Abstract
Palmitic acid (PA) in root exudates or decaying residues can reduce the incidence of soil-borne diseases and promote the growth of some crop plants. However, the effects of PA on soil-borne pathogens and microbial communities are poorly understood. Here, we investigate the effects of PA on overall watermelon microbial communities and the populations of Fusarium oxysporum f.sp. niveum (Fon). The effects of PA on the mycelial growth and spore production of Fon were tested in vitro, while its effects on Fon, total bacteria and total fungi populations, and microbial communities were evaluated in a pot experiment. The results revealed that all test concentrations of PA inhibited Fon mycelia growth and spore production. The pot experiment showed that 0.5 mM and 1 mM PA reduced Fon but increased total bacteria populations, and 0.5 mM and 1 mM PA 0.5 mM and 1 mM PA promoted the change to a soil type of bacteria soil. Meanwhile, 0.5 mM PA and 1 mM PA altered the community composition of the rhizosphere microorganisms and reduced the relative abundance of two bacterial operational taxonomic units (OTUs) and the two fungal OTUs that were significantly (p < 0.01) related with disease severity and increased that of four bacterial OTUs and the two fungal that were highly significantly (p < 0.01) negatively correlated with the disease severity. These results suggest that application of PA decreased the populations of Fon, changed the rhizosphere microbial composition, reduced the disease severity of Fusarium wilt, and promoted the growth of watermelon.
Keywords: PA, Watermelon, Fon, Antifungal effects, Microbial abundance, Rhizosphere
1. Introduction
Watermelon is one of the most important horticultural crops with high economic value, and hence widely cultivated throughout the world. However, continuous monoculture has disrupted soil microbial balance, rapidly increasing the incidence of Fusarium wilt disease. Due to this disease, the yearly yield of watermelon crop is reduced by approximately 35.1–58.5 million tons worldwide (Tang et al., 2019). Fusarium wilt of watermelon is caused by the soil-borne fungal pathogen Fusarium oxysporum f.sp. niveum (Fon) which can rest in the soil for up to 20 years making it very difficult to control (Larkin et al., 1996, Xu et al., 2015). For the past 20 years, several measures have been taken to mitigate the occurrence of this disease, such as soil fumigation (Xue et al., 2019), chemical agents (Everts et al., 2014), biological control (Liu et al., 2019, Wu et al., 2019), and other agricultural methods (Li et al., 2019, Tang et al., 2019). But, an effective biological measure to curb this disease is still lacking, and hence more studies are required.
Fatty acids are essential molecules that play crucial roles in almost all organisms, and act as signaling agents in plant-plant, plant-microbe and plant-environment interactions (Tumlinson and Engelberth, 2008, Upchurch, 2008, Raffaele et al., 2009). Several studies have revealed that fatty acids and their derivatives directly inhibit the growth of plant pathogens within the rhizosphere, and improve the surrounding environment of plant rhizosphere to reduce the occurrence of crop diseases and promote crop growth (Davis et al., 1997, Liu et al., 2008, Liu et al., 2012). Palmitic acid (PA) is a saturated long-chain fatty acid compound that is widely found in the seeds of beans, sunflowers and cotton (Lukonge et al., 2007, Perez-Vich et al., 2016, Zhao et al., 2019). Also, it is found in high levels in many plant root exudates and residues (Guo et al., 2010, Pan et al., 2013). Previous studies have shown that PA can inhibit the growth of plant soil-borne pathogens and promote the growth of seedlings (Davis et al., 1997, Abdel-Naime et al., 2019, Ding et al., 2019). However, the effects of PA on watermelon growth and its rhizosphere microbial community have rarely been explored.
Previous studies have reported that root exudates of different wheat varieties exhibit different inhibitory abilities on pathogens, and PA isolated by gas chromatography-mass spectrometry has the ability to inhibit the growth of Fon mycelium in vitro (Pan et al., 2013). Therefore, we hypothesize that application of PA at different concentration would alter soil microbial communities and reduce the Fon populations in watermelon rhizosphere. The changes in absolute abundances of Fon and changes in microbial communities in watermelon rhizosphere were studied using quantitative PCR and high-throughput sequencing. Our results provide new insights and a theoretical reference for treating and alleviating the soil-borne disease of watermelon wilting.
2. Materials and methods
2.1. Fungal strains
Fon was isolated from the infected roots of watermelon in Xiangfang farm of Northeast Agricultural University, Harbin, China (45° 72′ N, 126° 76′E) and identified as physiologically race No. 1 via 16S rRNA gene sequencing analysis and light microscopy observation. All the strains were preserved at 4 °C in refrigerator on potato dextrose agar (PDA) slants by regular transfer, which consists of potato 200 g, dextrose 20 g and agar 20 g per liter (pH = 7).
2.2. Soil sampling
Monoculture watermelon soil with sandy loam texture was collected in the spring from the top layer (0–15 cm) of a greenhouse at the Xiangfang farm of Northeast Agricultural University, Harbin, China (45° 72′ N, 126° 76′E). Watermelon has been planted in this greenhouse for five years and was infected by Fon. After removing the plant residues and large stones by using a <6 mm sieve, the soil was thoroughly mixed and transported to a greenhouse. The physicochemical properties of the soil were as follows: Organic matter, 34.65 g·kg−1; Inorganic N, 82.09 g·kg−1; Available P, 73.37 mg·kg−1; Available K, 137.25 mg·kg−1; EC, 0.23 mS cm−1 (1:2.5, w/v); pH, 6.89 (1:2.5, w/v), and were determined by the method described by Zhou et al. (2018).
