Abstract
In an epoch of escalating number of antibiotic-resistance bacteria, there is a dire need to develop efficient and novel feeding strategies for animal nutrition as alternatives to antibiotics. Here, implicating nutrigenomic approach, phytobiotics and organic acids were used to evaluate ghrelin gene expression levels, gut microflora composition, performance parameters and intestinal histomorphological changes in broiler chickens. One-day-old chicks (n = 315) were reared for 42 days and distributed randomly into five experimental groups; each with three replicates (21 birds per replicate). Experimental groups were control: basal diet only, antimicrobial growth promoter: 40 g/metric ton of basal diet (virginiamycin), organic acids: 4 kg/metric ton of basal diet, phytobiotics: 3 kg/metric ton of basal diet, combination: 7 kg/metric ton of basal diet (organic acids 4 kg and phytobiotics 3 kg metric ton of feed). Growth performance, histological and ghrelin gene expression analysis were executed on 21 and 42 days while, quantitative bacterial analysis of cecum and ileum was performed on day 42. Increased feed intake and body weight (p < 0.05) were noticed in phytobiotics group. Addition of phytobiotics significantly improved (p < 0.05) villus height and ratio of villus height/crypt depth in ileum, jejunum, and duodenum and down-regulated ghrelin gene expression levels. Total coliform and Escherichia coli in cecal and ileal digesta were decreased significantly (p < 0.05) in organic acids group. Correlation analysis revealed Lactobacillus spp. were positively correlated to villus height/crypt depth ration in duodenum. The findings indicated the importance of gene-nutrient-microbiota interactions based on nutrigenomics approach. Hence, phytobiotics and organic acids might be suitable alternatives to antibiotics for improved performance and immunity, along with healthier meat production in poultry.
Keywords: Antibiotic resistance, Broiler, Gut bacteria, Intestinal histology, Natural growth promoters
1. Introduction
Nutrigenomics is an emerging science in animal sciences which played a significant role in exploring the effect of individual nutrients on gene expression levels, metabolic processes, and epigenetic factors in the cells. It provides a tool for evaluating the production performance of improved nutrient digestion and absorption in order to maintain animal health and production performance (Nowacka-Woszuk, 2020). Along with their role as building blocks and energy providers, nutrients also have multiple biological functions including radical scavenging (antioxidants) and potent signaling molecules (nutritional hormones). Hence, a modified diet is used in livestock systems to infer the role of nutrients in host on gene expression, protein levels and metabolites production (Vilar da Silva et al., 2020).
Antibiotics have been extensively used in animal husbandry for decades. Some are used to treat the bacterial infections as therapeutically for the improvement of health and well-being of food producing animals. Mostly antibiotics used as growth promoting agents for prophylactic purposes to improve growth performance, reduce the bacterial pathogens and increase feed conversion efficiency which are termed as antibiotic growth promoters (AGPs) (Hernández et al., 2006). The continuous use of AGPs in animal production raised the concern associated with emergence of microbial resistance to antibiotics which are used to treat animal human infections (Yakhkeshi et al., 2011). After the ban of AGPs, many alternatives have been searched out and being used such as enzymes, probiotics, prebiotics, organic acids, phytobiotics and antioxidants feed additives. Organic acids (OA) are strong alternatives for AGPs (Smulikowska et al., 2010), in successfully reducing proliferation of acid-tolerant microorganisms such as E. coli, Salmonella and Campylobacter spp. in host gut. OA tend to enhance nutrient absorption and Lactobacillus spp. population by increasing height of villus and crypt depth (García et al., 2007, Liu et al., 2014). Phytobiotics contain compounds extracted from medicinal plants with beneficial effect on production performance and animal health. For phytobiotics, whole plant, parts of plants and their essential oils may be used (Stevanović et al., 2018). Plant based feed additives known to exert positive effects on performance parameters, feed consumption, gene expression levels, metabolism, and feed efficiency along with better nutrient absorption in broilers. Natural feed additives extracted from plants are generally considered to be safe, healthy and less hazards to living systems (Ertas et al., 2005). The supplementation of phytobiotics in chicken diet increased villus height with deeper crypt depth and improved digestive enzymes secretions such as proteases and amylases to enhanced growth performance with increased absorptive cell growth in the gastrointestinal tract (Jamroz et al., 2006).
The chicken gastrointestinal tract harbors various population of microorganisms present in symbiotic relationships which ultimately affects the nutrition, metabolism, and immunity in host (Sohail et al., 2012). Nutrient digestion, assimilation and absorption process in broiler depends upon the maturation of small intestine. Mucus production, ecological balance between pathogenic and non-pathogenic bacteria and intestinal epithelial integrity mostly affects the efficacy of intestinal villi. Intestinal villi are considered as natural protection/barrier which prevents entry of any toxic material and pathogenic bacteria in the intestinal lumen (Cheled-Shoval et al., 2011). These factors may cause destabilization of microflora balance and altered intestinal barrier. The altered intestinal barrier expedites the absorption of unwanted substances that reduces absorptive and digestive activities of gastrointestinal tract. Intestinal morphology played a significant role in nutrient absorption by the application of feed additives for increasing the absorptive efficiency of intestine. Since the intestinal morphology is crucial in nutrients absorption thus, certain feed additives are being used for improving intestinal absorptive efficacy (Pelicano et al., 2005, Song et al., 2019a). The current study aimed to analyzed effects of phytobiotics and organic acids on growth performance, gut microflora composition, gut pH, ghrelin gene expression levels, intestinal morphology to decipher their potential as alternatives to antimicrobials in chicken diet.
2. Methodology
2.1. Experimental design
A total of 315 one day old unsexed chicks were procured from local hatchery. Chicks were randomly distributed among five treatment groups (63 chicks/treatment) with three replicates per treatment (21 birds/replicate). The replicates reared in separate pen (size of each pen was 24 ft2). The standard operating procedures of animal experiments were performed according to Care and Use of Agricultural Animals in Research (McGlone, 2010), after approval from institutional ethical committee (DG/AA-089) at The Karachi Institute of Biotechnology and Genetic Engineering (KIBGE), University of Karachi.
