Abstract
Ulcerative colitis (UC) is an inflammatory bowel disease with high morbidity. Acetic acid-induced damage of colonic mucosa in rats is a commonly used experimental animal model of UC. This research aimed to explore for the first time the ameliorative effect of dietary supplementation with fava bean on the incidence of UC in rats fed with sucrose containing diet. Rats were divided into five groups as follows: G1, control healthy rats; G2, colitic rats; G3, colitic rats fed diets containing 30% sucrose, G4, healthy rats fed diets containing 30% sucrose and G5, colitic rats fed diets containing 30% sucrose supplemented with dried ground fava bean. Colonic injury and inflammation were evaluated through a disturbance of oxidative biomarkers, a significant increase in inflammatory biomarkers and inflammatory cytokines, and histological abnormalities in colonic tissues accompanied by colonic mucosal DNA damage. Colitic rats fed on sucrose containing diet demonstrated additional histological, biochemical, and DNA alterations in colonic mucosa of rats. Dietary supplementation with dried ground fava bean significantly corrected the impaired oxidative and inflammatory biomarker levels and modulated histological features and DNA alterations. Finally, fava bean attenuated the oxidative damage and colonic injury induced by acetic acid, which confirmed its high anti-oxidant and anti-incendiary properties.
Keywords: Acetic acid, Fava bean, Sucrose, Ulcerative colitis
1. Introduction
Inflammatory intestinal disease is a chronic gastrointestinal tract disease (Rohr et al., 2018). It has two main profiles: epithelial ulceration and ulcerative colitis (UC). Various etiological factors may predispose to UC, such as genetic, environmental and immunological factors (Jiao et al., 2018). It affects the quality of a patient’s life and increases the incidence of colon cancer (Abraham et al., 2017).
UC only includes the rectum and colon. Although its aetiology is not fully understood, UC has been linked to lowered anti-oxidant capacity and raised production of free radicals, which are responsible for lipid peroxidation (LPO) and may lessen the ability of intracellular anti-oxidants, thus ultimately leading to prominent colonic inflammation. In numerous studies, UC pathogenesis indicated an important relationship between excessive inflammation and oxidative stress. Improving LPO and free scavenging of radicals afford a valuable, protective, and therapeutic treatment for UC (Scioli et al., 2014). Various risk factors are associated with UC, including long duration of the disease, low energy activity, complicated primary sclerosing cholangitis or stenotic disease and insufficient pharmacological therapy (Abdin, 2013).
Approximately 50% of patients with UC undergo removal of part of their colons because of failure of conservative medicinal treatments. In addition, these patients are not sufficiently strong to maintain long-term remission (Santos et al., 2020). However, treatments are linked to serious adverse side effects, such as abdominal cramps, diarrhoea, fever and increased blood pressure. New therapeutic selections with lower toxicity and slight side effects need to be developed (Nguyen et al., 2020).
Colonic inflammation has been widely used in numerous chemically induced animal models to elucidate its primary pathophysiological mechanisms and to assess the potential of chemicals that induce UC, such as acetic acid (AA) (Dothel et al., 2013). Colitis induced by AA is a common model of experimental colitis used to examine inflammatory bowel disease. This model is generated via intra-rectal injection of AA, to induce inflammation and ulceration in the rat rectum and colon. Oxidative destruction in this situation is thought to be a pathogenic factor, and this damage is also observed in the colon’s gross morphology (Cagin et al., 2016).
It has been demonstrated that dietary factors are responsible for the pathogenesis of different conditions, including colon cancer and celiac disease; thus, the diet presumably involves in etiology of inflammatory bowel disease (Costello et al., 2020). Diets rich in fibre, such as those rich in cereals, fruits and vegetables have a positive effect on health, due to their effect on reducing the incidence of different types of diseases as it increase the volume of fecal bulk, reducing the time of intestinal transit, cholesterol and glycaemic levels, also fibres was able to trap harmful substances (mutagenic and carcinogenic agents), stimulating the proliferation of the intestinal flora. (Dhingra et al., 2012).