2.3. In vitro evaluation of antifungal ability
To determine the effects of PA on Fon mycelial growth and spore production in vitro, 5 different concentrations of PA were used according the previous study by Pan et al. (2013). PA (0, 0.25, 0.5, 1, and 2 mmol) was added to 40 ml acetone, transferred to the PDA medium and potato dextrose broth (PDB) medium (potato 200 g and dextrose 20 g per liter), and the pH of the solution was adjusted to 7 using 0.1 M NaOH solution. Finally, the volume was fixed to one liter with distilled water and configured into PDA medium and potato dextrose broth (PDB) containing different concentrations of PA, respectively; And 0 mmol L−1 PA (including 4 ml acetone) was used as control. The plugs (5 mm in diameter) was taken from the Fon mycelium edge of growth after growing for five days, inoculated separately in PDA (20 ml) plate and PDB (100 ml) with different concentrations of PA. All the plates with PDA medium were placed in an incubator at 28 °C for 5 days in dark conditions, and all the flasks with PDB medium were placed in shaking table at 28 °C, 150 rpm for 5 days in dark conditions. The mycelial diameter and spore production were measured using a ruler and a hemacytometer, respectively, and each treatment was repeated five times as described previously (Bardia and Rai, 2007, Williamson and Richardson, 1988). The effective concentration (EC50) values were calculated by estimating the regression of colony growth inhibition and spore production inhibition probability against the logarithmic value of PA concentration (Liu et al., 2019).
2.4. Greenhouse pot experiment
The experiment was carried out in the greenhouse of the Horticultural experiment Center of Northeast Agricultural University. Watermelon seeds (Cv. Cuiyu) were soaked in 2.5% sodium hypochlorite solution for 20 min to perform disinfection and germinated at 28 °C in the hole trays (one seed per hole) containing vermiculite. When the seedlings sprouted, they were kept in a greenhouse (30 °C day/20 °C night, relative humidity of 60% and 16 h light/8 h dark).
PA was added to distilled water and the pH was adjusted to 7 using 0.1 M NaOH solution, which was chosen to not change the existing soil pH (Fierer and Jackson, 2006). The monoculture soil of watermelon was homogenously poured with PA solution at concentrations of 0 mM, 0.25 mM, 0.5 mM, 1 mM, 2 and 4 mM. The watermelon seedlings during the four-leaf stage were transplanted into the pots (24 cm × 18 cm) containing 3 kg of soil, with each pot containing a single seedling. Watermelon was harvested after 60 days of planting. Watermelons were subjected to typical management and water content of the soil was regulated with distilled water every 2 days (about 60% of its moisture holding capacity). In total, 360 pots were used for this experiment (6 treatments × 20 pots × 3 replicates). Harvesting was done after 60 days of planting and samples were collected.
2.5. Plant weight, yield and disease severity
To determine the Plant fresh weight and dry weight of the whole watermelon plant, 5 watermelon plants from each replication were selected and carefully removed from the soil on days 60 after transplanting. These plants were weighed after cleaning and drying for 72 h at 70 °C to investigate the plant fresh weight and dry weight. The ripened fruit was harvested to investigate the fruit yield. Twenty plants from each replicate were used to calculate the disease severity of Fusarium wilt by using the method prescribed by Chu et al. (2014). The scores were provided such that 0 represents no obvious yellowing symptoms, all leaves green; 1 represents leaf yellowing area of 1–25%; 2 represents leaf yellowing area of 26–50% and slight wilting; 3 represents leaf yellowing area of 50–75%and wilting; And 4 represents yellowing area greater than 75% and severe wilting.
2.6. Soil sample collection
The rhizosphere soil samples of watermelon were collected from 5 plants from each replication and uniformly mixed to form a composite soil sample for each replicate on days 20, 40 and 60 after transplanting as described previously by Zhou and Wu (2012).
2.7. DNA extraction and quantitative PCR amplification
To assess the effectiveness of PA, the populations of soil microorganisms including total bacteria, total fungi and Fon were determined. A 0.250 mg watermelon rhizosphere soil was used for extraction of soil DNA using the PowerSoil DNA Isolation Kit (MOBIO Laboratories, Carlsbad, USA) following the manufacturer's protocol. DNA was extracted thrice from each soil sample replicate and the product was combined to make a composite DNA sample. Quantitative PCR was used in an IQ5Real-time PCR system (Bio-Rad Lab, LA, United States) to detect the population of bacteria, fungi and Fon in the rhizosphere soil of watermelon. The specific primer pair sets used were as follows: bacteria (338-F: 5′-ACTCCTACGGGAGGCAGCAG-3′/518-R: 5′-ATTACCGCGGCTGCTGG-3′), fungi (ITS1-F: 5′-CTTGGTCATTTAGAGGAAGTAA-3′/ITS4-R: 5′-TCCTCCGCTTATTGATATGC-3′), and Fon (Fon-1: 5′-CGATTAGCGAAGACATTCACAAGACT-3′/Fon-2:5′-ACGGTCAAGAAGATGCAGGGTAAAGGT-3′) (Lin et al., 2010). The quantitative PCR reaction system (20 ul) was as follows: For bacteria/fungi, template DNA (2 μl), primers (1 μl), 2 × Taq PCR MasterMix (10 μl; Tiangen Biotech, Beijing, China) and sterile water (6 μl); For Fon, template DNA(2 μl), primers (0.5 μl), 2 × Taq PCR MasterMix (10 μl) and sterile water(7 μl). The quantitative PCR reaction conditions were as follows: for bacteria/fungal, (1) 94 °C for 5 min (2) 26 cycles of 94 °C for 60 s, 65 °C for 90 s and 72 °C for 90 s (3) 72 °C for 10 min; For Fon, (1) 94 °C for 5 min (2) 35 cycles of 94 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s (3) 72 °C for 3 min. A target gene from bacteria, fungi and Fon genomic DNA were used to create the plasmid standard for their quantification. The bacteria, fungi and Fon DNA copy concentration was calculated based on the method previously described by Jin et al. (2019). PCR assays were conducted in triplicates.
2.8. Illumina Miseq sequencing and raw data processing
To further determine the effects of PA on soil microbial community composition, DNA from all composite soil samples at 40 days extracted by Illumina Miseq were sequenced. The V4-V5 regions of the bacterial 16SrDNA gene and the ITS1 regions of the fungal rRNA gene were amplified using the primer sets of 515F/907R and ITS1F/ITS2R, respectively (Fazzini et al., 2011, Derakhshani et al., 2016). Both the forward and reverse primers also had a 6-bp unique barcode to each sample. The amplification products of the three PCR reactions were combined and purified using the Agarose Gel DNA purification kit (TaKaRa), and were then mixed accordingly to achieve the same molar concentration of the final mixture.
