experiment was chosen based on previous reports that 200 mg/kg MP is an effective protective dose against paracetamol-induced hepatic damage (Azim et al., 2017). Additionally, MO was shown to exert potent hypolipidemic and anti-obesity effects in a similar animal model when were haven by doses of 200, 400, and 600 mg/kg (Bais et al., 2014, Metwally et al., 2017).
2.3.3. Glucose and insulin tolerance
By the end of the experimental process, each of the rats was exposed to either OGTT (oral glucose tolerance test; 2 g/kg; day 57) or IPITT (intraperitoneal insulin tolerance test; 0.75 units/kg; day 59) as described by Gallou-Kabani et al. (2007). In both cases, the rats were being fasted for a night (12 h) before being tested. Blood samples (250 µl/each) have been collected using a cannula in the tail at 0, 15, 30, 60, and 120 min, in EDTA (10 µl of K3-EDTA10% /ml of blood) Eppendorf tubes and then centrifuged at 1,000 × g for 5 min to collect plasma samples. All plasma samples were frozen at –20 ℃ until use. The glucose and insulin levels in serum were determined at 0 min and considered the fasting levels. The homeostasis model assessment of IR index (HOMA-IRI) (a marker of IR) was calculated according to the equation as follows: HOMA-IRI = [FPG (mg/dL) × fasting IRI (µU/mL)/405].
2.3.4. Serum, plasma, and tissue collection
After the IPITT (day 60), all rats have been anesthetized by an intraperitoneal dose of ketamine hydrochloride/xylazine hydrochloride solution (1.9 mg/kg) (Cat. No. K-113, Sigma Aldrich, St Louis, MO, USA). The samples of blood (2 ml/rat) were collected from the heart directly using cardiac puncture procedure, centrifuged at 3000 rpm for 10 min to obtain the serum and plasma. Serum and plasma samples were deposited at –20 °C until further biochemical analysis. All experimental rats were ethically slaughtered using cervical dislocation and their fat pads (subcutaneous visceral (retroperitoneal, perineal, and mesenteric depots), and epidydimal) and livers were rapidly removed, put in the ice, and their weights were recorded. Pieces of these liver were placed directly in 10% buffered formalin and processed for routine histology as described below. By the liquid nitrogen, Additional liver samples were snap-frozen in and then it persevered at –80 °C until analysis.
2.3.5. Liver, obesity, and adiposity indices
To calculate the adiposity and liver indices, the total weight of the fat pads and livers were divided by the final body weight, respectively (Arika et al., 2019). Obesity (Lee index) was assessed as described by Lee, 1925, Arika et al., 2019; the third square of the final body weight of each animal was divided over the length of the animal (from nose to tail).
2.3.6. Liver homogenate preparation and hepatic lipid extraction
To prepare total liver homogenates, 100 mg frozen liver samples were homogenized in 0.5 ml of ice-cold phosphate-buffered saline (PBS; pH 7.4) (Cat. No. 20012043, Thermo Fisher Scientific) that containing 10 µl of protease inhibitor cocktail (Cat. No A32965, Thermo Fisher Scientific). All samples were then centrifuged at a speed of 1,300 × g for 10 min at 4 °C. The collected supernatants were frozen at –80 °C for future analysis. To measure the lipid fractions in the livers, hepatic lipids were extracted as described in the method of Folch et al. (1957). Briefly, the frozen liver samples were homogenized with 19- vol of the mixture of methanol and chloroform (1:2, v/v). This step was repeated three times for 3 min each. Each time, the tubes were centrifuged at a speed of 1,300 × g for 10 min, and the superior layer containing the lipid phase was isolated. Lastly, the organic solvent was eliminated by reduced pressure and the dried lipids were re-dissolved in 250 µl of isopropanol, then stored at –20° until further analysis.
2.3.7. Plasma, serum, and liver homogenate biochemical measurements
Glucose and insulin plasma levels were quantified by using assays and ELISA kits (Cat. No. 81693; Cat. No. 90010, Crystal Chem, IL, USA). Hepatic and serum rates of total cholesterol (TC) and the high-density lipoproteins (HDL-C) were determined using a special fluorometric kit for rats (Cat. No. STA-384, Cellbiolabs, CA, USA). Serum and hepatic levels of triglycerides (TGs) were measured using a rat assay kit (Cat. NO. STA-396, Cellbiolabs, CA, USA); serum and hepatic levels of low-density lipoproteins (LDL-C) were measured using a rat assay kit (Cat. No. 80069, Crystal Chem, CA, USA). Hepatic glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD) activities, and malondialdehyde (MDA) level were measured using rat ELISA kits (Cat. No. MBS774703; Cat. No. MBS036924; Cat. No. MBS9712526; Cat. No. MBS73868, MyBioSource, CA, USA). Hepatic levels of TNF-α, IL-6 were assessed using special rat ELISA kits (Cat. No. K1052; Cat. No. K4145, BioVision, CA, USA, respectively). The C-reactive protein (CRP) and adiponectin levels in serum were determined by ELISA (Cat. No. ab108827; Cat. No. Ab239421, Abcam, Cambridge, UK). The levels of resistin and leptin in plasma were measured using an ELISA kit (Cat. No. MBS013451 and Cat. No. MBS730855, MyBioSource, CA, USA).
2.3.8. Hematoxylin and eosin staining
Fresh liver tissue samples were fixed in buffer solution (10% formalin). The tissues have been dehydrated in ascending concentrations of ethanol (70–100%), it then cleared with Xylene. All tissues were then embedded in the paraffin wax and slice into 4-μm thick sections. All samples were then stained with hematoxylin and eosin and examined by an independent investigator under a light microscope (Olympus Optical, Tokyo, Japan).
2.4. Statistical analysis
The statistical analyses were accomplished by using Graph Pad Prism analysis software (Version 8, Australia). The changes in the food intake and final body weight were analyzed by two-way ANOVA followed by Post Hoc. Tukey’s test. All other comparison procedures were done using one-way ANOVA followed by Tukey’s t-test. Differences were considered significant statistically at p < 0.05.