Legumes are an important source of nutrients and occupy an important part in traditional diets in many countries. In addition, they can be eaten in processed or raw forms (Singh et al., 2013). They are rich in dietary fibre, protein, starch, fatty acids, vitamins, trace minerals and phenolic anti-oxidants (Alcázar-Valle et al., 2020).
The fava bean (Vicia faba L.) FB, commonly referred as broad bean, belongs to the Fabaceae family. In the Mediterranean, it is considered a chief winter crop, whereas in other regions of Europe and South America it is considered a spring crop. Fava beans consist of 12% fibre, 22.4%–36% protein, 1.2%–4% lipids and 57.8%–61% carbohydrates (Bouchenak and Lamri-Senhadji, 2013). They are considered an appropriate food for diabetic patients and can protect against heart disease and reduce blood glucose levels (Lonnie et al., 2020). Moreover, they are rich in l-3,4 dihydroxyphenylalanine (l-DOPA), which is a precursor to neurotransmitter, catecholamine (Ramya and Thaakur, 2007). This study aimed to examine for the first time the effect of dietary supplementation of fava beans in experimental UC rats fed on high-sucrose diet.
2. Materials and methods
2.1. Materials
Fava beans were purchased from local markets in Jeddah, Makkah Province, washed, let to dry, and then ground to a fine powder before use.
2.2. Induction of ulcerative colitis
Colitis was induced in colon tissues via an intracolonic single administration of 4% AA (2 mL) through a soft paediatric lubricated catheter under a low dose of ether anaesthesia. Rats were kept in a horizontal position after AA administration for 2 min, to prevent the draining of AA. The same procedure was performed in control animals using equal volumes of saline solution; treatments were started after colitis induction (Shanmugam et al., 2020).
2.3. Experimental design
Fifty male albino rats weighing 150–175 g were housed at the King Fahd Medical Research Center Animal Facility Breeding Colony were allowed free access to basal diet (the basal diet was prepared according to AOAC (2000)) containing 10% corn seed oil, 10% casein, 70% corn starch; 4%salt mixture, 5% cellulose, 1% vitamin mixture, 1%; and water ad libitum. The Ethical Committee of King Fahd Medical Research Center, Jeddah, KSA. approved the study.
Rats were randomly divided into five groups (10 animals/group) as detailed below. Group 1: a healthy control that received normal saline (2 mL) once intrarectally and was fed the basal diet for 21 days; Group 2: a colitic group that received the AA intraperitoneally as a single administration and was fed the basal diet for 21 days; Group 3: colitic rats fed the basal diet supplemented with 30% sucrose (sucrose was replaced with corn starch, to keep the concentrations of other nutrients and carbohydrates constant) for 21 days; Group 4: healthy rats that were fed the basal diet supplemented with 30% sucrose, with dietary administration of dried ground fava seeds as a 252 g/kg diet according to León-Espinosa et al. (2016) (dried ground fava seeds were replaced with casein to keep the concentration of protein in the diet constant) for 21 days; and Group 5: colitic rats that were fed the 30% sucrose containing basal diet as well as dried ground fava seeds (252 g/kg) for 21 days.
At the end of the experimental regimen, the final body weight and the food intake were recorded. Rats were sacrificed, and serum was separated from the obtained blood samples by centrifugation. Serum samples were stored at − 20 °C for later use in biochemical analyses and a tissue specimen was obtained from the distal colon and stored in saline solution for DNA analysis and histological investigation.
2.4. Assessments of colitis
2.4.1. Calculation colon/body weight ratio
Eight-cm-long part from the rear of the colon was removed and longitudinally opened. Colon was emptied of faecal materials by spraying 0.9% saline blotted dried and weighed. The oedema index was determined from ratio of colon segment to body weight (Distrutti et al., 2009).