The mixture was subjected to sequencing in an Illumina MiSeq instrument (USA) at Major Bio-Pharm Technology Co., Ltd., Shanghai, China. The raw sequence reads were de-multiplexed, quality-filtered, and processed using FLASH as described previously (Magoc and Salzberg, 2011, Zhou et al., 2017). Briefly, the chimeric sequences were processed in QIIME (Caporaso et al. 2010). The sequences were clustered into operational taxonomic units (OTUs) at 97% sequence similarity with UPARSE against an agglomerative clustering algorithm (Edgar, 2013). Taxonomic assignments to OTUs were carried out using BLAST against the SILVA (Quast et al., 2013) (bacterial) and Unite (Koljalg et al., 2013) (fungal) databases. All sequence data have been archived in the NCBI Sequence Read Archive (SRA) with the Accession Number SUB7295634. A total of 24219 16SrDNA gene sequences and 31594 ITS gene sequences were randomly selected for further analysis.
2.9. Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL) was used for data analysis. One-way ANOVA and Duncan's multiple range tests were used for detecting statistical significance. Correlation analysis was carried out using bivariate correlation and Pearson correlation coefficients. P < 0.05 and P < 0.01was considered to be statistically significant.
3. Results
3.1. In vitro antifungal ability evaluation
The results of the antifungal ability evaluation revealed that all concentrations of PA had significantly inhibitory effects on mycelial growth and the spore production ability of Fon when compared with control, and the inhibitory effects increased with increasing concentrations of PA after incubating for 5 days (Table 1 and Table 2), (P < 0.05). A concentration of 2 mM PA showed the strongest antifungal ability, and the inhibition of mycelial growth and spore production reached 37.2% and 71.7% as compared to control, respectively.
Table 1.
Effects of PA on Fon mycelium growth after incubating for 5 days.
| Treatment | Mycelial growth inhibition |
|||
|---|---|---|---|---|
| Colony diameter/mm | Toxicity regression equation | R2 | EC50/mM L−1 | |
| 0 mM PA(Control) | 74.9 ± 1.27a | y = 1.31x-0.68 | 0.968 | 3.357 |
| 0.25 mM PA | 69.6 ± 0.55b | |||
| 0.5 mM PA | 64.7 ± 1.30c | |||
| 1 mM PA | 54.7 ± 2.21d | |||
| 2 mM PA | 47.0 ± 2.10e | |||
Letters indicate values (means ± SE, n = 3) with significant differences within the same column (P < 0.05, Duncan’s multiple range test).
Table 2.
Effects of PA on Fon Spore production.
| Treatment | Spore production inhibition |
|||
|---|---|---|---|---|
| Number of spore (106 cfu·ml−1) | Toxicity regression equation | R2 | EC50/mM L−1 | |
| 0 mM PA (Control) | 1.84 ± 0.15a | Y = 0.64x-0.34 | 0.864 | 0.296 |
| 0.25 mM PA | 0.92 ± 0.08b | |||
| 0.5 mM PA | 0.85 ± 0.04b | |||
| 1 mM PA | 0.71 ± 0.04c | |||
| 2 mM PA | 0.52 ± 0.06d | |||
Letters indicate values (means ± SE, n = 3) with significant differences within the same column (P < 0.05, Duncan’s multiple range test).
3.2. Plant weight and yield
Watermelon fresh weight, dry weight and fruit yield with 1 mM PA treatment were highest among rest of the treatments (Table 3). when compared with control, 1 mM PA treatment showed a significant increase in the seedling fresh weight, dry weight and fruit yield by 43.8%, 46.0% and 20.2%, respectively (Table 3), (P < 0.05).
Table 3.
Effects of PA on plant weight and yield of watermelon.
| Treatment | Whole plant fresh weight/g | Whole plant dry weight/g | Fruit yield/g plant−1 |
|---|---|---|---|
| 0 mM PA(Control) | 116.54 ± 7.53c | 15.78 ± 2.19b | 406.4 ± 22.2cd |
| 0.25 mM PA | 135.66 ± 11.86b | 16.78 ± 1.46b | 437.3 ± 18.2bc |
| 0.5 mM PA | 142.76 ± 11.11b | 18.22 ± 1.22b | 454.6 ± 30.5bc |
| 1 mM PA | 167.57 ± 11.70a | 21.46 ± 0.98a | 488.3 ± 26.1a |
| 2 mM PA | 140.95 ± 7.59b | 16.96 ± 1.54b | 411.2 ± 16.4d |
| 4 mM PA | 104.16 ± 6.22c | 10.85 ± 1.22c | 364.2 ± 34.8e |
Letters indicate values (means ± SE, n = 3) with significant differences within the same column (P < 0.05, Duncan’s multiple range test.
3.3. Watermelon Fusarium wilt disease severity
The watermelon began to develop symptoms of Fusarium wilt gradually after 30 days of transplantation, while no disease severity was observed in watermelons treated with 1 mM PA after 30 days. During the harvest period, the watermelon treated with 1 mM PA demonstrated the lowest disease severity, which decreased by 71.8%, when compared with the control treatment (Fig. 1), (P < 0.05).
Fig. 1.
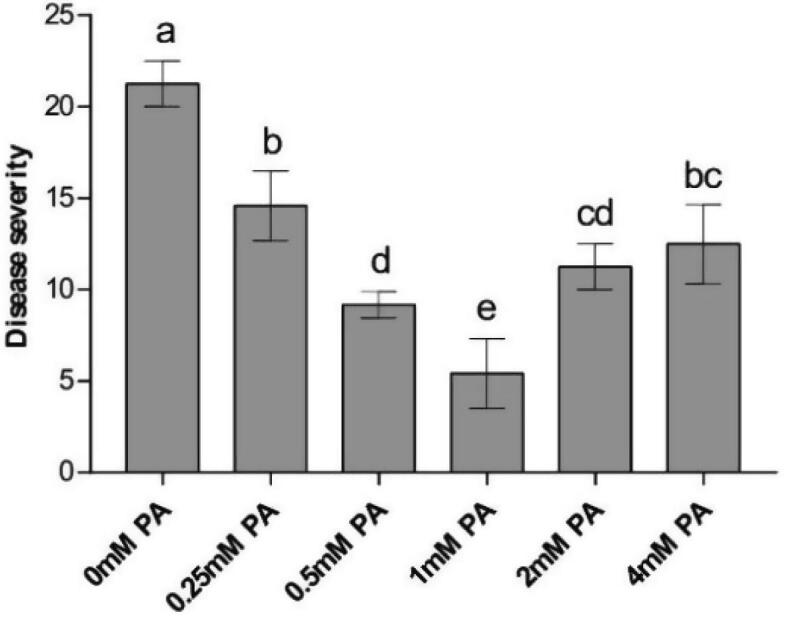
Effects of PA on the disease severity in watermelon. Letters indicate values (means ± SE, n = 3) with significant differences within the same group (P < 0.05, Duncan’s multiple range test).