2.2. Experimental feeds
Two experimental rations were formulated including starter and finisher diets according to hubbard nutritional requirements of broilers and each diet was analyzed for proximate analysis (AOAC, 2005). Experimental ration with ingredients and nutrients composition is mentioned in Table 1. The starter and finisher diets were formulated and calculated to contain crude protein 21.3% and 19.02%, respectively. Rations were formulated in iso-nitrogenous and iso-caloric form with the growth enhancers and divided into five treatments:
-
•
Control: (basal diet without additives)
-
•
Antimicrobial growth promoter (AGP): (basal diet + virginiamycin, 40 g/metric ton)
-
•
Organic acids: (basal diet + blend of formic acid, (45%), propionic acid (11.5%) and citric acid (15%); 4 kg/metric ton)
-
•
Phytobiotics: (basal diet + mixture of dried powder of rhizome of ginger (10%), liquorice (10%), ashwagandha roots (10%), black seeds (10%) and leaves of green tea (15%); 3 kg/metric ton)
-
•
Combination: (basal diet + combination of phytobiotics and organic acids; 4 kg/metric ton)
Table 1.
Composition of starter and finisher diets.
| Feed ingredients | Starter | Finisher | Nutrients | Calculated composition |
|
|---|---|---|---|---|---|
| Inclusion (kg) | Inclusion (kg) | Starter Diet | Finisher Diet | ||
| Corn | 590 | 620 | ME8, Kcal /kg | 2900 | 3000 |
| Soya bean meal (44%) | 194.3 | 280 | Crude protein (%) | 21.5 | 19.17 |
| Canola meal | 100 | 0 | Crude fiber (%) | 4.34 | 3.98 |
| Fish meal (55% crude protein) | 30 | 0 | Ether extract (%) | 3.97 | 4.71 |
| Corn gluten (60% crude protein) | 30 | 25 | Ash (%) | 3.99 | 3.7 |
| PPM1 (50% crude protein) | 20 | 20 | Calcium (%) | 1 | 0.9 |
| Limestone | 13 | 14 | Available. Phosphorous (%) | 0.47 | 0.4 |
| Vegetable oil | 3.6 | 16 | Dig. Lysine (%) | 1.19 | 1.07 |
| DL-Methionine | 1.8 | 2.6 | Dig. Methionine (%) | 0.54 | 0.56 |
| Lysine sulphate 55% | 4.7 | 3.9 | Dig. Methionine + Cysteine (%) | 0.88 | 0.88 |
| Vitamin premix2 | 0.5 | 0.5 | Dig. Tryptophan (%) | 0.0.23 | 0.2 |
| Mineral premix3 | 0.5 | 0.5 | Dig. L-threonine (%) | 0.9 | 0.8 |
| L-threonine | 1.6 | 1.5 | |||
| Salt (NaCl) | 2 | 2 | Proximate analysis | ||
| Sodium-bi-carbonate | 2.5 | 2.3 | ME8, Kcal /kg | 2898 | 2996 |
| *Anti –coccidial4 | 0.2 | 0 | Crude protein (%) | 21.3 | 19.02 |
| *Anti –coccidial5 | 0 | 0.5 | Crude fiber (%) | 4.21 | 3.76 |
| * MDCP6 (21%) | 3 | 9.9 | Ether extract (%) | 3.99 | 4.78 |
| Choline chloride 60% | 2 | 1 | Ash (%) | 3.87 | 3.73 |
| Quantum blue (Phytase) | 0.2 | 0.2 | |||
| Seldox7 (antioxidant) | 0.15 | 0.15 | |||
| Total | 1000 | 1000 | |||
PPM1 = Poultry by Product Meal, Vitamin premix2: composition per kg (vitamin A, 20,000 KIU/kg; vitamin E, 48,000 mg/kg; vitamin D3,5,400 KIU/kg; vitamin B1,4,000 mg/kg; vitamin K34,000 mg/kg; vitamin B2,9,000 mg/kg; vitamin B6, 7,600 mg/kg; vitamin B12,20 mg/kg; Niacine,60,000 mg /kg; Folic acids, 1,600 mg/kg; Pantothenic Acid,20.000 mg/kg; Biotin, 200 mg/kg); Mineral premix3: composition per kg (Manganese,130.000 mg/kg, Iron , 60.000 mg/kg; Iodine, 1,800 mg/kg; Zinc, 120,000 mg/kg; Copper, 10,000 mg/kg; Selenium, 360 mg/kg ; Cobalt , 400 mg/kg); *Anti-coccidial4: maduramicin 1%; *Anti –Coccidial5 Diclazuril 0.5%; * MDCP6 21% mono dicalcium phosphate; Seldox7: contained butylated hydroxy anisole, butylated hydroxy toluene, ethoxyquin and citric acid; ME8: metabolizable energy.
2.3. Growth performance analysis
All experimental diets were administered to birds ad libitum. The growth performance analysis including body weight, feed intake, and feed conversion ratio were executed at 21 and 42 days during the trial.
2.4. Sample collection
On day 21 and 42, three chicks from every replicate pen were randomly selected and slaughtered for histomorphological and microflora analysis. The distal portion of ileum, jejunum and duodenum were collected for histomorphological analysis. The cecal and ileal digesta of slaughtered birds on day 42, were aseptically collected in cryo-protective broth. All samples were stored at −80 °C for further analyses.
2.5. Histological analysis
Three chicks from every replicate were selected for histomorphological analysis and slaughtered for collection of distal portions of ileum, jejunum, and duodenum at 21- and 42 days. The portions were initially fixed in buffered formalin saline (10%) followed by dehydration in various alcohol concentrations in a range from 70–100%. Later, samples were infiltrated with xylene and then embedded in paraffin wax. Section cutting of samples were performed through microtome (section size 5 μm). The sections were placed on a glass slide, fixed in wax ribbon and stained with hematoxylin-eosin. Slides were analyzed under a microscope and pictures were taken. These images were analyzed using the (Image J,1.50i) software. The depth of the crypt was calculated by measuring the distance between the basolateral membrane and individual villi. (Baurhoo et al., 2007).