2.4.2. Estimation of anti-oxidant and inflammatory biomarkers in colon homogenates
The collected colorectal tissue was homogenised in 10 mM Tris-HCl buffer (pH 7.1). The obtained colon homogenate was used to measure anti-oxidant enzyme levels, including the following: non-protein sulfhydryl (NP-SH) and total glutathione sulfhydryl (T-GSH), according to Sedlak and Lindsay (1968), and catalase (CAT) activity and nitric oxide (NO) using commercial kits (Biovision, USA). Protein carbonyl contents (PCOs) in colon homogenates were determined spectrophotometrically according to Levine et al. (1990) based on the reaction of the carbonyl group with 2,4-dinitrophenylhydrazine. Colonic myeloperoxidase (MPO) activity was determined according to Krawisz et al. (1984), whereas colonic alkaline phosphatase (ALP) activity was measured using disodium p-nitrophenol phosphate as the substrate (Dorai and Bachhawat, 1977).
2.4.3. Determination of colonic mucosal cytokines
Inflammatory mediators including IL-6, IL-β and TNF-α and prostaglandin E2 (PGE2), were determined in colon tissues by ELISA based assays in accordance with the (Kamiya Biomedical Co. CA. USA) supplier’s instructions.
2.4.4. Determination of colon mucosal DNA
Colonic DNA was isolated from colon mucosal tissue following the guidelines of manufacturer (QIAGEN, USA). About 25 mg of colon tissues were minced into smaller pieces to facilitate complete lysis. To the tissue lysate, 180 μl of ATL buffer, 20 μl of proteinase K were added and incubated at 56 °C. The sample was vortexed during incubation, to homogenate it, or placed in a shaking water bath and then vortexed for 20 s. Next, to this sample mixture, 200 μl of AL buffer and 200 μl of absolute was added, and vortexed to mix thoroughly. Finally, DNA purity and content were measured and further analysed by agarose gel electrophoresis.
2.4.5. Macroscopic assessment
Several parameters were assessed for microscopic scoring. Each of the studied parameters including mucosal and submucosal inflammatory cell infiltration, mucosal oedema, and necrosis of the mucosa was allotted a grade (+++, severe; ++, moderate; +, mild; and –, no change) based on the shift in its severity in relation to respective parameter in control.
2.4.6. Histopathological investigations
Following excision, colon tissue was fixed in 10% formalin. Tissue samples were embedded into blocks of paraffin wax and cut into thin sections. They were then stained with haematoxylin and eosin H&E and examined under light microscope.
2.5. Statistical analysis
Data were analysed by (SPSS program for windows, version 20) (SPSS Inc., Chicago, IL, USA). The difference between groups was made using One Way ANOVA (LSD) test. The data were presented as means ± SD. The p < 0.01 was considered as statistically significant.
3. Results
3.1. Anthropometric parameters and food intake
Data on the effects of treatments on anthropometric parameters and food intake in control and colitic rats are presented in Table 1. Food intake in colitic rats was not affected by the presence of colitis, as it was comparable to that observed in control rats. However, food intake in normal and colitic rats fed a diet with 30% sucrose-supplementation was significantly (p < 0.01) augmented compared with normal diet fed control and colitic rats without supplementation. The final body weights of normal rats that fed on a 30% sucrose supplemented diet was significantly elevated (p < 0.01) compared those in control rats. In contrast, body weights of colitic rats were significantly decreased (p < 0.01) compared with control rats. However, the administration of a 30%-sucrose-supplemented diet to colitic rats led to a significant increase (p < 0.01) in their body weights compared with colitic rats. The body weights of colitic rats fed a sucrose-supplemented diet were further increased after the supplementation of their diets with FB, compared with colitic rats fed a diet with or without supplementation with sucrose. The colon-to-body weight ratio was significantly (p < 0.01) higher in normal rats fed on a diet supplemented with 30%-sucrose. Similarly, matched to control rats, colitic rats fed with or without the sucrose-supplemented diet had significantly (p < 0.01) elevated colon to body weight ration. Conversely, colitic rats fed a diet added with both sucrose and FB had a significantly (p < 0.01) lower colon-to-body weight ratio.
Table 1.