3.4. Absolute abundances of total bacteria, total fungi and Fon
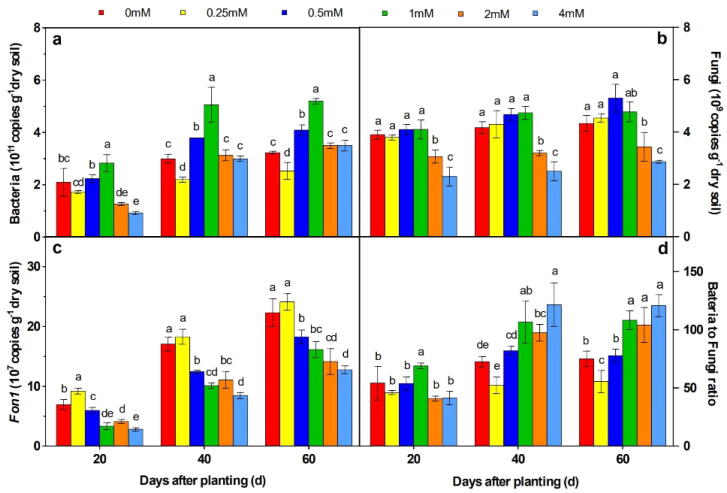
The total bacteria in the soil initially showed an increasing trend and then a decreasing trend in each period with increasing concentrations of PA (Fig. 2a). Among different concentrations of PA, the total bacteria after treatment with 1 mM PA concentration was the highest and significantly increased in each period (Fig. 2a), (P < 0.05). Compare with control, the total bacteria increased by 33.3%, 70% and 66.7% on days 20, 40 and 60 after planting, respectively (Fig. 2a). As for the total fungi in soil, the addition of 2 mM PA and 4 mM PA concentrations significantly reduced the total fungal population when compared with the control, and no significant difference was found from the remaining treatments (Fig. 2b), (P < 0.05). With respect to the Fon population present in the soil, all treatments, except the one with 0.25 mM PA concentration, significantly decreased the population and the inhibitory ability increased with increasing concentrations of PA during each period (Fig. 2c), (P < 0.05). The reduction of Fon population by treatment with 4 mM PA concentration remained the lowest during all stages, which decreased by 59.4%, 50.6% and 42.6%, respectively, when compared with control treatment. The bacterial to fungal ratio of the group treated with 1 mM PA concentration on day 20 after planting treatment and the ones treated with 1 mM PA, 2 mM PA and 4 mM PA concentrations on days 40 and 60 after planting were significantly higher when compared to control (Fig. 2a), (P < 0.05).
Fig. 2.
Effects of PA on soil bacteria (a), fungi (b), Fon (c) populations and the bacteria to fungi ratio (d) in watermelon, and these results were detected using quantitative PCR. Letters indicate values (means ± SE, n = 3) with significant differences within the same group (P < 0.05, Duncan's multiple range test).
3.5. Relative abundance of most influential bacteria and fungi
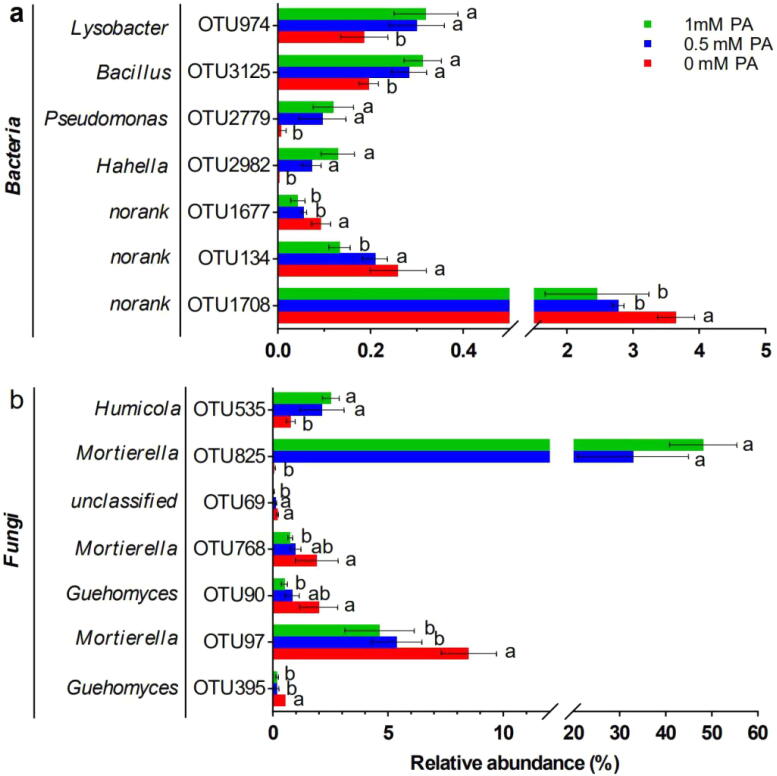
To further clarify the effect of PA on the composition of microbial community in the rhizosphere of watermelon, the relative abundance of bacterial and fungal OTUs of ≥0.1% in the rhizosphere of watermelon and significantly correlation with watermelon Fusarium wilt disease severity were analyzed (Table S1 and S2), (P < 0.05). At OTU level, there were 3 bacterial OTUs (i.e., OTU1708, OTU134 and OTU1677) with significant positive correlation with watermelon Fusarium wilt disease severity, which were all unclassified members at the genus level (S1), (P < 0.01). The relative abundance of the bacteria OTU1708 and OTU1677 within 0.5 mM PA and 1.0 mM PA treatments, and OTU134 within 1 mM PA treatment were significantly decreased when compared to control treatment (Fig. 3a), (P < 0.05). The relative abundance of the 3 bacterial OTUs treated with 1.0 mM PA decreased by 32.6%, 50.0% and 55.5%, respectively, as compared to control treatment. However, there were four bacterial OTUs, OTU2982, OTU2779, OTU3125 and OTU974, that showed a significantly negative correlation with watermelon Fusarium wilt disease severity, were belonging to Hahella, Pseudomonas, Bacillus and Lysobacter genera of bacterial genera, respectively (S1), (P < 0.01). The relative abundance of these four bacteria were all significantly increased within 0.5 mM PA and 1.0 mM PA treatments and the relative abundance treated within 1.0 mM PA increased by 30 times, 13.35 times, 0.62 and 0.71 times, respectively, when compared with control (Fig. 3a), (P < 0.05).