3. Results
3.1. Moringa peregrina leaf extract (MPLE) prevents weight gain, increased food intake, and fat mass and exerts an anti-hyperlipidemic effect on HFD-fed rats
The effects of HFD and MP leaf and bark extracts on final body mass, weekly food intake, and subcutaneous, visceral, and epididymal fat pad weight are presented in Table 2. Rats fed HFD and HFD + MP leaf or bark extracts had markedly higher final body mass, weekly food intake, and subcutaneous, visceral, and epididymal fat pad weight than control group rats fed AIN 93 (Table 2). No significant differences were observed among rats fed HFD + MP bark extracts (300 or 600 mg/kg) and rats fed HFD + 300 mg/kg MP leaf extract. Interestingly, rats fed HFD + 600 mg/kg MP leaf extracts had significantly lower visceral and subcutaneous fat deposit weights compared to other groups fed HFD. Similarly, serum, hepatic, and fecal lipid levels followed the same pattern (Table 3). The levels of serum total CHOL, TGs, LDL-C, and levels of hepatic CHOL and TGs of rats fed HFD were higher significantly than that of the control group but did not differ significantly from that of rats fed HFD + MP bark extracts (300 or 600 mg/kg) and rats fed HFD + 300 mg/kg MP leaf extract. However, feeding rats HFD with oral administration of 600 mg/kg of MP leaf extract significantly reduced serum and hepatic lipid levels and increased serum HDL compared to groups fed HFD and did not differ significantly from those fed the AIN 93 control diet. Fecal TG and CHOL in the HFD group did not differ significantly from rats fed HFD with MP bark and leaf extracts but differed significantly from the control.
Table 2.
Final body weight, average food intake, and weight of various fat pads in all groups of rats.
| Control | HFD | HFD + MPLE **(300 mg/kg) | HFD + MPLE **(600mg/kg) | HFD + MPBE **(300 mg/kg) | HFD + MPBE **(600 mg/kg) | |
|---|---|---|---|---|---|---|
| Final body weight (g) | 332 ± 15.6 | 456 ± 24.2 a | 398 ± 17.5ab | 258 ± 18.7abc | 465 ± 22.5acd | 453 ± 32.1acd |
| Weekly food intake (g/rat) | 146.4 ± 22.5 | 265.8 ± 31.2 | 205± 16.3ab | 167 ± 18.7ab | 245 ± 26.5acd | 259 ± 25.2acd |
| Fat deposits (g) | ||||||
| a. Epididymal | 1.4 ± 0.63 | 3.1 ± 0.73a | 2.2 ± 0.65ab | 1.8 ± 0.59abc | 3.6 ± 0.78acd | 3.2 ± 0.45acd |
| b. Visceral | 6.3 ± 0.89 | 11.5 ± 1.5a | 8.2 ± 1.1ab | 6.8 ± 0.59bc | 10.3 ± 1.7acd | 10.8 ± 1.3acd |
| c. Subcutaneous | 2.1 ± 0.34 | 3.9 ± 0.78a | 2.9 ± 0.59ab | 2.3 ± 0.43bc | 4.1 ± 1.1acd | 3.5 ± 0.83acd |
Data were analysed by 1-way ANOVA and presented as mean ± SD. Values were considered significantly different at p < 0.05. a: vs. control-fed standard diet; b: vs. HFD, c: vs. HFD + MP leaf ethanolic extract (300 mg/kg), d: vs. MP leaf ethanolic (600 mg/kg). MPLE: Moringa peregrina leaf extract, MPBE: Moringa peregrina bark extract.
Table 3.
Levels of serum, hepatic and fecal lipids in all groups of rats.
| Lipid Profile | Control | HFD | HFD + MPLE (300 mg/kg) |
HFD + MPLE (600 mg/kg) |
HFD + MPBE (300 mg/kg) |
HFD + MPBE (600 mg/kg) |
|
|---|---|---|---|---|---|---|---|
| Serum | Triglycerides (mg/dl) | 38.5 ± 6.5 | 78.7 ± 11.2a | 58.3 ± 7.3ab | 41.3 ± 7.8bc | 73.2 ± 7.8acd | 76.5 ± 9.3acd |
| CHOL (mg/dl) | 84.3 ± 6.3 | 158.3 ± 13.4a | 102 ± 9.1ab | 87.9 ± 7.6bc | 167.2 ± 12.1acd | 153 ± 8.9acd | |
| LDL-C (mg/dl) | 31.2 ± 6.5 | 78.7 ± 6.5a | 48.7 ± 5.2ab | 35.6 ± 7.2bc | 69.8 ± 8.9acd | 73.3 ± 8.5acd | |
| HDL-C (mg/dl) | 22.3 ± 5.4 | 12.3 ± 3.4a | 17.6 ± 4.1ab | 21.3 ± 3.5bc | 11.7 ± 3.6acd | 13.2 ± 2.9acd | |
| Liver | Triglycerides (mg/g) | 0.67 ± 0.03 | 0.95 ± 0.04a | 0.78 ± 0.05ab | 0.64 ± 0.06bc | 1.03 ± 0.08acd | 0.89 ± 0.06acd |
| CHOL (mg/g) | 2.4 ± 0.36 | 6.3 ± 0.31a | 4.3 ± 0.56ab | 2.8 ± 0.67bc | 5.9 ± 0.87acd | 5.7 ± 0.83 acd | |
| Fecal | Triglycerides (mg/g dried) | 2.4 ± 0.4 | 3.8 ± 0.8a | 5.8 ± 1.3ab | 7.2 ± 1.4abc | 4.1 ± 0.8acd | 3.3 ± 1.1acd |
| CHOL (mg/g dried) | 3.7 ± 0.9 | 5.6 ± 1.3a | 7.2 ± 1.1ab | 9.8 ± 1.2abc | 6.1 ± 1.7acd | 5.8 ± 1.6 acd |
Data were analysed by 1-way ANOVA and presented as mean ± SD. Values were considered significantly different at p < 0.05. a: vs. control-fed standard diet; b: vs. HFD, c: vs. HFD + MP leaf ethanolic extract (300 mg/kg), d: vs. MP leaf ethanolic (600 mg/kg). Moringa peregrina leaf extract, MPBE: Moringa peregrina bark extract. CHOL: Total cholesterol. TGs: triglycerides. LDL-C: Low-density lipoprotein-cholesterol. HDL-C: High-density lipoprotein-cholesterol.