Effects of treatments on body weight and food intake in control and colitic rats.
| Control | 30% sucrose | Colitic | Colitic + 30% sucrose | Colitic + 30% sucrose + FB | |
|---|---|---|---|---|---|
| Food intake (g) | 260 ± 18.7 | 285 ± 19.9ab* | 264 ± 17.9 | 280 ± 19.5ab* | 282 ± 18.4ab* |
| Final body weight (g) | 310 ± 21.2 | 350 ± 19.4a* | 250 ± 17.8a* | 266 ± 19.5b* | 280 ± 20.4bc* |
| Colon/body weight ratio | 5.1 ± 0.94 | 6.2 ± 0.96a* | 6.8 ± 0.99a* | 7.1 ± 0.81a* | 5.8 ± 0.91d* |
FB, fava beans; acompared with the control; bcompared with the colitic group; ccompared with the colitic + 30% sucrose group; dcompared with the 30% sucrose, colitic + 30%sucrose and colitic + 30% sucrose + FB groups. The data are shown as the mean ± SD (N = 10). *p < 0.01.
3.2. Oxidative stress markers
The effects of different treatments on oxidative stress markers in colon tissues are listed in Table 2. Compared with the control animals, T-GSH, NP-SH and CAT levels were significantly decreased (p < 0.01), and NO, PCO, MPO and ALP levels were significantly increased (p < 0.01) in colitic rats. Feeding of a 30%-sucrose-supplemented diet to colitic rats further downregulated GSH, NP-SH and CAT levels and further augmented the NO, PCO, MPO and ALP levels significantly compared with colitic rats that were fed a normal diet. Conversely, supplementation of the diet of colitic rats with FB together with 30% sucrose led to a significant reversal (p < 0.01) in the levels of the studied oxidative stress markers compared with those detected in colitic rats fed on a diet with or without supplementation with 30% sucrose. Compared with control, normal healthy rats fed on a sucrose-supplemented diet showed no significant change in any of the studied oxidative stress markers.
Table 2.
Effect of different treatments on oxidative stress parameters in rat colon tissues.
| Control | 30% sucrose | Colitic | Colitic + 30% sucrose | Colitic + 30% sucrose + FB | |
|---|---|---|---|---|---|
| T-GSH (nmol/L/mg protein) | 5.2 ± 0.94 | 4.9 ± 1.1a* | 3.4 ± 0.82b* | 1.8 ± 0.09abc* | 4.2 ± 1.2d* |
| NP-SH (nmol/L/mg protein) | 3.34 ± 0.9 | 3.1 ± 0.77a* | 2.6 ± 0.82b* | 1.5 ± 0.72abc* | 2.9 ± 0.9d* |
| CAT (U/mg protein) | 22.7 ± 7.5 | 20.3 ± 7.3a* | 9.1 ± 1.9b* | 6.8 ± 1.2abc* | 15.5 ± 5.5d* |
| NO (μg/mg wet tissue) | 23.5 ± 8.4 | 21.5 ± 7.5a* | 64.6 ± 12.5b* | 79.4 ± 15.7abc* | 16.3 ± 4.7d* |
| PCO (nmol/mg protein) | 0.9 ± 0.04 | 1.1 ± 0.92a* | 1.89 ± 0.09b* | 2.6 ± 0.99abc* | 1.2 ± 0.94d* |
| MPO (U/mg protein) | 64.6 ± 15.3 | 79.7 ± 16.2a* | 152.3 ± 22.2b* | 188.4 ± 26.7abc* | 138.5 ± 24.3d* |
| ALP (U/I) | 4.9 ± 1.9 | 5.3 ± 1.9a* | 23.5 ± 8.6b* | 30.7 ± 9.4abc* | 13.7 ± 2.7d* |
FB, fava beans ,T-GSH, total glutathione sulfhydryl; NP-SH, non-protein sulfhydryl; CAT, catalase; NO, nitric oxide; PCO, protein carbonyl content; MPO, myeloperoxidase, ALP, alkaline phosphatase. aCompared with the control; bcompared with the control and 30% sucrose group; ccompared with the colitic group; dcompared with the colitic and colitic + 30% sucrose group. The data are expressed as the mean ± SD (N = 10). *p < 0.01).