Fig. 3.
Comparison of relative abundance of most influential bacteria and fungi. The bacteria in (a) showed significant positive or negative correlation with the disease severity, the fungi in (b) showed significant positive or negative correlation with the disease severity (P < 0.01). Letters indicate values (means ± SE, n = 3) with significant differences within the same OTU (P < 0.05, Duncan's multiple range test).
Meanwhile, there were five fungal OTUs, OTU395, OTU97, OTU90, OTU768 and OTU69, that showed a significantly positive correlation with watermelon Fusarium wilt disease severity, and these belonged to Guehomyces, Mortierella, Guehomyces, Mortierella and unclassified of fungal genera, respectively (S2) (P < 0.01). The relative abundance of the fungi OTU395 and OTU97 within 0.5 mM PA and 1.0 mM PA, and OTU90, OTU768 and OTU69 within 1.0 mM PA were significantly decreased when compared to control treatment, and their abundance within 1.0 mM PA were decreased by 45.5%, 74.8%, 78.8%, 60.6% and 64.4% (Fig. 3b), (P < 0.05). However, there were also two fungal OTUs, OTU825 and OTU535, that showed a significantly negative correlation with Fusarium watermelon wilt disease severity, were belonging to Mortierella and Humicola spp., respectively (S2), (P < 0.01). The relative abundance of the two fungal OTUs were all significantly increased within 0.5 mM PA and 1.0 mM PA treatments and the relative abundance treated within 1.0 mM PA increased by 787.4 times and 2.2 times, respectively, when compared with control (Fig. 3b), (P < 0.05).
4. Discussion
Many studies have reported the excellent antifungal potential of PA (Liu et al., 2008). Previous study showed that PA is the main component of wheat root exudates, which inhibited the growth of Fon mycelium in vitro, and the current study further confirmed the findings (Pan et al., 2013). Also, the spore production capacity of Fon was strongly inhibited by all PA treatments. Previous studies have shown that fatty acids can be transfer to the fungal membrane, which exacerbates the disorder of the plasma membrane, and further alters the protein conformation and increases the membrane permeability, eventually disintegrating the cytoplasm (Bergsson et al., 2001). Moreover, some studies have shown that acetyl fatty acids interfere with the biosynthesis of Fon sphingolipids and affect the normal synthetic metabolism of the fungi (Li et al., 2003). These evidences may be vital reasons of antifungal mechanism of PA, we need to further verify this mechanism.
As an exogenous carbon source material, PA inevitably caused changes in soil microbial composition. In this experiment, the low concentrations of PA (0.25–1 mM) increased the levels of watermelon rhizosphere bacteria when compared with the control, which was similar to the results of the study conducted by Zhang et al. (2017). Notably, the relatively high concentrations of PA (2–4 mM) decreased the total bacteria and fungi in the soil, and this might be due to the formation of osmotic pressure by high concentrations of PA that either destroyed or disintegrated some microbial plasma membranes and tissues (Avis and Belanger, 2001). Controlling the growth of pathogens might be an important measure to alleviate soil-borne diseases (Zhao et al., 2017). The results of our study showed that all treatments except at the concentration of 0.25 mM PA reduced the Fon population of soil, indicating the inhibitory ability of PA on Fon and in potentially managing the disease. More interestingly, the population of Fon gradually increased with the growth of watermelon at all treatment concentrations. This might be due to the fact that some compounds, such as cinnamic acid, ferulic acid and p-hydroxybenzoic acid released by the roots of watermelon during continuous monocropping, which in turn promoted the growth of Fon (Lv et al., 2018). PA was shown to reduce the population of Fon during the harvest stage when compared to the control, indicating the positive effect of PA. The ratio of bacteria to fungi is also considered as important indicators for assessing soil health (Janvier et al., 2007). Compared with the control, the concentration of 1 mM PA increased the bacteria to fungi ratio in the rhizosphere of watermelon and improved the health of the soil. Zhou et al. (2010) have also shown that exogenous PA could increase the bacteria to fungi ratio of the rhizosphere, improve soil microbial composition and promote the growth of eggplant.
Soil microbial community composition directly or indirectly affects the growth and disease occurrence in plants (Mendes et al., 2011). As a result, many researchers have sought to optimize soil health and facilitate plant growth by changing the composition of soil microbial communities, such as by using soil fumigation combined with organic fertilizers and the use of intercropping instead of continuous monoculture (Ren et al., 2016, Xue et al., 2019). In this study, the results of Miseq sequencing indicated that PA improved microbial community composition in the rhizosphere of watermelon. The relative abundance of the OTU1708 and OTU1677 in the rhizosphere soil of 0.5 mM PA and 1 mM PA were decreased when compared to control and they have positive correlation with the disease severity, which indicates the two bacteria might be harmful bacteria to the health of watermelon. On the contrary, the relative abundance of the OTU2982, OTU2779, OTU3125 and OTU974 in the rhizosphere soil of 0.5 mM PA and 1 mM PA, belonging to the genus Hahella, Pseudomonas, Bacillus and Lysobacter, respectively, were increased and showed a negative correlation with the disease severity. Hahella are more commonly found in petroleum sediments and can degrade hydrocarbons (Abed et al., 2014), and some studies reported that some species of Hahella can produce antifungal compounds, such as prodiginines (Kim et al., 2007). Besides, Bacillus and Pseudomonas are the two most common biocontrol bacteria that can inhibit Fon, and manage Fusarium wilt and promote plant growth (Zhang et al., 2020a, Zhang et al., 2020b). Lysobacter play a key role in controlling soil-borne pathogens and there was a higher relative in the rhizosphere of vanilla and apple with amending of bio-fertilizers (Wang et al., 2016, Xiong et al., 2017).