2.6. Microflora composition
For bacterial enumeration, 1 g sample was transferred in 9 mL phosphate buffer saline (PBS) and serially diluted. The Lactobacillus spp., Escherichia coli, Salmonella spp., total coliform, and total viable bacteria (aerobes and anaerobes) were quantified on de Man Rogosa and Sharpe (CM0359, Oxoid UK), xylose lysine deoxycholate (CM0469, Oxoid UK), eosin methylene blue (CM0069, Oxoid UK), MaConkey’s (CM0505, Oxoid UK) and plate count agar (CM0325, Oxoid UK), respectively. The colonial counts were estimated through log cfu/g using spread plate technique (Andrews et al., 2014).
2.7. Gastrointestinal tract pH analysis
For determination of pH, 10 g of gut contents (crop, proventriculus, duodenum, jejunum, ileum and gizzard) were taken and diluted in PBS to make 1:10 dilution and pH was observed using pH meter (Al-Natour and Alshawabkeh, 2005).
2.8. Gene expression analysis
The expression of ghrelin gene was determined using real-time quantitative polymerase chain reaction (RT-qPCR). Primers used in the study are forward 5′ CCTTGGGACAGAAACTGCTC 3′ and reverse 5′ CACCAATTTCAAAAGGAACG 3′ designed using Primer 5.0 software and manufactured from commercial company (Promega, USA). Total RNA was extracted from proventriculus tissue (100 mg) using TRIzol ((Invitrogen/Life Technologies, Isogene Co, Russia). The quantitative and qualitative analysis of extracted RNA was performed using Nanodrop P 360 (Implen, Germany) and agarose gel electrophoresis, respectively. RNA (2 µg) was initially reverse-transcribed using commercial kit (Thermo Fisher Scientific, UK) and real-time PCR was performed using SYBR green master mix (Applied Biosystems, Warrington, UK) on ABI 7300 system (Applied Biosystems, USA). The reaction conditions were denaturation at 95 °C for 30 s, annealing at 55 °C for 1 min and extension at 72 °C for 1 min. The ghrelin and actin (housekeeping) gene expressions were analyzed in all samples through ddCt relative quantification plate study software.
2.9. Statistical analysis
Analysis of variance (one-way ANOVA) was performed for statistical analysis of data and Duncan multiple range (DMR) test was used for comparing means. The significance was assumed at p < 0.05. Pearson’s test was conducted to find correlation among changes associated with gut microflora composition and correlation coefficient (r) was indicated with significance at p < 0.05.
3. Results
3.1. Performance analysis
The performance parameters were significantly different (p < 0.05) in all groups (Fig. 1.). The highest feed intake, weight gain and improved feed efficiency were observed in phytobiotics at 21 (Fig. 1A) and 42 days (Fig. 1B) followed by combination group. Similarly, elevated body weight gain (p < 0.05) was also observed in combination and phytobiotics. Phytobiotics group also showed feed conversion ratio of 1.85 which indicated better nutrient efficiency.
Fig. 1.
Growth performance of broiler chickens at 21 (A) and 42 days (B). Significance assumed at p < 0.05. Barsa,b,c with similar letter represents non-significant differences (p > 0.05). Error bars represents standard error of mean. FCR: Feed conversion ratio.
3.2. Histological analysis
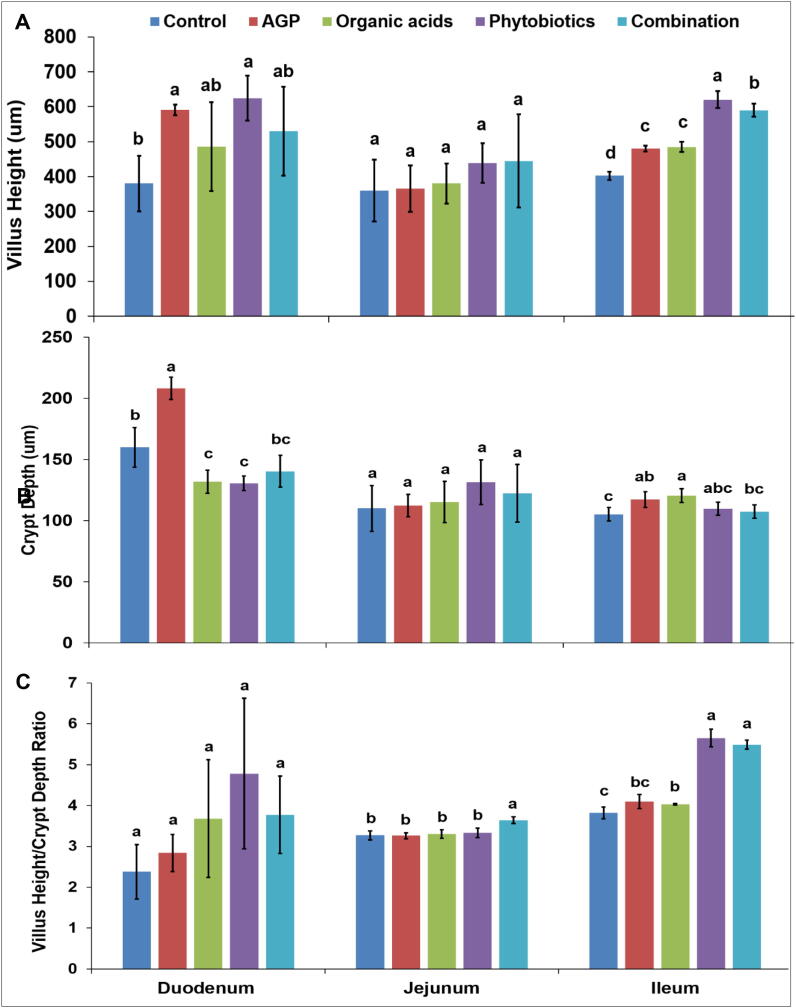
Non-significant effects (p > 0.05) of different feed groups for villus height (Fig. 2A) and crypt depth (Fig. 2B) in jejunum and duodenum at 21 days were noted. In ileum, highest villus height (620.5 µm) was observed in phytobiotics supplemented group. Higher depth of crypt in duodenum (140.4 µm) and ileum (120.4 µm) was recorded in organic acids and combination groups, respectively. Phytobiotics displayed significantly (p < 0.05) different crypt depth (109.7 µm) in all feed groups. Furthermore, phytobiotics and combination groups showed highest ileum (5.6 µm) and jejunum (3.6 µm) ratio (Fig. 2C). At 21 days, villus height to crypt ratio was unaffected (p < 0.05) in duodenum among feed groups. Among natural growth enhancers, non-significant (p > 0.05) effects on ileum villus height were exhibited (Fig. 3.).