3.3. Inflammatory markers
The effects of various treatments on inflammatory indices are presented in Table 3. Colitic rats had significantly elevated levels of IL-6, TNF-α, PGE2 and IL-1β (p < 0.01) compared with control rats. Colitic rats fed on a diet containing 30%-sucrose exhibited a further increase (p < 0.01) in these markers matched to those in colitic rats that were fed a normal diet. In contrast, colitic rats fed on a diet supplemented with sucrose as well as FB exhibited significantly decreased levels (p < 0.01) of all the studied inflammatory markers compared with colitic rats fed on diet with or without sucrose supplementation. Conversely, the levels of studied inflammatory markers were comparable between control and normal rats that were given a 30%-sucrose containing diet.
Table 3.
Effects of different treatments on colonic mucosal inflammatory markers.
| Control | 30% sucrose | Colitic | Colitic + 30% sucrose | Colitic + 30% sucrose + FB | |
|---|---|---|---|---|---|
| TNF-α (pg/g of tissue) | 1715 ± 34 | 1748 ± 36a* | 3910 ± 48b* | 4160 ± 54c* | 2235 ± 39d* |
| IL-1β (pg/g of tissue) | 2250 ± 32 | 2230 ± 37 a* | 4653 ± 59b* | 4875 ± 52c* | 3125 ± 42 d* |
| IL-6 (pg/g of tissue) | 76 ± 9.5 | 80 ± 9.4 a* | 320 ± 18.3b* | 390 ± 18.6c* | 180 ± 11.5 d* |
| PGE2 (pg/mg of tissue) | 252 ± 11.9 | 270 ± 12.5 a* | 1456 ± 21.2b* | 1570 ± 24.5c* | 950 ± 17.7 d* |
FB, fava beans, TNF-α, tumour necrosis alpha; IL-1β, interleukin-beta; IL-6, interleukin-6; PGE2, prostaglandin-E2. a Compared with the control; b compared with the control and 30% sucrose group; ccompared with the colitic group; d compared with the colitic and colitic + 30% sucrose group. The data are expressed as the mean ± SD (N = 10) *p < 0.01.
3.4. Histopathological analysis
The scores of the histological analysis of colonic mucosa are illustrated in Table 4. A significant necrosis of colonic mucosa, infiltration of inflammatory cells in mucosa and submucosa and submucosal oedema was noted in colitic rats as well as in colitic rats fed a 30%-sucrose-supplemented diet. Necrosis of the mucosa and submucosal inflammatory cell infiltration were more severe in colitic rats that were fed a normal diet compared with colitic rats fed on a diet with 30% sucrose. In contrast, submucosal oedema was more pronounced in colitic rats that were fed a sucrose containing diet compared with colitic rats fed with a diet without 30% sucrose supplementation. Moreover, rats fed with a diet mixed with sucrose as well as FB exhibited a significant improvement in necrosis of mucosa, oedema and mucosal inflammatory cell infiltration. No significant changes were noticed in normal healthy rats fed a 30%-sucrose containing diet compared with control rats (Table 4).
Table 4.
Scores of histopathological parameters in control and different treatment groups.
| Histological observations | Control | 30% sucrose | Colitic | Colitic + 30% sucrose | Colitic + 30% sucrose + FB |
|---|---|---|---|---|---|
| Necrosis of the mucosa | − | − | +++ | ++ | − |
| Mucosal inflammatory cell infiltration | − | − | +++ | +++ | + |
| Submucosal inflammatory cell infiltration | − | − | +++ | ++ | + |
| Submucosal oedema | − | − | ++ | +++ | + |
FB, fava beans; (−), no change; (+), mild change; (++), moderate change; (+++), severe change
3.5. Histology of mucosal tissue
The histological observations performed via H&E staining of mucosal tissues of rats collected after the different treatments are provided in Fig. 1. A significant mucosal necrosis accompanied by submucosal and mucosal infiltration of inflammatory cells and oedema in submucosa were observed in both colitic rats fed on a normal diet and in colitic rats fed with a normal diet supplemented with 30% sucrose compared with control rats, in which normal histology of colonic mucosa and absence of submucosal oedema and inflammatory cell infiltration were observed. In turn, colitic rats that were given a normal diet exhibited more severe mucosal necrosis and submucosal infiltration of inflammatory cells compared with colitic rats that were provided with a diet with sucrose supplementation. Submucosal oedema was more pronounced in colitic rats fed a sucrose diet than it was in those without the sucrose diet. In contrast, a significant improvement in mucosal necrosis, submucosal and mucosal inflammatory cell infiltration and oedema in submucosa were noted in colitic rats given a diet supplemented with FB.