3.2. MPLE improves liver architecture and ameliorates hepatic steatosis
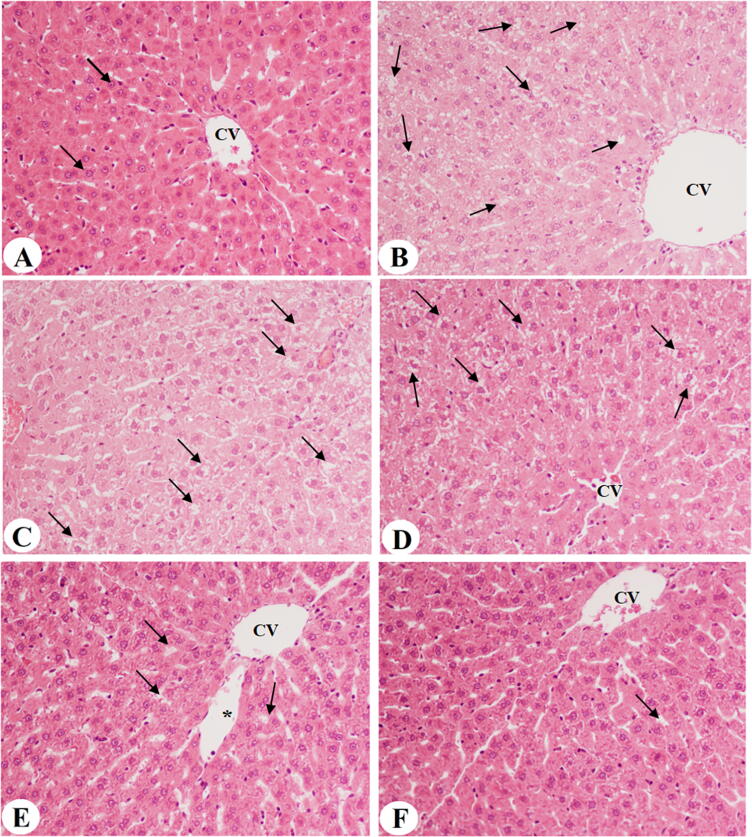
Rats fed the control diet revealed the normal structure of the liver with an intact central vein and normal hepatocytes with curved euchromatic nuclei radiating from the central vein (Fig. 1A). An increase in small and medium fat vacuoles with dilated central veins was observed in the liver tissues of rats fed HFD and HFD + MP bark extract of both doses (300 and 600 mg/kg) (Fig. 1B, C&D). In contrast, an obvious reduction in fat vacuoles with improvement in the hepatocytes structure and normal-sized central veins was observed in the rats fed HFD + MPLE (300 mg/kg) livers (Fig. 1E). Interestingly, the group fed HFD + 600 mg/kg MPLE exhibited almost normal liver structure, with the absence of fat vacuoles, similar to those of the control diet rats (Fig. 1F).
Fig. 1.
Photomicrographs of liver sections obtained from all groups of rats at 200x. A: Control rat fed standard diet, showing normal liver architecture with an intact normal-sized central vein (CV) and sinusoids with normally-sized rounded euchromatic nuclei hepatocytes (arrow) radiating from the CV. B: Rat fed high-fat diet (HFD), showing a dilated CV with increased accumulation of small- and medium-sized fat droplets (arrow). C: Rat fed HFD + Moringa peregrina bark extract (300 mg/kg), showing accumulation of fat small- and medium-sized fat droplets D: Rat fed HFD + Moringa peregrina bark extract (600 mg/kg), showing accumulation of small- and medium-sized fat droplets E: Rat fed HFD + Moringa peregrina leaf extract (300 mg/kg), showing a reduction of fat droplets (arrow) with the presence of some dilated sinusoids (star). F: Rat fed HFD + Moringa peregrina leaf extract (600 mg/kg), showing almost normal liver architecture, mostly lacking fat droplets (arrow).
3.3. MPLE lowers fasting glucose and insulin levels and improves glucose homeostasis and insulin sensitivity in HFD-fed rats
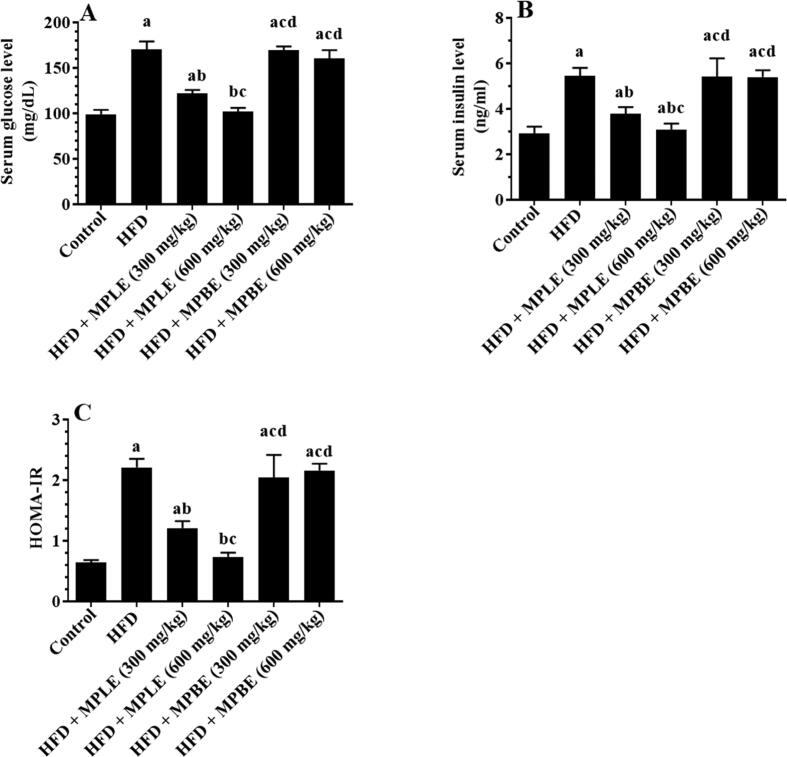
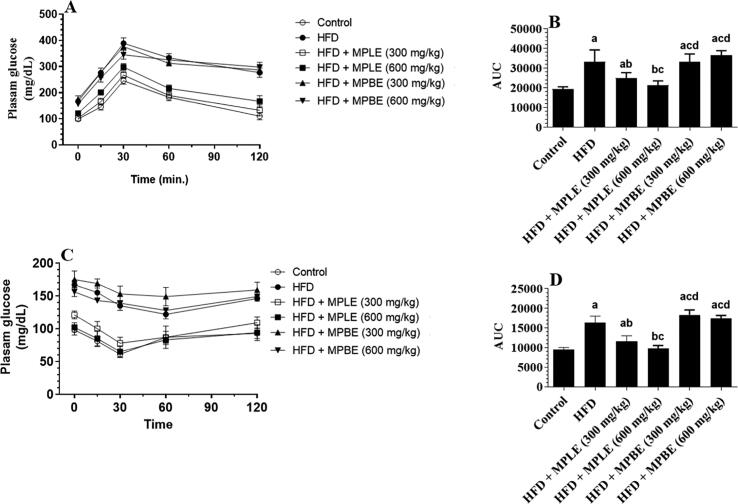
Fig. 2 A&B shows the effects of an HFD diet and HFD with MP bark and leaf extracts on glucose and insulin levels. Fasting glucose and insulin levels were significantly increased in the HFD-fed group compared to that of control rats (Fig. 2A&B). Similarly, the HOMA-IR index was increased significantly in HFD-fed rats in comparison with control rats, indicating an insulin resistance status (Fig. 2C). These results were confirmed by the higher glucose in plasma and the calculated area under the curve (AUC) at