Fig. 2.
Villus height (A), crypt depth (B) and villus height/crypt depth ratio (C) of broiler chickens at 21 days. Significance assumed at p < 0.05. Barsa,b,c with similar letter represents nonsignificant differences (p > 0.05).
Fig. 3.
Histomorphology of jejunum, duodenum, and ileum showing villus height at 21 days in broiler chickens.
On the other hand, villus height (Fig. 4A) and crypt depth (Fig. 4B) of jejunum, duodenum and ileum affected significantly (p < 0.05) by different feed groups at 42 days. Organic acids fed group exhibited highest duodenum crypt depth (188.2 µm). Combination group showed highest crypt depth of jejunum (182.1 µm) and ileum (170.0 µm) among all groups. The higher ratio of jejunum (7.4 µm) and ileum (7.3 µm) was observed in phytobiotics supplemented group. Villus height and crypt depth ratio of duodenum was non-significant (p > 0.05) among feed groups at 42 days (Fig. 4C). Phytobiotics showed highest duodenum (1390.4 µm), jejunum (1345.0 µm) and ileum (1235.9 µm) villus height compared to other groups (Fig. 5).
Fig. 4.
Villus height (A), crypt depth (B) and villus height/crypt depth ratio (C) of broiler chickens at 42 days. Significance assumed at p < 0.05. Barsa,b,c with similar letter represents nonsignificant differences (p > 0.05).
Fig. 5.
Histomorphology of jejunum, duodenum, ileum showing villus height at 42 days in broiler chickens.
3.3. Quantitative analysis of gut bacteria
Microflora composition of cecum and ileum were enumerated to examine the effects of various feed groups. Total viable anaerobes, total viable coliform bacteria, Escherichia coli and Lactobacillus spp. counts were considerably affected (p < 0.05) by feed variations in both cecal and ileal regions. The highest total anaerobes and Lactobacillus spp. counts were evident in both phytobiotics and combination groups whereas, decreased population of Escherichia coli and total coliform was noted in organic acids group with 5.3 and 5.4 log cfu/g respectively (Table 2). In particular, Salmonella spp. was not detected from cecal and ileal digesta in any study group.
Table 2.
Microbial enumeration (log cfu/g) of cecal and ileal digesta of broilers at 42 days.
| Study groups | Total aerobes | Total anaerobes | Total coliform | Lactobacillus spp. | Escherichia coli | Salmonella spp. |
|---|---|---|---|---|---|---|
| Cecal digesta | ||||||
| Control | 6.5a | 5.9d | 5.9a | 5.2d | 6.1a | ND |
| AGP | 6.4ab | 6.1c | 5.6b | 5.3cd | 5.5bc | ND |
| Organic acids | 6.2ab | 6.4b | 5.4c | 5.5bc | 5.3c | ND |
| Phytobiotics | 6.2b | 6.9a | 5.4bc | 5.9ab | 5.6b | ND |
| Combination | 6.4a | 6.7a | 5.5b | 5.8a | 5.6b | ND |
| SEM | 0.05 | 0.05 | 0.05 | 0.09 | 0.08 | |
| p-value | 0.16 | <0.001 | 0.01 | <0.001 | <0.001 | |
| Ileal digesta | ||||||
| Control | 6.3a | 5.9b | 6.1a | 5.1d | 5.2a | ND |
| AGP | 6.3a | 6.1b | 5.2b | 5.3cd | 4.6b | ND |
| Organic acids | 6.1a | 6.5a | 4.9c | 5.6bc | 4.5b | ND |
| Phytobiotics | 6.2a | 6.9a | 4.9c | 5.8ab | 4.7b | ND |
| Combination | 6.2a | 6.6a | 5.1bc | 5.7a | 4.7b | ND |
| SEM | 0.09 | 0.1 | 0.07 | 0.09 | 0.12 | |
| p-value | 0.54 | <0.001 | <0.001 | <0.001 | 0.01 | |
Means bearing different letters in a row differ significantly (p < 0.05). SEM: Standard error of mean. ND: Not detected.
3.3.1. Gastrointestinal pH
Dietary variation affected (p < 0.05) gastrointestinal pH at 21 and 42 days in crop and gizzard (Table 3). The pH of proventriculus and small intestine (jejunum, duodenum, and ileum) remained unaffected (p < 0.05) with the changes in diet.
Table 3.
Gastrointestinal pH of broiler chickens at 21 and 42 days.
| Study groups | Crop | Proventriculus | Gizzard | Duodenum | Jejunum | Ileum |
|---|---|---|---|---|---|---|
| 0–21 days | ||||||
| Control | 5.1a | 2.9a | 2.8a | 5.7a | 6.7a | 7.5a |
| AGP | 5.3a | 2.9a | 2.6ab | 5.5ab | 6.5ab | 7.3ab |
| Organic acids | 4.0b | 2.7b | 2.7c | 5.4b | 6.4b | 7.2b |
| Phytobiotics | 5.0a | 2.8ab | 2.5b | 5.6ab | 6.6ab | 7.3ab |
| Combination | 4.2b | 2.7ab | 2.3c | 5.5b | 6.5ab | 7.4ab |
| SEM | 0.19 | 0.05 | 0.06 | 0.07 | 0.07 | 0.10 |
| p-value | <0.001 | 0.10 | <0.001 | 0.06 | 0.10 | 0.31 |
| 21–42 days | ||||||
| Control | 5.1a | 3.0a | 2.9a | 5.8a | 6.8a | 7.7a |
| AGP | 4.8b | 2.8ab | 2.8ab | 5.7a | 6.6ab | 7.5ab |
| Organic acids | 4.1d | 2.7b | 2.1d | 5.3b | 6.0d | 7.3b |
| Phytobiotics | 4.7bc | 2.9ab | 2.6bc | 5.7a | 6.4bc | 7.5ab |
| Combination | 4.5c | 2.8b | 2.3cd | 5.6ab | 6.2cd | 7.4ab |
| SEM | 0.06 | 0.07 | 0.10 | 0.10 | 0.09 | 0.12 |
| p-value | <0.001 | 0.06 | <0.001 | 0.06 | 0.01 | 0.28 |
Means bearing different letters in a row differ significantly (p < 0.05). SEM: Standard error of mean.