For fungi, the relative abundance of OTU395 and OTU97 in the rhizosphere soil of 0.5 mM PA and 1 mM PA was decreased when compared with control and showed positive correlation with the disease severity. OTU395 were assigned to Guehomyces, which can produce ligninase and have strong ability to degrade some carbon sources that are difficult to be degraded, which indicates that exogenous fatty acids carbon source, such as PA, can reduce microorganisms that feed on carbon source (Zhang et al., 2020a, Zhang et al., 2020b). Interestingly, both Guehomyces and Fusarium were increased in the fields of strawberry for many years, which might exist as a harmful fungal genus in the rhizosphere of watermelon (Li and Liu, 2019). Most notably, the relative abundance of OTU825 and OTU535 in the rhizosphere soil of 0.5 mM PA and 1 mM PA were dramatically increased, which belonged to the genus Mortierella and Humicola, and demonstrated a negative correlation with the disease severity. Previous studies have shown that Mortierella and Humicola are usually recognized as beneficial fungi, Mortierella was negatively correlated with Fusarium in watermelon crop system and chili pepper-banana rotation system, and Humicola is also found in soils with high organic matter content (Hong et al., 2020, Lang et al., 2012, Meng et al., 2019, Wang et al., 2020). These evidences indicate that PA might directly or indirectly improve the rhizosphere environment of watermelon, reduce the population of Fon, suppress the Fusarium disease and promoting plant growth by enhancing the relative abundance of beneficial fungi. Moreover, Mortierella has also been shown to use fatty acid carbon sources for metabolism to produce long-chain unsaturated fatty acids, such as linoleic acid and arachidonic acid, enhancing the systemic resistance of plants (Dye et al., 2020, Hao et al., 2014, Sumayo et al., 2014). However, a decrease of OTU97 from Mortierella was also observed in the rhizosphere soil of 0.5 mM PA and 1 mM PA, but the decrease was totally covered by OTU825. Therefore, we speculated that OTU825 was more sensitive to fatty acids to the carbon source response, and inter-genera competition inhibits OTU97 growth. Overall, these evidences suggested that PA can aggregate and increase bacteria or fungi that are capable of metabolizing fatty hydrocarbons. Not only do they play an important role in the metabolism of fatty acids, but most of them are regarded as beneficial bacteria or fungi which improved the rhizosphere of watermelon, reduced the disease severity of Fusarium wilt, and promoted the growth of watermelon. Nevertheless, the sustainable effects of PA to microbial community still warrants further investigation and we also need to pay more attention to whether other fatty acids have the similar effects.
5. Conclusion
Our results showed that PA, a kind of aliphatic compound that is seen in the root exudates of wheat, altered the amount of soil microorganisms, increased the amount of bacteria in the rhizosphere and reduced the populations of Fon. It also changed the rhizosphere microbial community composition, and increased the relative abundance of beneficial rhizosphere microorganisms. All these changes might promote watermelon growth and alleviate Fusarium wilt disease, but should be further verified by conducting studies in future. In summary, our results provided a novel treatment approach with PA, which promotes watermelon growth and alleviates wilt disease by inducing positive plant-microbial interactions.
Ethics approval
This paper has not been submitted elsewhere for publication. This research did not involve any human participants and/or animals.
Consent to participate
The authors declare their consent to participate.
Consent for publication
The authors declare their consent for publication.
Availability of data and material (data transparency)
The datasets generated and/or analyzed during the current study will be made available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
This work was funded by a program of the National Vegetable Industry Technical System of China (project CARS-25), and a program of the Collaborative Innovation System of Modern Agricultural Industry Technology of Heilongjiang of China (project HNWJZTX201701). All authors express their gratitude to anyone who helped with the work.
Authors' contributions
KXM, FZW, WHL and KP came up with the ideas and designed methodology; KXM, WTD and XYL collected the data; KXM, JMK analyzed the data. KXM, JMK and MKR did the writing of the manuscript. All authors contributed crucially to the drafts and gave final approval for publication.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.03.040.
Contributor Information
Wenhui Li, Email: eau2014@163.com.
Kai Pan, Email: pankai001@neau.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdel-Naime W.A., Fahim J.R., Fouad M.A., Kamel M.S. Antibacterial, antifungal, and GC-MS studies of Melissa officinalis. S. Afr. J. Bot. 2019;124:228–234. [Google Scholar]
- Abed R.M.M., Al-Sabahi J., Al-Maqrashi F., Al-Habsi A., Al-Hinai M. Characterization of hydrocarbon-degrading bacteria isolated from oil-contaminated sediments in the Sultanate of Oman and evaluation of bioaugmentation and biostimulation approaches in microcosm experiments. Int. Biodeterior. Biodegrad. 2014;89:58–66. [Google Scholar]
- Avis T.J., Belanger R.R. Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001;67(2):956–960. doi: 10.1128/AEM.67.2.956-960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardia P.K., Rai P.K. In vitro and field evaluation of biocontrol agents and fungicides against wilt of cumin caused by Fusarium oxysporum f. sp. cumini. Biophys. J . 2007;98(11):2469–2477. [Google Scholar]
- Bergsson G., Arnfinnsson J., Steingrimsson O., Thormar H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001;45(11):3209–3212. doi: 10.1128/AAC.45.11.3209-3212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7(5) doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L.R., Pan K., Wu F.Z., Tao L., Wang Y. Effects of Hexadecanoic acid on Fusarium oxysporum f. sp niveum control and on growth of watermelon (Citrullus lanatus) Allelopathy J. 2014;34(2):241–252. [Google Scholar]
- Davis E.L., Meyers D.M., Dullum C.J., Feitelson J.S. Nematicidal activity of fatty acid esters on soybean cyst and root-knot nematodes. J. Nematol. 1997;29(4):677–684. [PMC free article] [PubMed] [Google Scholar]
- Derakhshani H., Tun H.M., Khafipour E. An extended single-index multiplexed 16S rRNA sequencing for microbial community analysis on MiSeq illumina platforms. J. Basic Microbiol. 2016;56(3):321–326. doi: 10.1002/jobm.201500420. [DOI] [PubMed] [Google Scholar]
- Ding L.S., Guo W.B., Chen X.H. Exogenous addition of alkanoic acids enhanced production of antifungal lipopeptides in Bacillus amyloliquefaciens Pc3. Appl. Microbiol. Biotechnol. 2019;103(13):5367–5377. doi: 10.1007/s00253-019-09792-1. [DOI] [PubMed] [Google Scholar]
- Dye S.M., Yang J., Bostock R.M. Eicosapolyenoic fatty acids alter oxylipin gene expression and fatty acid hydroperoxide profiles in tomato and pepper roots. Physiol. Mol. Plant Pathol. 2020;109:11. [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10(10):996-+. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Everts K.L., Egel D.S., Langston D., Zhou X.G. Chemical management of Fusarium wilt of watermelon. Crop Prot. 2014;66:114–119. [Google Scholar]
- Fazzini R.A.B., Levican G., Parada P. Acidithiobacillus thiooxidans secretome containing a newly described lipoprotein Licanantase enhances chalcopyrite bioleaching rate. Appl. Microbiol. Biotechnol. 2011;89(3):771–780. doi: 10.1007/s00253-010-3063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer, N., Jackson, R.B., 2006. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. United States of America, 103(3), 626-631. [DOI] [PMC free article] [PubMed]
- Guo, X.W., Kun, L.I., Sun, Y.N., Zhang, L.H., Xi-Xi, H.U., Hong-Gang, et al., 2010. Allelopathic effects and identification of allelochemicals in grape root exudates. Acta Horticulturae Sinica, 37(6), 861-868.