all measured time intervals from (15, 30, 60, and 120 min) after the OGTT and IPITT in HFD-fed rats compared to the control rats (Fig. 3A–D). However, feeding rats HFD + MPLE significantly lowered fasting glucose as well as insulin levels, HOMA-IR and concomitantly reduced plasma glucose levels and AUC at all tested intervals after the OGTT and IPITT compared to HFD with oral administration of MP bark extracts (MPBE) (Fig. 2A–C and Fig. 3A–D). The improvement in fasting glucose and insulin levels as well as the reduction in glucose levels after OGTT and IPITT were more profound when MPLE was administered at the highest dose (600 mg/kg as compared to 300 mg/kg). The concentration of fasting glucose, insulin, and HOMA-IR index, and plasma glucose levels and the corresponding AUC of both OGTT and IPITT were not significantly different between rats of the HFD group and those fed HFD with MPBE (300 and 600 mg/kg) (Fig. 2A-C and Fig. 3A-D).
Fig. 2.
Fasting levels of glucose (A) and insulin (B), as well as the calculated homeostatic model assessment for insulin resistance index (C) in the plasma of all groups of rats. Data were analyzed by one-way ANOVA and presented as mean ± SD. Values were considered significantly different at p < 0.05. a: vs. control-fed standard diet; b: vs. HFD, c: vs. HFD + MP leaf extract (300 mg/kg), d: vs. MP leaf extract (600 mg/kg). MPLE: Moringa peregrina leaf extract, MPBE: Moringa peregrina bark extract.
Fig. 3.
Glucose levels and the calculated area under the curve (AUC) after the oral glucose tolerance test (OGTT, A and B) or intraperitoneal insulin tolerance test (IPITT, C and D) in all groups of rats. Data were analyzed by one-way ANOVA and presented as mean ± SD. Values were considered significantly different at p < 0.05. a: vs. control-fed standard diet; b: vs. HFD, c: vs. HFD + MP leaf extract (300 mg/kg), d: vs. MP leaf extract (600 mg/kg). MPLE: Moringa peregrina leaf extract, MPBE: Moringa peregrina bark extract.
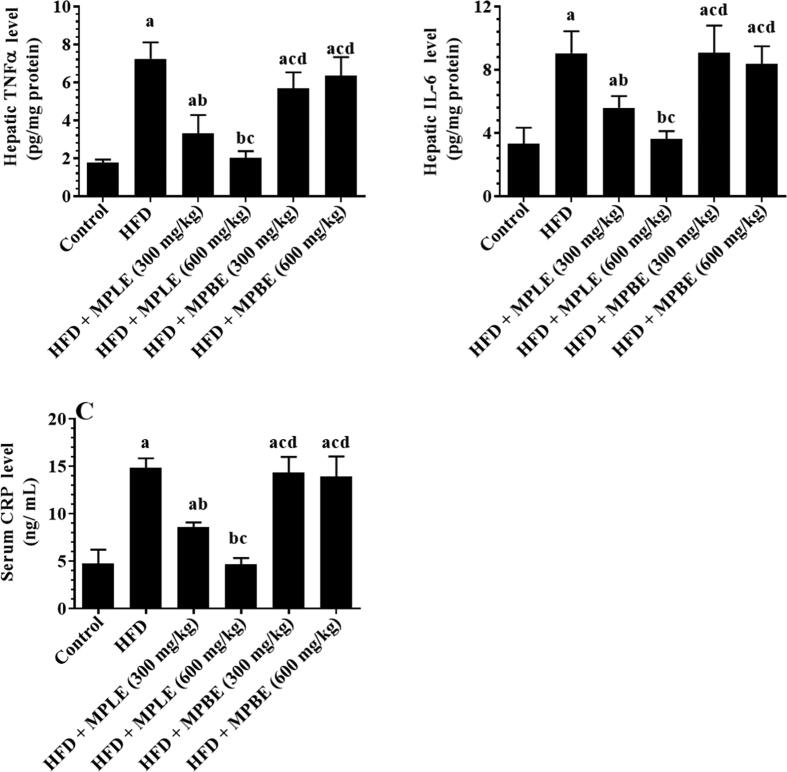
3.4. MPLE suppresses hepatic inflammation and oxidative stress and lowers CKMB serum levels in HFD-fed rats
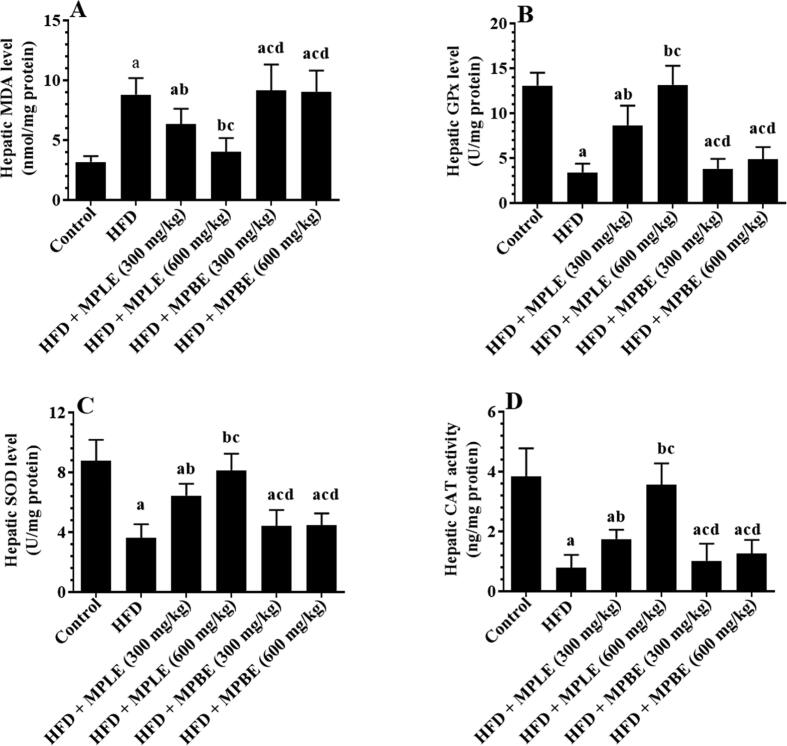
Feeding rats an HFD significantly increased hepatic levels of MDA, TNF-α, IL-6, and serum levels of CRP and significantly decreased hepatic levels of SOD and GPx compared to that of rats fed a control diet (Fig. 4, Fig. 5). In contrast, feeding rats an HFD + 300 or 600 mg/kg MPLE significantly decreased MDA, TNF-α, and IL-6 levels and significantly increased CRP serum levels and SOD, GPx, and CAT