3.4. Gene expression analysis
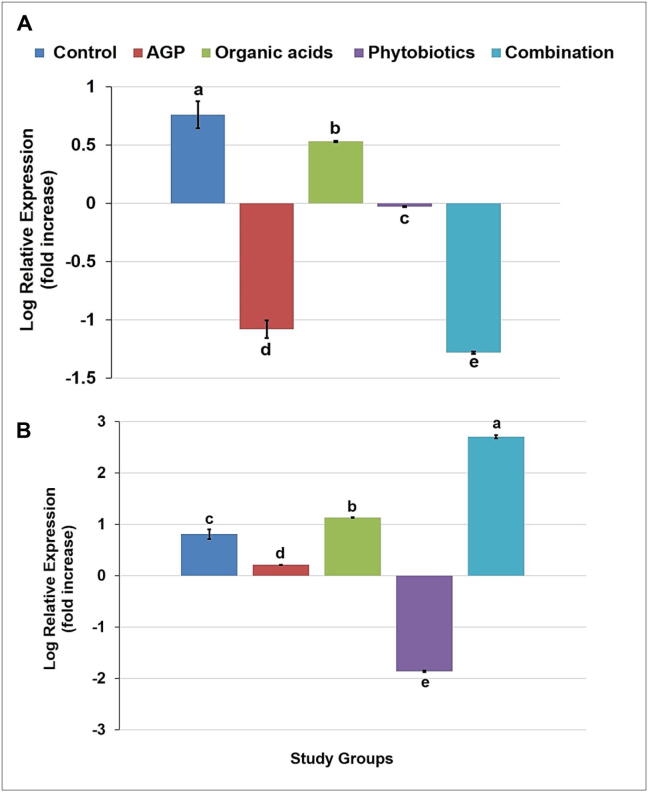
Avian ghrelin gene expression was carried by RT-qPCR. real time polymerase chain reaction at 21 and 42 days of age. The ghrelin gene expression was significantly (p < 0.05) different among all treatment groups at both stage of broiler production; 21 (Fig. 6A) and 42 (Fig. 6B) days. The expression level of ghrelin gene was up-regulated in control and organic acids groups among all treatments at 21 days whereas, down-regulation of gene was observed only in phytobiotics at 42 days.
Fig. 6.
Ghrelin gene expression at 21 (A) and 42 (B) days in broiler chickens. Significance assumed at p < 0.05. Barsa,b,c with similar letter represents nonsignificant differences (p > 0.05).
3.5. Correlation analysis
The association of cecal/ileal microflora composition with ghrelin gene expression, growth performance parameters and small intestinal histomorphology was executed using Pearson’s correlation test. Correlation analysis showed cecal Lactobacillus spp. were positively correlated to villus height/crypt depth ratio in duodenum (r = 0.8, p < 0.05) (Table 4). Moreover, cecal and ileal total anaerobes are also positively correlated to villus height/crypt depth ratio of duodenum, jejunum, and ileum. Interestingly, cecal Lactobacillus spp. population correlated positively (r = 0.9, p < 0.05) with body weight of broiler chickens (Table 5).
Table 4.
Correlation analysis of cecal/ileal microflora and villus height/crypt depth ratio of broilers at 42 days of age.
| Parameters | Villus height/Crypt depth ratio |
||
|---|---|---|---|
| Duodenum | Jejunum | Ileum | |
| Cecal digesta | |||
| Total aerobes | −0.5 | −0.8 | −0.8 |
| Total anaerobes | 0.8* | 0.8 | 0.9* |
| Total coliform | −0.5 | −0.8 | −0.8 |
| Lactobacillus spp. | 0.8* | 0.7 | 0.8 |
| Escherichia coli | −0.1 | −0.5 | −0.5 |
| Ileal digesta | |||
| Total aerobes | −0.1 | −0.3 | −0.4 |
| Total anaerobes | 0.8 | 0.8* | 0.9* |
| Total coliform | −0.5 | −0.7 | −0.8 |
| Lactobacillus spp. | 0.7 | 0.7 | 0.8 |
| Escherichia coli | −0.2 | −0.5 | −0.6 |
Correlation significant at p < 0.05 level.
Table 5.
Correlation analysis among microflora, ghrelin gene expression and growth parameters in broiler chickens.
| Parameters | Feed intake | Body weight gain | FCR |
|---|---|---|---|
| Cecal coliform | −0.8 | −0.7 | 0.7 |
| Cecal Lactobacillus spp. | 0.8 | 0.9* | −0.9* |
| Ileal coliform | −0.9* | −0.8 | 0.8 |
| Ileal Lactobacillus spp. | 0.8 | 0.8 | −0.8* |
| Ghrelin gene expression | −0.2 | −0.3 | 0.3 |
Correlation significant at p < 0.05 level. FCR: Feed conversion ratio.
4. Discussion
The findings of current study evinced significantly increased (p < 0.05) body weight in chickens fed with organic acids and phytobiotics in diet. The histomorphological changes revealed increased villus height in phytobiotics and combination groups. It also affected the gut microbial dynamics of broiler chickens with numerically increased population of Lactobacillus spp. and decreased total coliform and Escherichia coli growth. Inclusion of natural feed additives maintained pH in various regions of gastrointestinal tract similar to antibiotic growth promoter. Organic acids exhibited up-regulated levels of ghrelin gene in broiler at 21 and 42 days whereas, down-regulated levels of ghrelin were noticed in phytobiotics on both days. Correlation analysis further validated that increased Lactobacillus spp. population is positively correlated to villus height/crypt depth ratio in broiler chickens and body weight gain in broiler.