- Hao G.F., Chen H.Q., Du K., Huang X.Y., Song Y.D., Gu Z.N. Increased fatty acid unsaturation and production of arachidonic acid by homologous over-expression of the mitochondrial malic enzyme in Mortierella alpina. Biotechnol. Lett. 2014;36(9):1827–1834. doi: 10.1007/s10529-014-1546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Jv H., Lu M., Wang B., Ruan Y. Significant decline in banana fusarium wilt disease is associated with soil microbiome reconstruction under chilli pepper-banana rotation. Eur. J. Soil Biol. 2020;97:103154. [Google Scholar]
- Janvier C., Villeneuve F., Alabouvette C., Edel-Hermann V., Mateille T., Steinberg C. Soil health through soil disease suppression: which strategy from descriptors to indicators? Soil Biol. Biochem. 2007;39(1):1–23. [Google Scholar]
- Jin X., Wu F., Zhou X. Different toxic effects of ferulic and p -hydroxybenzoic acids on cucumber seedling growth were related to their different influences on rhizosphere microbial composition. Biol. Fertil. Soils. 2019;56(1):125–136. [Google Scholar]
- Kim D., Lee J.S., Park Y.K., Kim J.F., Jeong H., Oh T.-k. Biosynthesis of antibiotic prodiginines in the marine bacterium Hahella chejuensis KCTC 2396. Journal of Applied Microbiology. 2007;102(4):937–944. doi: 10.1111/j.1365-2672.2006.03172.x. [DOI] [PubMed] [Google Scholar]
- Koljalg U., Nilsson R.H., Abarenkov K., Tedersoo L., Taylor A.F.S., Bahram M. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013;22(21):5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
- Lang J., Hu J., Ran W., Xu Y., Shen Q. Control of cotton verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol. Fertil. Soils. 2012;48(2):191–203. [Google Scholar]
- Larkin R.P., Hopkins D.L., Martin F.N. Suppression of Fusarium wilt of watermelon by nonpathogenic Fusarium oxysporum and other microorganisms recovered from a disease-suppressive soil. Phytopathology. 1996;86(8):812–819. [Google Scholar]
- Li C.X., Fu X.P., Zhou X.G., Liu S.W., Xia Y., Li N.H. Treatment with wheat root exudates and soil microorganisms from wheat/watermelon companion cropping can induce watermelon disease resistance against fusarium oxysporum f. sp. niveum. Plant Dis. 2019;103(7):1693–1702. doi: 10.1094/PDIS-08-18-1387-RE. [DOI] [PubMed] [Google Scholar]
- Li W.H., Liu Q.Z. Changes in fungal community and diversity in strawberry rhizosphere soil after 12 years in the greenhouse. J. Integrative Agric. 2019;18(3):677–687. [Google Scholar]
- Li X.C., Jacob M.R., ElSohly H.N., Nagle D.G., Smillie T.J., Walker L.A. Acetylenic acids inhibiting azole-resistant Candida albicans from Pentagonia gigantifolia. J. Nat. Prod. 2003;66(8) doi: 10.1021/np030196r. [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Chen K.S., Chang J.Y., Wan Y.L., Hsu C.C., Huang J.W. Development of the molecular methods for rapid detection and differentiation of fusarium oxysporum and f. oxysporum f. sp. niveum in taiwan. New Biotechnol. 2010;27(4):409–418. doi: 10.1016/j.nbt.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Liu F.Y., Zhu Q., Yang H.R., Zhou J., Dai C.C., Wang X.X. An integrated prevention strategy to address problems associated with continuous cropping of watermelon caused by Fusarium oxysporum. Eur. J. Plant Pathol. 2019;155(1):293–305. [Google Scholar]
- Liu P., Liu Z.H., Wang C.B., Guo F., Wang M., Zhang Y.F. Effects of three long-chain fatty acids present in peanut (Arachis hypogaea L.) root exudates on its own growth and the soil enzymes activities. Allelopathy J. 2012;29(1):13–24. [Google Scholar]
- Liu S.Y., Ruan W.B., Li J., Xu H., Wang J.G., Gao Y.B. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia. 2008;166(2):93–102. doi: 10.1007/s11046-008-9124-1. [DOI] [PubMed] [Google Scholar]
- Lukonge E., Labuschagne M.T., Hugo A. The evaluation of oil and fatty acid composition in seed of cotton accessions from various countries. J. Sci. Food Agric. 2007;87(2):340–347. [Google Scholar]
- Lv H.F., Cao H.S., Nawaz M.A., Sohail H., Huang Y., Cheng F. Wheat intercropping enhances the resistance of watermelon to fusarium wilt. Front. Plant Sci. 2018;9:15. doi: 10.3389/fpls.2018.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoc T., Salzberg S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T.Z., Wang Q.J., Abbasi P., Ma Y. Deciphering differences in the chemical and microbial characteristics of healthy and fusarium wilt-infected watermelon rhizosphere soils. Appl. Microbiol. Biotechnol. 2019;103(3):1497–1509. doi: 10.1007/s00253-018-9564-6. [DOI] [PubMed] [Google Scholar]
- Mendes R., Kruijt M., de Bruijn I., Dekkers E., van der Voort M., Schneider J.H.M. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332(6033):1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- Pan K., Xu L.H., Wu F.Z., Han Z., Chu L.R., Qiu C.F. Fungicidal effects of wheat root exudates on Fusarium oxysporum f. sp niveum. Allelopathy Journal. 2013;32(2):257–265. [Google Scholar]