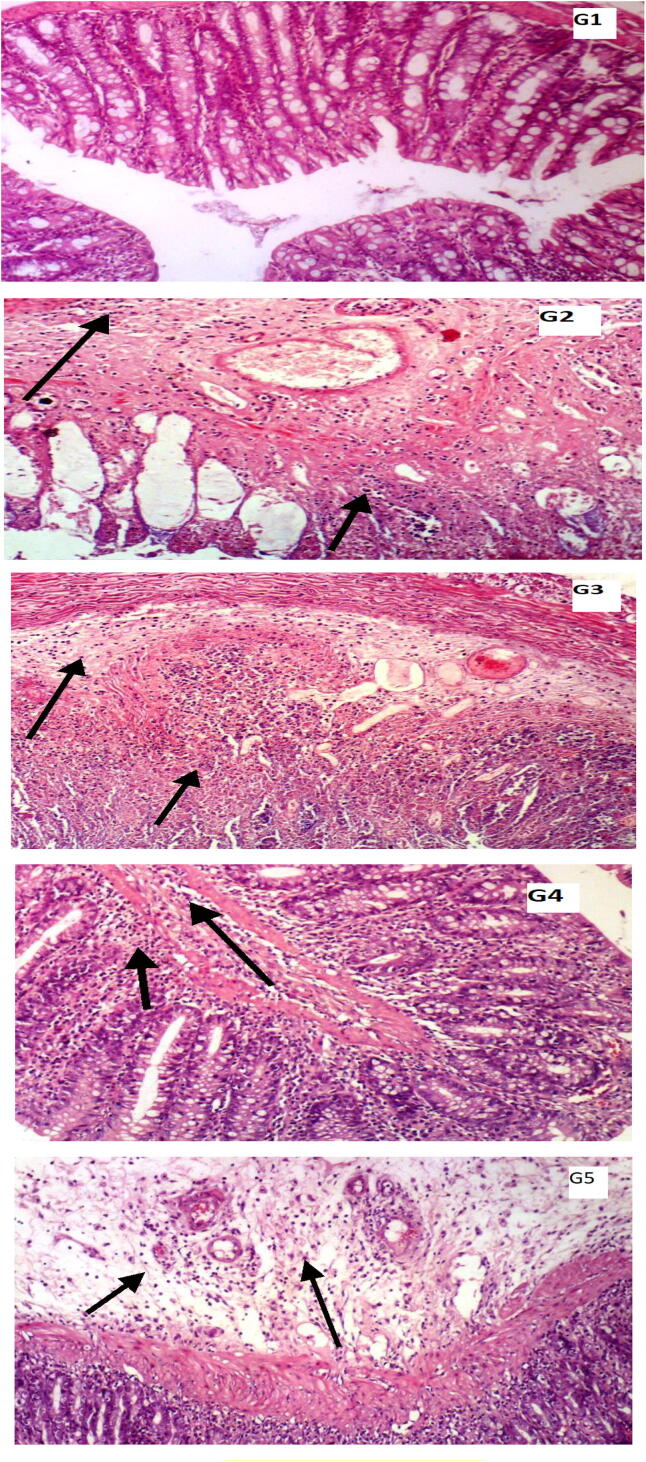
Fig. 1.