In current study the mixture of phytobiotics such as Camellia sinensis (green tea), Withania somnifera (ashwagandha), Zingiber officinale (ginger), Nigella sativa (black seed) and Glycyrrhiza glabra (liquorice) were used which showed improved feed efficiency. It may be due to the numerous biologically active compounds present in the phytobiotics which has antimicrobial, immunomodulatory, antioxidative properties along with growth promoting effects which ultimately improved host weight gain (Reis et al., 2018). Aswagandha contains withanine and withanolides, these compounds may stimulate the gastrointestinal tract secretions which help to increase nutrient digestion and absorption to improve weight gain (Jyotsana and Berwal, 2019). Ginger, a potential phytobiotics, having bioactive compounds such as shogals, ginerdiol, and gingerdione acting as pharmacological, antioxidant, and antimicrobial agent (Teles et al., 2019, El-Hack et al., 2020). Addition of ginger powder improved nutrient digestion and absorption in the gastrointestinal tract of chicken by stimulating the disaccharidase, lipase and maltase secretions which may lead to improved feed intake and feed efficiency (Idoko et al., 2020). The feeding of phytobiotics and organic acids in combination proved to enhance body weight gain and feed conversion efficiency in broiler compared to AGPs. The synergistic effect of these two additives is linked with gut physiology because mostly organic acids are active in crop, gizzard and upper portion of digestive tract while, essential oils are active in later segment of the intestinal tract or distal portion of the gut. Both of these additives exhibited promising results in broiler gut when administered as alternatives to diet (Gole et al., 2020).
The findings of current research regarding gut microflora populations proved that the dynamics of intestinal bacteria are influenced by the supplementation of organic acids in chickens feed and responsible for significantly reduced growth of harmful bacterial populations such as Clostridium perfringens, Campylobacter & E. coli (Markowiak and Śliżewska, 2017). The application of propionic acid suppresses E. coli and Salmonella growth in cecal and fecal microorganism populations without exerting negative effects on the growth and counts of Lactobacillus spp. in chickens (Ding et al., 2019). Morphological changes of gastrointestinal tract are influenced by variation and composition of feeds. Morphological parameters i.e. villus height and crypt depth are responsible for vigorous functioning of intestine by facilitating larger surface area for better absorption of nutrient (Awad et al., 2009). Higher villus height/crypt depth ratio is linked with epithelial cell turn over while decreased ratio is associated with decreased intestinal bacterial population along with reduced surface area for nutrients absorption (Laudadio et al., 2012). Present work demonstrated that application of organic acids enhanced villus height of duodenum and jejunum and reduced growth of pathogenic bacterial population with increased beneficial bacterial counts. It has already been reported that the application of formic and citric acids enhanced villus height and crypt depth width and enhanced the growth performance of broilers (Ragaa and Korany, 2016). Chaveerach et al. (2004) (Chaveerach et al., 2004) reported that propionic, acetic and formic acids are considered to improve villus height/length and crypt depth and ultimtaly improved the production performance. These short chain fatty acids (SCFAs) may be used as antibacterial agents in poultry feed to decrease the proliferation of Salmonella, Campylobacter, E. coli and total coliform in the broiler gut. The application of phytobiotics in poultry diets increases villus height with deeper crypt and enhanced the digestive enzymes secretion such as proteases and amylases, to promote the growth and production performance of chickens along with enhanced absorptive cell growth in the gastrointestinal tract (Abudabos et al., 2018). Phytobiotics or plant derived compounds alone and in combinations have been proved to enhance growth performance, production, and health of animals (Rahimi et al., 2011). Phytobiotics having antimicrobial activity came in contact with the microbial cell membrane, alters permeability for H+ and K+ cations resulting in decreased entero-pathogens population. Moreover, phytobiotics also help in maintaining microflora balance that will involve in modifying digestive organs functioning, nutrient absorbance and weight gain of chicken (Ferdous et al., 2019). Phytobiotics significantly modulates gastrointestinal microflora and intestinal epithelium integrity by reducing the pathogenic bacterial load and stabilizing intestinal health (Talbott et al., 2018). Antibacterial activity of black cumin against E. coli, Shigella sonnei & Streptococcus mutans has already been established (Khan et al., 2012). Black seed contains essential oils (carvacrol and thymol) and fatty acids which enhanced digestive secretions and maintained intestinal integrity. These effects eventually improved weight gain in host (Alagawany et al., 2015). Ginger and green tea supplementation in chicken diet improved villus height surface area for nutrient absorption and cell division processes in various sections of intestine along with decreased pathgenic load (Cao et al., 2005, Gadang et al., 2008). It may be associated with the presence of bioactive molecules in green tea against numerous bacteria. The polyphenols present in green tea known to increased Lactobacilli with reduced population of Proteus spp. and Enterobacteriaceae at 56 days of age in poultry. Moreover, green tea leaves contain L-theanine which might be responsible for improved weight gain in birds (Williams et al., 2016)).
In current study, we analyzed avian ghrelin gene expression among treatment groups. Physiological status including feed consumption was different among all treatment groups in broiler chicken during experimental period of research which shown noteworthy role of avian ghrelin gene expression through the different satiety level. The supplementation of organic acids group depicted up-regulation of avian ghrelin gene expression in broilers which revealed enhanced nutrients digestion and absorption process in gastrointestinal tract with noticeable hunger signs in broilers. At the result, more feed consumed to attain body weight gain and eventually improved feed conversion ratio (Song et al., 2019b). Down-regulation of avian ghrelin gene expression was observed in phytobiotics group. It may be due to enhanced amino acids, vitamins, mineral absorption, protein digestibility, carbohydrate metabolism and fat digestibility through optimum bile acid production which displayed postprandial satiety response in gastrointestinal tract to attain increased body weight and improved feed conversion (Khwatenge et al., 2020, Shahryar and Lotfi, 2020). However, the combination fed group showed up- and down-regulation of avian ghrelin gene expression at 21 and 42 days of age of broilers. It might be due to irreconcilability of doses, smell, and taste of certain organic acids and phytobiotics. Phytobiotics and organics acids has antimicrobial effects to maintain beneficial microflora populations, improvements in histomorphology of intestine and ultimately improved digestion and absorption of nutrients produced competitive exclusion resulting metabolic energy may intervene with energy homeostasis to illuminate up- and down-regulation in combination fed group as well.