hepatic levels compared to those fed a control diet (Fig. 4, Fig. 5). No significant differences were detected between the rats fed HFD and rats fed HFD with MPBE extracts in terms of inflammation and oxidative stress biomarkers. (Fig. 4, Fig. 5). While the levels of these markers remained statistically varied with the MPLE dose of 300 mg/kg, normal levels of all markers were observed in HFD + MPLE (600 mg/kg) rats, which did not differ significantly from those measured in the control rats (Fig. 4, Fig. 5).
Fig. 4.
Malondialdehyde (MDA) (A), glutathione peroxidase (GPx) (B), superoxide dismutase (SOD) (C), and catalase (CAT) (D) levels in the livers of all groups of rats. Data were analyzed by one-way ANOVA and presented as mean ± SD. Values were considered significantly different at p < 0.05. a: vs. control-fed standard diet; b: vs. HFD, c: vs. HFD + MP leaf extract (300 mg/kg), d: vs. MP leaf extract (600 mg/kg). MPLE: Moringa peregrina leaf extract, MPBE: Moringa peregrina bark extract.
Fig. 5.
Tumor necrosis factor-α (TNF-α) (A) and interleukin-6 (IL-6) (B) hepatic levels and C-reactive protein (CRP) (C) serum levels in all groups of rats. Data were analyzed by one-way ANOVA and presented as mean ± SD. Values were considered significantly different at p < 0.05. a: vs. control-fed standard diet; b: vs. HFD, c: vs. HFD + MP leaf extract (300 mg/kg), d: vs. MP leaf extract (600 mg/kg). MPLE: Moringa peregrina leaf extract, MPBE: Moringa peregrina bark extract.
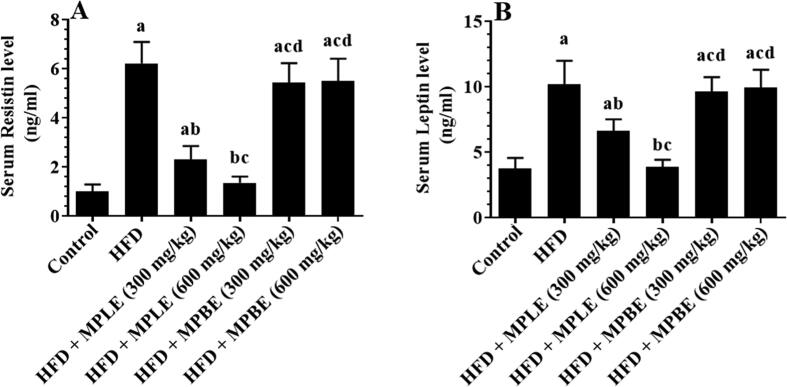
3.5. MPLE reduces serum leptin and resistin in HFD-fed rats
Leptin and resistin serum were higher in HFD-fed rats with significant differences than the control group (Fig. 6A&B). Administration of MPLE significantly reduced the HDF-mediated increase in leptin and resistin levels, which compared significantly with a control group (Fig. 6A&B). No significant differences in leptin and resistin levels were observed among rats fed the control diet and HFD + MPLE (600 mg/kg). However, there are no significant differences were appeared in adipokine serum levels was observed between HFD-fed rats and HFD + MPBE (300 mg and 600 mg/kg) (Fig. 6A&B).
Fig. 6.
Resistin (A) and leptin (B) levels in serum of all groups of rats. Data were analyzed by one-way ANOVA and presented as mean ± SD. Values were considered significantly different at p < 0.05. a: vs. control-fed standard diet; b: vs. HFD, c: vs. HFD + MP leaf extract (300 mg/kg), d: vs. MP leaf extract (600 mg/kg). MPLE: Moringa peregrina leaf extract, MPBE: Moringa peregrina bark extract.