Histopathological examination of haematoxylin and eosin H&E-stained ulcerative colitis rat tissues. G1: A normal histology of mucosa, submucosa, mucosa and serosa and absence of inflammatory cell infiltration were found in control rats; G2: colitic rats exhibited a marked mucosal necrosis associated with inflammatory cell infiltration in submucosa and mucosa; G3: colitic rats + 30% sucrose showed a more severe mucosal necrosis accompanied by nuclear enlargement as an indication of degenerative changes in response to the sucrose-supplemented diet; G4: colitic rats + 30% sucrose displayed a normal histology of mucosa, comparable to that of the control; G5: colitic rats + 30% sucrose + FB dietary supplementation exhibited a significant reversal in the mucosal necrosis and mucosal and submucosal inflammatory cell infiltration (Magnification X100).
3.6. DNA damage
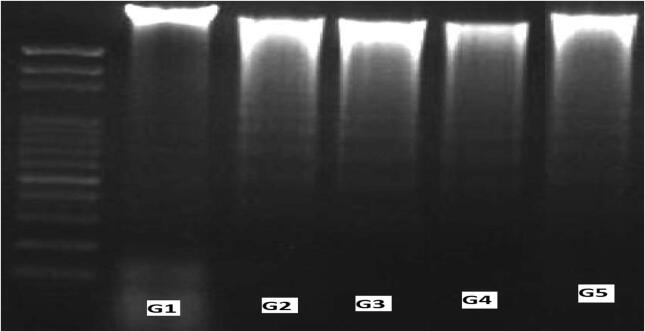
The effects of the different treatments on mucosal DNA fragmentation are presented in Fig. 2. Relatively, 55% and 68% fragmentation of mucosal DNA was noticed in colitic rats fed a normal diet and in colitic rats given a normal diet supplemented with 30% sucrose, respectively, compared with the 1% fragmentation of colonic DNA detected in control rats. Colitic rats fed on a diet supplemented with 30% sucrose and FB showed a marked reduction in colonic DNA fragmentation, as only 42% of DNA was found to be fragmented, indicating a significant protection of FB against DNA damage. Rats that were fed a 30% sucrose diet exhibited 7% DNA fragmentation, which was comparable to the 1% fragmentation of colonic DNA found in control rats.
Fig. 2.
Mucosal DNA fragmentation analysis by agarose gel electrophoresis. Lane 1, 2000 bp DNA marker; lane 2, G1 normal healthy rats, 1% fraction; lane 2, G2 colitic rats, 55% fraction; lane 3, G3 colitic rats + 30% sucrose, 68% fraction; lane 4, colitic rats + 30% sucrose, 7% fraction; and lane 5, G5 colitic rats + 30% sucrose + FB dietary supplementation, 42% fraction.
4. Discussion
Acetic-acid-induced UC is a widely used animal model for ulcerative colitis (Ashry et al., 2016). It is similar to human UC in terms of histopathological characteristics, pathogenesis and the inflammation, rendering it to be a useful tool for anti-colitic drug screening (Randhawa et al., 2014).
In this study, AA elicited an inflammatory response in colon tissues, as evident from the reduction of anti-oxidant biomarkers (T-SH, NP-SH and CAT) and the increase of NO, PCO, inflammatory cytokines and inflammatory markers. Here, a high-sucrose diet was administered and the results of the G2 colitic group and the G3 colitic group were compared. We found that this diet generated multiple effects on the occurrence of colon inflammation caused by a single dose of AA. Because of the hydrolysis of sucrose into monosaccharides, a rat feeding study reported that sucrose increased the proliferation of colon cells to a greater extent than fructose and glucose did shortly after its ingestion (Wang et al., 2009). A study performed by Chen et al. (2015) confirmed that sucrose acts as a co-initiator or promoter of colon tumour formation. Pettan-Brewer et al. (2011) demonstrated that sucrose augments inflammation markers and acts as a proinflammatory agent. Epidemiological studies presented important correlations between inflammation of the colon and high intake of sucrose. Different studies have recently demonstrated that increased consumption of sucrose correlated with a raised colon cancer risk (Rawla et al., 2019). In an animal study sucrose elevated the rate of mutations in the colon, and also changed oxidative stress by glycoxidation and changed the colonic environment, leading to a variation in pH and short-chain fatty acids, which are fermentation products, a central supply of colonic epithelium energy (Gu et al., 2019).