5. Conclusion
In conclusion, phytobiotics and organic acids can be supplemented as dietary substitutes to antibiotics for preventing possible emergence of microbial resistance in humans. These alternatives might reduce intestinal pathogenic bacteria and increased population of Lactobacillus spp. and maintained satiety levels through regulation of ghrelin gene with improvement in intestinal histological changes attributed to increased digestion and nutrient absorptions resulted in increased performance and host health. It is assumed that phytobiotics meet the biological safety requirements of poultry products without developing resistance in human and poultry microorganisms.
6. Ethics approval
The study protocol was approved by ethicalcommittee of KIBGE, University of Karachi (Pakistan) and performed by the owner of poultry farm-house. Every effort was made to minimize animal suffering.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors (SM, KAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. 1440-0138.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Syed Muddassar Hussain Gilani, Email: gilani1294@gmail.com.
Saddia Galani, Email: sadia.galani@kibge.edu.pk.
Sitwat Zehra, Email: sitwat.zehra@kibge.edu.pk.
S. Mahboob, Email: mushahid@ksu.edu.sa.
References
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. J. App. Animal Res. 2018;46(1):691–695. doi: 10.1080/09712119.2017.1383258. [DOI] [Google Scholar]
- Al-Natour M.Q., Alshawabkeh K.M. Using varying levels of formic acid to limit growth of Salmonella gallinarum in contaminated broiler feed. Asian-Austr. J. Animal Sci. 2005;18(3):390–395. doi: 10.5713/ajas.2005.390. [DOI] [Google Scholar]
- Alagawany M., El-Hack M., Farag M.R., Tiwari R., Dhama K. Biological effects and modes of action of carvacrol in animal and poultry pro-duction and health-a review. Adv. Animal Veterinary Sci. 2015;3:73–84. doi: 10.14737/journal.aavs/2015/3.2s.73.84. [DOI] [Google Scholar]
- Andrews W.H., Jacobson A., Hammack T. Bacteriological Analytical Manual (BAM) 8th ed. Center for Food Safety and Applied Nutrition; U.S. FDA, College Park, MD: 2014. Salmonella. [Google Scholar]
- AOAC . 18th rev. ed. Association of Official Analytical Chemists International; Gaithersburg (MD, USA): 2005. Official Methods of Analysis. [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poultry Sci. 2009;88(1):49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Baurhoo B., Phillip L., Ruiz-Feria C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poultry Sci. 2007;86(6):1070–1078. doi: 10.1093/ps/86.6.1070. [DOI] [PubMed] [Google Scholar]
- Cao B.H., Karasawa Y., Guo Y.M. Effects of Green Tea Polyphenols and Fructo-oligosaccharides in Semi-purified Diets on BroilersPerformance and Caecal Microflora and Their Metabolites. Asian-Austr. J. Animal Sci. 2005;18(1):85–89. doi: 10.5713/ajas.2005.85. [DOI] [Google Scholar]
- Chaveerach P., Keuzenkamp D.A., Lipman L.J.A., Van Knapen F. Effect of organic acids in drinking water for young broilers on Campylobacter infection, volatile fatty acid production, gut microflora and histological cell changes. Poultry Sci. 2004;83(3):330–334. doi: 10.1093/ps/83.3.330. [DOI] [PubMed] [Google Scholar]
- Cheled-Shoval S.L., Amit-Romach E., Barbakov M., Uni Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre-and posthatch periods in chickens. Poultry Sci. 2011;90(10):2301–2310. doi: 10.3382/ps.2011-01488. [DOI] [PubMed] [Google Scholar]
- Ding S., Wang Y., Yan W., Li A., Jiang H., Fang J. Effects of Lactobacillus plantarum 15–1 and fructooligosaccharides on the response of broilers to pathogenic Escherichia coli O78 challenge. Plos One. 2019;14 doi: 10.1371/journal.pone.0212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hack A., Mohamed E., Alagawany M., Shaheen H., Samak D., Othman S.I., Allam A.A., Taha A.E., Khafaga A.F., Arif M. Ginger and its derivatives as promising alternatives to antibiotics in poultry feed. Animals. 2020;10:452. doi: 10.3390/ani10030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertas O.N., Guler T., Çiftçi M., DalkIlIç B., Simsek U.G. The effect of an essential oil mix derived from oregano, clove and anise on broiler performance. Inter. J. Poultry Sci. 2005;4:879–884. [Google Scholar]
- Ferdous M.F., Arefin M.S., Rahman M.M., Ripon M.M.R., Rashid M.H., Sultana M.R., Hossain M.T., Ahammad M.U., Rafiq K. Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. Adv. Veterinary Animal Res. 2019;6:409. doi: 10.5455/javar.2019.f361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadang V., Hettiarachchy N., Johnson M., Owens C. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey frankfurter system. J. Food Sci. 2008;73:M389–M394. doi: 10.1111/j.1750-3841.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- García V., Catalá-Gregori P., Hernández F., Megías M.D., Madrid J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. App. Poultry Res. 2007;16(4):555–562. doi: 10.3382/japr.2006-00116. [DOI] [Google Scholar]
- Gole M., Manwar S., Chaudhary S., Kawitkar S., Khose K. The impact of feeding clove essential oils and organic acids on immunity, gut health and economics of broiler production. J. Pharmacogn. Phytochem. 2020;9:1417–1422. [Google Scholar]
- Hernández F., García V., Madrid J., Orengo J., Catalá P., Megías M.D. Effect of formic acid on performance, digestibility, intestinal histomorphology and plasma metabolite levels of broiler chickens. Br. Poultry Sci. 2006;47(1):50–56. doi: 10.1080/00071660500475574. [DOI] [PubMed] [Google Scholar]
- Idoko A., Zaharaddeen A., Imam N., Nura S., Abdulazeez B., Sunday H., Ugwu K., Olaiya H. Physicochemical assessment of broiler chickens fed diets supplemented with a mixture of ginger, garlic and cinnamon. J. Appl. Sci. Environ. Manag. 2020;24:809–814. doi: 10.4314/jasem.v24i5.12. [DOI] [Google Scholar]
- Jamroz D., Wertelecki T., Houszka M., Kamel C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Animal Physiol. Animal Nutri. 2006;90(5-6):255–268. doi: 10.1111/j.1439-0396.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- Jyotsana, P.K., Berwal, R., 2019. Supplementation of Ashwagandha root powder improving gut micro flora and immune response of broilers.