4. Discussion
The results of this study demonstrate the anti-obesity, hypolipidemic, and hepatoprotective effects of MP leaf ethanolic extract in the fed HFD rats. Feeding rodents an energy-dense diet (i.e., HFD) is the most effective strategy to induce obesity that resembles human obesity (Madsen et al., 2010, Lutz and Woods, 2012, Arika et al., 2019). Hyperphagia (over-feeding) is the main mechanism responsible for HFD-induced obesity and fat deposits (Hall et al., 2014, Licholai et al., 2018, Arika et al., 2019). The precise mechanism by which HFD induces hyperphagia remains unclear, however, it is largely accepted that the process involves homeostatic (endocrine and neural), hedonic (palatability), and cognitive feedback stimulation mechanisms (Hall et al., 2014, Licholai et al., 2018). Treatment of HFD-induced obesity with ethanolic extracts of the leaves of MP (300 and 600 mg/kg) not only reduced body and fat weight gains and prevented hepatic steatosis but also improved glucose concentration and insulin hemostasis, suppressed oxidative stress and inflammation in the liver, and attenuated the changes in adipokine levels in HFD-fed rats. Additionally, the positive effects of MP leaf extract on the measured parameters were more profound when the HFD-fed rats were treated with the upper dose (600 mg/kg), which returned most of the measured parameters, including obesity, adiposity index, food intake, and leptin level to normal levels. The MP leaf extracts also reduced TGs and CHOL in the feces of HFD-fed rats, suggesting suppression the intestinal fat absorption. The mechanism by which MP limits fat absorption may be via the suppression of the activity of pancreatic lipase, an effect that is similar to that exerted by orlistat (Viner et al., 2010). These effects were not achieved with the administration of MP bark extract, indicating that the anti-obesity and anti-hyperlipidemic properties of MP are only present in the leaves.
The anti-obesity effects of MP leaf ethanolic extracts could be attributed to the suppression of absorption of fat in the intestine and/or food intake. The observed reduction in leptin levels appears secondary to the reduction of fat intake; however, the MP leaf extract may modulate the activity of leptin through the suppression of transcription factors that regulate adipose tissue metabolism and leptin expression (i.e., PPARγ). Therefore, we can reasonably assume that the observed metabolic and hepatoprotective effects of MP in this study are the results of the suppression of dietary fat intake. In agreement with our findings, Metwally et al., 2017, Bais et al., 2014 reported anti-obesity effects of M. oliefera ethanolic and methanolic leaf extract on HFD-fed rats. Similarly, jaboticaba (Plinia cauliflora) peel and aqueous extract significantly reduced the bodyweight of HFD-fed rats. Although many reports have demonstrated the anti-obesity potential of different plant extracts, the precise mechanism is not clearly understood. The anti-obesity action could be attributed to flavonoids and tannins that reduce palatability and subsequently food intake, inhibiting micelle formation and α-glucosidase, which results in reduced TGs intestinal absorption and suppression of gastric emptying and pancreatic lipase activity (Kim et al., 2010, Zhang et al., 2015, Arika et al., 2019).
Hyperlipidemia, hyperglycemia, low-grade inflammation, and hepatic and systemic IR are major hallmarks of obesity. These common comorbidities in obese individuals and animal models lead to T2DM as well as several cardiovascular and cerebrovascular disorders (Jarolimova et al., 2013, Apovian, 2016, Wu and Ballantyne, 2020). White adipose tissue acts as an endocrine organ, secreting numerous adipokines, including IL-1β, TNF-α, IL-6, and chemoattractants such as MCP-1 and MIF (McArdle et al., 2013). Obese individuals experience increased nutrient intake and adipose tissue mass and alterations in intestinal microbiota and immune cell milieu of the adipose tissue, which initiates low-grade and local inflammation (i.e., metabolic inflammation). This inflammation can extend to other tissues, such as the liver and skeletal muscles, causing tissue inflammation and observed IR (McArdle et al., 2013). Furthermore, the increase in adipose tissue mass stimulates the release of resistin, which induces IR in the skeletal muscles and liver and stimulates hepatic gluconeogenesis (Huang and Yang, 2016). Moreover, to IR, obesity leads to hepatic morphological changes, hepatic steatosis, and NAFLD (Recena et al., 2019, Velázquez et al., 2019). Higher influx of FFAs, overproduction of ROS, oxidative stress, inflammation, and IR were shown to be the major players in HFD-induced NAFLD (Masarone et al., 2018, Lasker et al., 2019).
In this study, HFD-fed rats exhibited increased circulatory levels of LDL-c, lower levels of HDL, hepatic steatosis with increased accumulation of CHOL, TGs and higher circulatory levels of liver injury markers, including CRP, MDA, and inflammatory cytokines (TNF-α & IL-6), with a reduction in antioxidant enzyme levels (CAT, SOD, and GPx). Moreover, IR was demonstrated by the fasting hyperglycemia, hyperinsulinemia, higher HOMA-IR index, and impaired OGTT and IPITT results, which coincided with a significant increase in circulatory resistin levels, reflecting the increase in fat mass and its crucial participation in the observed IR. These data confirm that obesity-induced oxidative stress and inflammation are two crucial mechanisms for hepatic steatosis, liver damage, and IR development.
The present study demonstrated that administration of MP ethanolic leaf extract orally at doses of 300 and 600 mg/kg to HFD-fed rats diminished fasting glucose and insulin rates significantly, increased insulin sensitivity, and decreased circulatory levels of resistin, indicating an attenuation of hyperlipidemia and hepatic oxidative stress, inflammation, and steatosis associated with HFD. The 600 mg/kg MP dose was more effective for attenuating marker levels. Although these effects could be secondary to appetite reduction and inhibition of fat absorption, it could also be possible that MP leaf extract exerts independent hypoglycemic, antioxidant, and anti-inflammatory effects. Similar findings were reported by Zou et al., 2006, Kaffashi Elahi, 2013, and Halaby et al. (2015).
Several investigators have demonstrated the hypoglycemic, antioxidant, and anti-inflammatory effects of many species of Moringa. Khan et al. (2017) reported that oral administration of 100 and 200 mg/kg body weight of M. oleifera leaf ethanolic extraction to STZ-induced diabetic rats and HFD-rats restored the fasting blood glucose, liver marker enzyme, and lipid levels. Similarly, Bais et al., 2014, Metwally et al., 2017 demonstrated significant reductions in fasting glucose and insulin levels with oral feeding of M. Oleifera leaf extract to HFD-fed rats. MP and MO have both shown potent antioxidant, hypolipidemic, anti-atherosclerosis, and hepatoprotective effects in various animal models, suppressing ROS production, inhibiting lipid peroxidation, and upregulating antioxidant gene expression (El-Alfy et al., 2011, Fakurazi et al., 2012, Minaiyan et al., 2014, Fard et al., 2015, Asgari-Kafrani et al., 2020). The anti-inflammatory possibility of MO was demonstrated in several animal models of inflammation, including obesity, colitis, egg albumin-induced inflammation, lipopolysaccharides-induced inflammation, and experimental immune inflammation, which was attributed to the ability of MO to inhibit the activity of nuclear factor Kappa-beta (NF-κB) and prostaglandins and leukotrienes synthesis (Mahajan and Mehta, 2010, Koheil et al., 2011, Minaiyan et al., 2014).