- Perez-Vich B., del Moral L., Velasco L., Bushman B.S., Knapp S.J., Leon A. Molecular basis of the high-palmitic acid trait in sunflower seed oil. Mol. Breed. 2016;36(4):12. [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(D1):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaele S., Leger A., Roby D. Very long chain fatty acid and lipid signaling in the response of plants to pathogens. Plant Signaling Behav. 2009;4(2):94–99. doi: 10.4161/psb.4.2.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L.X., Huo H.W., Zhang F., Hao W.Y., Xiao L., Dong C.X. The components of rice and watermelon root exudates and their effects on pathogenic fungus and watermelon defense. Plant Signaling Behav. 2016;11(6):9. doi: 10.1080/15592324.2016.1187357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumayo M.S., Kwon D.K., Ghim S.Y. Linoleic acid-induced expression of defense genes and enzymes in tobacco. J. Plant Physiol. 2014;171(18):1757–1762. doi: 10.1016/j.jplph.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Tang L.L., Nie S.R., Li W.H., Fan C., Wang S.Q., Wu F.Z. Wheat straw increases the defense response and resistance of watermelon monoculture to Fusarium wilt. BMC Plant Biol. 2019;19(1):15. doi: 10.1186/s12870-019-2134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlinson J.H., Engelberth J. Fatty Acid-Derived Signals that Induce or Regulate Plant Defenses Against Herbivory. In: Schaller A., editor. Induced Plant Resistance to Herbivory. Springer; Dordrecht: 2008. pp. 389–407. [Google Scholar]
- Upchurch R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008;30(6):967–977. doi: 10.1007/s10529-008-9639-z. [DOI] [PubMed] [Google Scholar]
- Wang L., Li J., Yang F., Yaoyao E., Shen Q. Application of bioorganic fertilizer significantly increased apple yields and shaped bacterial community structure in orchard soil. Microb. Ecol. 2016;73(2):1–13. doi: 10.1007/s00248-016-0849-y. [DOI] [PubMed] [Google Scholar]
- Wang M., Xue J., Ma J., Feng X., Xu H. Streptomyces lydicus m01 regulates soil microbial community and alleviates foliar disease caused by alternaria alternata on cucumbers. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson G.B., Richardson D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988;14(1):181–187. doi: 10.1007/BF01022540. [DOI] [PubMed] [Google Scholar]
- Wu Y.C., Zhou J.Y., Li C.G., Ma Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiology Open. 2019;8(8):14. doi: 10.1002/mbo3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Guo S., Jousset A., Zhao Q., Shen Q. Bio-fertilizer application induces soil suppressiveness against fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 2017;114:238–247. [Google Scholar]
- Xu W.H., Liu D., Wu F.Z., Liu S.W. Root exudates of wheat are involved in suppression of Fusarium wilt in watermelon in watermelon-wheat companion cropping. Eur. J. Plant Pathol. 2015;141(1):209–216. [Google Scholar]
- Xue C., Shen Z.Z., Hao Y.W., Yu S.T., Li Y.C., Huang W.J. Fumigation coupled with bio-organic fertilizer for the suppression of watermelon Fusarium wilt disease re-shapes the soil microbiome. Appl. Soil Ecol. 2019;140:49–56. [Google Scholar]
- Zhang D.Q., Yan D.D., Cheng H.Y., Fang W.S., Huang B., Wang X.L. Effects of multi-year biofumigation on soil bacterial and fungal communities and strawberry yield. Environ. Pollut. 2020;256:10. doi: 10.1016/j.envpol.2019.113415. [DOI] [PubMed] [Google Scholar]
- Zhang F., Chen Y., Wu C., Xiao C., Wu C., Yang Y. Effects of exogenous fatty acids on growth and rhizosphere soil of pepper plants. Acta Agriculturae Zhejiangensis. 2017;29(5):760–766. [Google Scholar]
- Zhang H.Y., Chen W., Zhao B.P., Phillips L.A., Zhou Y., Lapen D.R. Sandy soils amended with bentonite induced changes in soil microbiota and fungistasis in maize fields. Appl. Soil Ecol. 2020;146:12. [Google Scholar]
- Zhao J., Mei Z., Zhang X., Xue C., Zhang C.Z., Ma T.F. Suppression of Fusarium wilt of cucumber by ammonia gas fumigation via reduction of Fusarium population in the field. Sci. Rep. 2017;7:8. doi: 10.1038/srep43103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Chang H., Feng L., Jing Y., Teng W.L., Qiu L.J. Genome-wide association mapping and candidate gene analysis for saturated fatty acid content in soybean seed. Plant Breeding. 2019;138(5):588–598. [Google Scholar]
- Zhou B., Han L., Yin Y., Wu J., Sun C., Ye X. Effects of allelochemicals hexadecanoic acid on soil microbial composition and biomass in rhizosphere of eggplant. J. Shenyang Agric. Univ. 2010;41(3):275–278. [Google Scholar]
- Zhou X.G., Liu J., Wu F.Z. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil. 2017;415(1–2):507–520. [Google Scholar]
- Zhou X.G., Wu F.Z. p-Coumaric acid influenced cucumber rhizosphere soil microbial communities and the growth of fusarium oxysporum f.sp cucumerinum owen. PLoS ONE. 2012;7(10):11. doi: 10.1371/journal.pone.0048288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.G., Zhang J.H., Pan D.D., Ge X., Jin X., Chen S.C. p-Coumaric can alter the composition of cucumber rhizosphere microbial communities and induce negative plant-microbial interactions. Biol. Fertil. Soils. 2018;54(3):363–372. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.