- Khan S.H., Ansari J., Haq A.U., Abbas G. Black cumin seeds as phytogenic product in broiler diets and its effects on performance, blood constituents, immunity and caecal microbial population. Italian J. Animal Sci. 2012;11(4):e77. doi: 10.4081/ijas.2012.e77. [DOI] [PubMed] [Google Scholar]
- Khwatenge C.N., Kimathi B.M., Taylor-Bowden T., Nahashon S.N. Expression of lysine-mediated neuropeptide hormones controlling satiety and appetite in broiler chickens. Poultry Sci. 2020;99:1409–1420. doi: 10.1016/j.psj.2019.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudadio V., Passantino L., Perillo A., Lopresti G., Passantino A., Khan R.U., Tufarelli V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poultry Sci. 2012;91(1):265–270. doi: 10.3382/ps.2011-01675. [DOI] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T., Bravo D., Maddox C., Pettigrew J. Effects of capsicum oleoresin, garlic botanical, and turmeric oleoresin on gene expression profile of ileal mucosa in weaned pigs. J. Animal Sci. 2014;92:3426–3440. doi: 10.2527/jas.2013-6496. [DOI] [PubMed] [Google Scholar]
- Markowiak P., Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone J. Guide for the care and use of agricultural animals in research and teaching. Fed. Animal Sci. Societies. 2010 [Google Scholar]
- Nowacka-Woszuk J. Nutrigenomics in livestock—recent advances. J. App. Genetics. 2020;61(1):93–103. doi: 10.1007/s13353-019-00522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano E.R.L., Souza P.A., Souza H.B.A., Figueiredo D., Boiago M., Carvalho S., Bordon V. Intestinal mucosa development in broiler chickens fed natural growth promoters. Brazil. J. Poultry Sci. 2005;7(4):221–229. doi: 10.1590/S1516-635X2005000400005. [DOI] [Google Scholar]
- Ragaa N.M., Korany R.M. Studying the effect of formic acid and potassium diformate on performance, immunity and gut health of broiler chickens. Animal Nutri. 2016;2(4):296–302. doi: 10.1016/j.aninu.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi, S., Teymouri, Z.Z., Karimi, T.M., Omidbaigi, R., Rokni, H., 2011. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens.
- Reis J.H., Gebert R.R., Barreta M., Baldissera M.D., Dos Santos I.D., Wagner R., Campigotto G., Jaguezeski A.M., Gris A., de Lima J.L. Effects of phytogenic feed additive based on thymol, carvacrol and cinnamic aldehyde on body weight, blood parameters and environmental bacteria in broilers chickens. Microb. Pathogenesis. 2018;125:168–176. doi: 10.1016/j.micpath.2018.09.015. [DOI] [PubMed] [Google Scholar]
- Shahryar H.A., Lotfi A. Effect of ghrelin peripheral administration on growth performance, carcass quality, and selected serum parameters in broiler chickens. J. Hellenic Veterinary Med. Soc. 2020;71:2047–2054. doi: 10.12681/jhvms.22965. [DOI] [Google Scholar]
- Smulikowska S., Czerwiński J., Mieczkowska A. Effect of an organic acid blend and phytase added to a rapeseed cake-containing diet on performance, intestinal morphology, caecal microflora activity and thyroid status of broiler chickens. J. Animal Physiol. Animal Nutri. 2010;94:15–23. doi: 10.1111/j.1439-0396.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Ijaz A., Sohail A., Shabbir M.Z., Rehman H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poultry Sci. 2012;91(9):2235–2240. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- Song J., Lei X., Luo J., Everaert N., Zhao G., Wen J., Yang Y. The effect of Epigallocatechin-3-gallate on small intestinal morphology, antioxidant capacity and anti-inflammatory effect in heat-stressed broilers. J. Animal Physiol. Animal Nutri. 2019;103:1030–1038. doi: 10.1111/jpn.13062. [DOI] [PubMed] [Google Scholar]
- Song X., Jiao H., Zhao J., Wang X., Lin H. Ghrelin serves as a signal of energy utilization and is involved in maintaining energy homeostasis in broilers. Gen. Comp. Endocrin. 2019;272:76–82. doi: 10.1016/j.ygcen.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Stevanović Z.D., Bošnjak-Neumüller J., Pajić-Lijaković I., Raj J., Vasiljević M. Essential oils as feed additives—future perspectives. Molecules. 2018;23:1717. doi: 10.3390/molecules23071717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott S., Stephens B., Talbott J., Oddou M. Effect of coordinated probiotic/prebiotic/phytobiotic supplementation on microbiome balance and psychological mood state in healthy stressed adults. FASEB J. 2018;32 lb122–lb122. [Google Scholar]
- Teles A.M., dos Santos B.A., Ferreira C.G., Mouchreck A.N., da Silva Calabrese K., Abreu-Silva A.L., Almeida-Souza F. IntechOpen; 2019. Ginger (Zingiber officinale) Antimicrobial Potential: A Review. Ginger Cultivation and Its Antimicrobial and Pharmacological Potentials. [Google Scholar]
- Vilar da Silva J.H., González-Cerón F., Howerth E.W., Rekaya R., Aggrey S.E. Alteration of dietary cysteine affects activities of genes of the transsulfuration and glutathione pathways, and development of skin tissues and feather follicles in chickens. Animal Biotech. 2020;31:203–208. doi: 10.1080/10495398.2019.1577253. [DOI] [PubMed] [Google Scholar]
- Williams J., Kellett J., Roach P.D., McKune A., Mellor D., Thomas J., Naumovski N. L-theanine as a functional food additive: Its role in disease prevention and health promotion. Beverages. 2016;2:13. doi: 10.3390/beverages2020013. [DOI] [Google Scholar]
- Yakhkeshi, S., Rahimi, S., Gharib, N.K., 2011. The effects of comparison of herbal extracts, antibiotic, probiotic and organic acid on serum lipids, immune response, GIT microbial population, intestinal morphology and performance of broilers.