Although the precise mechanisms by which MP leaf extract affords these beneficial effects can’t be concluded from this study, several lines of evidence have suggested that high contents of various hypoglycemic, hypolipidemic, anti-inflammatory, and antioxidant constituents such as phenolics, carotenoids, flavonoids, tannins, glycosides, vitamins, sterols, minerals, amino acids, and alkaloids contribute to the observed therapeutic effects (Lin et al., 2010, Alhakmani et al., 2013). Unfortunately, we did not conduct a phytochemical analysis in this study to determine the MP leaf compounds, which is considered a serious limitation that will be addressed in future research.
Despite these promising findings, the mechanisms by which the observed hypolipidemic, anti-inflammatory, anti-obesity, and antioxidant effects are induced by MP are unknown. Therefore, additional studies at the molecular level are warranted, targeting adipose tissue and liver antioxidant and anti-inflammatory pathways as well as fat absorption in both the control group and HFD-fed rats group to reveal the possible mechanisms of action. Besides, research focusing on glucose uptake, glucose hemostasis, and signaling sensitivity of insulin in both muscles and liver is required to reveal the hypoglycemic effect of MP extract.
5. Conclusion
The outcomes of this study demonstrate that the Moringa peregrina leaf extract possesses potent anti-obesity and hepatoprotective properties, mediated by increasing insulin sensitivity, reduced lipid absorption, anti-hyperlipidemic effects, and hepatic antioxidant effects in High-fat diet-induced obese rats. To propose the potential therapeutic use of MP leaf extract to combat obesity, additional research studies are required to illuminate the mode of action.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia, for funding this work through research group No (RG-1441-530).
Footnotes
Peer review under responsibility of King Saud University.
References
- Alhakmani F., Kumar S., Khan S.A. Estimation of total phenolic content, in-vitro antioxidant, and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac. J. Trop. Biomed. 2013;3(8):623–627. doi: 10.1016/S2221-1691(13)60126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Malki A.L., El Rabey H.A. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed. Res. Int. Article ID. 2015 doi: 10.1155/2015/381040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apovian C.M. Obesity: definition, comorbidities, causes, and burden. Am. J. Manag. Care. 2016;22(7):176–185. [PubMed] [Google Scholar]
- Arika W.M., Kibiti C.M., Njagi J.M., Ngugi M.P. Anti-obesity effects of dichloromethane leaf extract of Gnidia glauca in high fat diet-induced obese rats. Heliyon. 2019;5(11) doi: 10.1016/j.heliyon.2019.e02800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari-Kafrani A., Fazilati M., Nazem H. Hepatoprotective and antioxidant activity of aerial parts of Moringa oleifera in prevention of non-alcoholic fatty liver disease in Wistar rats. S. Afr. J. Bot. 2020;129:82–90. [Google Scholar]
- Azim S.A.A., Abdelrahem M.T., Said M.M., Khattab A. Protective effect of moringa peregrina leaves extract on acetaminophen -induced liver toxicity in albino rats. Afr. J. Tradit. Complement. Altern. Med. 2017;14(2):206–216. doi: 10.21010/ajtcam.v14i2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais S., Singh G.S., Sharma R. Antiobesity and hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Adv. Biol. 2014 [Google Scholar]
- Cheung B.M., Cheung T.T., Samaranayake N.R. Safety of anti-obesity drugs. Ther. Adv. Drug. Saf. 2013;4(4):171–181. doi: 10.1177/2042098613489721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Alfy T.S., Ezzat S.M., Hegazy A.K., Amer A.M., Kamel G.M. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (Family: Moringaceae) growing in Egypt. Pharmacogn. Mag. 2011;7(26):109–115. doi: 10.4103/0973-1296.80667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakurazi S., Sharifudin S.A., Arulselvan P. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen-induced hepatotoxicity in experimental rats through their antioxidant nature. Molecules. 2012;17(7):8334–8350. doi: 10.3390/molecules17078334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fard M.T., Arulselvan P., Karthivashan G., Adam S.K., Fakurazi S. Bioactive extract from moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages. Pharmacogn. Mag. 2015;11(4):556–563. doi: 10.4103/0973-1296.172961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fock K.M., Khoo J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013;28(4):59–63. doi: 10.1111/jgh.12407. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.H. simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Gallou-Kabani C., Vigé A., Gross M.S., Rabès J.P., Boileau C., Larue-Achagiotis C., Tomé D., Jais J.P., Junien C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity (Silver Spring) 2007;15(8):1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- Halaby M.S., El-Din M.M., Emara N.E. Influence of Moringa oleifera on non-alcoholic fatty liver in adult albino rats. Middle East. J. Appl. Sci. 2015;5(4):902–912. [Google Scholar]
- Hall K.D., Hammond R.A., Rahmandad H. Dynamic interplay among homeostatic, hedonic, and cognitive feedback circuits regulating body weight. Am. J. Public Health. 2014;104(7):1169–1175. doi: 10.2105/AJPH.2014.301931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan T.N., Ahmed S.N., Aalam S.M.M., Kumar C., Shafi G. Evaluation of cichorium extract for the growth supporting property in rat hepatocyte primary culture. Asian J. Plant Sci. 2007;6:431–434. [Google Scholar]
- Huang X., Yang Z. Resistin's, obesity and insulin resistance: the continuing disconnect between rodents and humans. J. Endocrinol. Invest. 2016;39(6):607–615. doi: 10.1007/s40618-015-0408-2. [DOI] [PubMed] [Google Scholar]
- Jarolimova J., Tagoni J., Stern T.A. Obesity: its epidemiology, comorbidities, and management. Prim Care Companion CNS Disord. 2013;15(5) doi: 10.4088/PCC.12f01475. PCC.12f01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung U.J., Choi M.S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffashi Elahi R. Preventive effect of turmeric powder the development of fatty liver in rats fed a high fat diet. J. Comp. Pathol. 2013;10:889–898. [Google Scholar]
- Kang J.G., Park C.Y. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab. J. 2012;36(1):13–25. doi: 10.4093/dmj.2012.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W., Parveen R., Chester K., Parveen S., Ahmad S. Hypoglycemic Potential of Aqueous Extract of Moringa oleifera Leaf and In Vivo GC-MS Metabolomics. Front Pharmacol. 2017;8:577. doi: 10.3389/fphar.2017.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Hiraishi A., Tsuchiya K., Sakamoto K. (-) Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology. 2010;62(3):245–255. doi: 10.1007/s10616-010-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koheil M.A., Hussein M.A., Othman S.M., El-Haddad A. Anti-inflammatory and antioxidant activities of Moringa peregrina Seeds. Free Radicals Antioxidants. 2011;1(2):49–61. [Google Scholar]
- Lasker S., Rahman M.M., Parvez F., Zamila M., Miah P., Nahar K., Kabir F., Sharmin S.B., Subhan N., Ahsan G.U., Alam M.A. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019;9(1):20026. doi: 10.1038/s41598-019-56538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.O. Determination of the Surface Area of the white rat with its application to the expression of metabolic results. AJP-Legacy Plus Other APS J. Content. 1925:24–33. [Google Scholar]
- Licholai J.A., Nguyen K.P., Fobbs W.C., Schuster C.J., Ali M.A., Kravitz A.V. Why do mice overeat high-fat diets? How high-fat diet alters the regulation of daily caloric intake in mice. Obesity (Silver Spring) 2018;26(6):1026–1033. doi: 10.1002/oby.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Racette S.B., Lefevre M., Spearie C.A., Most M., Ma L., Ostlund R.E., Jr. The effects of phytosterols present in natural food matrices on cholesterol metabolism and LDL-cholesterol: a controlled feeding trial. Eur. J. Clin. Nutr. 2010;64(12):1481–1487. doi: 10.1038/ejcn.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz T.A., Woods S.C. Overview of animal models of obesity. Curr. Protoc. Pharmacol. Chapter. 2012;5:Unit 5.61. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, A. N., Hansen, G., Paulsen, S. J., Lykkegaard, K., Tang-Christensen, M., Hansen, H. S., Levin, B. E., Larsen, P. J., Knudsen, L. B., Fosgerau, K., & Vrang, N., 2010. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J. Endocrinol. 2010; 206(3), 287-296. [DOI] [PubMed]
- Mahajan S.G., Mehta A.A. Immunosuppressive activity of ethanolic extract of seeds of Moringa oleifera Lam. in experimental immune inflammation. J. Ethnopharmacol. 2010;130(1):183–186. doi: 10.1016/j.jep.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Masarone M., Rosato V., Dallio M., Gravina A.G., Aglitti A., Loguercio C., Federico A., Persico M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell Longev. 2018 doi: 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle M.A., Finucane O.M., Connaughton R.M., McMorrow A.M., Roche H.M. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol. (Lausanne) 2013;4:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally F.M., Rashad H.M., Ahmed H.H., Mahmoud A.A., Abdol Raouf E.R., Abdalla A.M. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac. J. Trop. Biomed. 2017;7(3):214–221. [Google Scholar]
- Minaiyan M., Asghari G., Taheri D., Saeidi M., Nasr-Esfahani S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna. J. Phytomed. 2014;4(2):127–136. [PMC free article] [PubMed] [Google Scholar]
- Oussaada S.M., Galen K.A., Cooiman M.I., Kleinendorst L., Hazebroek E.J., Haelst M.M., Horst K.W., Serlie M.J. The pathogenesis of obesity. Metab. Clin. Exp. 2019;92:26–36. doi: 10.1016/j.metabol.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Rahman H.A., Sahib N.G., Saari N., Abas F., Ismail A., Mumtaz M.W., Hamid A.A. Anti-obesity effect of ethanolic extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat diet. BMC Complement Altern. Med. 2017;17(1):122. doi: 10.1186/s12906-017-1640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recena Aydos, L., Aparecida do Amaral, L., Serafim de Souza, R., Jacobowski, A. C., Freitas Dos Santos, E., Rodrigues Macedo, M.L., 2019. Nonalcoholic fatty liver disease induced by high-fat diet in C57bl/6 models. Nutrients. 11(12), 3067. [DOI] [PMC free article] [PubMed]
- Reeves P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. The Journal of nutrition. 1997;127(5):838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Ohtsu T., Ikeda Y., Tsubosaka M., Shibata S. Combination of meal and exercise timing with a high-fat diet influences energy expenditure and obesity in mice. Chronobiol Int. 2014;31(9):959–975. doi: 10.3109/07420528.2014.935785. [DOI] [PubMed] [Google Scholar]
- Tanti J.F., Ceppo F., Jager J., Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol (Lausanne). 2013;3:181. doi: 10.3389/fendo.2012.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya, V., 2006. Psychosocial aspects of obesity, in: Health and Treatment Strategies in Obesity. Adv. Psychosom. Med. Basel, Karger. 27, 73-85. [DOI] [PubMed]
- Velázquez K.T., Enos R.T., Bader J.E., Sougiannis A.T., Carson M.S., Chatzistamou I., Carson J.A., Nagarkatti P.S., Nagarkatti M., Murphy E.A. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019;11(8):619–637. doi: 10.4254/wjh.v11.i8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner R.M., Hsia Y., Tomsic T., Wong I.C. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obes Rev. 2010;11(8):593–602. doi: 10.1111/j.1467-789X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- WHO, 2015. World Health Organization, Obesity and Overweight Factsheet from the WHO. World. WHO Obesity Factsheet No. 311, 2015.
- Woods S.C., Seeley R.J., Rushing P.A., D'Alessio D., Tso P. A controlled high-fat diet induces an obese syndrome in rats. J. Nutr. 2003;133(4):1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- Wu H., Ballantyne C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020;126(11):1549–1564. doi: 10.1161/CIRCRESAHA.119.315896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J.W. Possible anti-obesity therapeutics from nature–a review. Phytochemistry. 2010;71(14–15):1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P., Li H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Li J., Lu C., Wang J., Ge J., Huang Y., Zhang L., Wang Y. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 2006;79(11):1100–1107. doi: 10.1016/j.lfs.2006.03.021. [DOI] [PubMed] [Google Scholar]