The results indicated that UC negatively affected the final body weight; this may be attributed to an impairment in the absorption of water in the inflamed mucosa, contributing to diarrhoea in this animal modelin cases of human inflammation of the intestines (Bitiren et al., 2020). The weight/length ratio was also used to determine the weight gain caused by oedema or chronic inflammation shortening, which are indices of the severity of colitis (Ağış et al., 2015). The increase in body weight in sucrose-supplemented groups may have led to an increase in palatability because of the effect of sucrose. In addition, a body weight increase was detected in G5 (colitic rats + 30% sucrose supplemented with FB), which was due to the effect of FB seeds on palatability and their high protein and carbohydrate content (Basheer-Salimia et al., 2014).
The increased colon to body weight ratio is considered a sensitive and reliable index for assessing severity of ulcers, oedema and increased levels of inflammatory cytokines, such as TNF-α and IL-6 (Palla et al., 2016). In this study, AA-treated rats exhibited a significant increase in the weight ratio of the colon to the body weight. Oxidative stress is a driving force in the initiation and pathogenesis of IBD. AA causes vascular dilation and the accumulation of white blood cells (WBCs), and elevates flow of blood, resulting in higher production of reactive oxygen species. Bitiren et al. (2020) demonstrated the role of free radicals in the etiology of mucosal injury. AA reduced the content of anti-oxidant biomarkers (T-SH, NP-SH, CAT, NO and PCO). In contrast, the absence of significant differences between the G1 and G4 control groups appears to indicate the role of AA in augmented oxidative stress in lieu of sucrose.
Earlier studies reported that sucrose causes colon, liver or plasma oxidative stress (Van Hecke et al., 2019). Several studies proved that intra-rectal AA administration decreases the defensive anti-oxidant system, including GSH and catalase activity (Krishnan et al., 2014). ROS produce several inflammatory cytokines that exacerbate tissue damage in different tissues. Free radicals cause severe cell damage due to lipid peroxidation (Jena et al., 2012). GSH is an important anti-oxidant intracellular enzyme that is expressed in the gut of mammals, and plays crucial role tissue repair because of its ability to inhibit ROS. Inflammation causes decrease in GSH, and the colon mucosa is severely degraded. GSH, therefore, plays pivotal role in intestinal cell protection and abrogating inflammation (Zińczuk et al., 2020). GSH or NP-SH peptide sulfhydryl groups are widely used in all biological systems and act as an enzyme-free anti-oxidant. GSH due to its sulfhydryl group directly negates ROS and protects oxidative related injuries and it can also indirectly participate in ROS detoxification as a coenzyme or cofactor (Bai et al., 2019).
Nitric oxide and nitric oxide synthase NOS are potential colitis facilitators. The inflammatory reactions observed during colitis, such as enhanced interstitial oedema and intestinal wall thickening, are linked to inflammatory molecules, such as NO (Gillberg et al., 2012). Clinically, colonic tissues of patients with colitis release ROS and NO, which lead to increased membrane peroxidation and DNA attacks (Venkatachalam et al., 2020). Our results indicate that dietary supplementation with dried fava beans can restore the oxidative status of colitic rats in colon homogenates. Ganesan and Xu (2017) indicated that the anti-proliferative and anti-oxidant potentials of legumes were correlated with the occurrence of different classes of phenolic compounds.
Fava bean pods also contain up to 100 mg/kg of caffeoyl-l-malic acid. Similarly, earlier studies (Xu and Chang, 2010) have demonstrated that pro-anthocyanidins are important phenolic compounds present in fava beans. Pro-anthocyanidins can, however, alleviate oxidative stress by scavenging free radicals and altering signalling pathways (Puiggròs et al., 2014).
In human, animal and culture studies, which have collectively demonstrated the potential of PCs to prevent or treat oxidative-stress-related diseases, the anti-oxidant efficacy of PCs has been verified (Casassa et al., 2013). MPO is a neutrophilic enzyme; its activity therefore, correlates to neutrophil population in the inflamed tissue. MPO activity measurement was, therefore, considered to be a sensitive test for the detection of acute intestinal inflammation (Molodecky et al., 2012). The increased activity of MPO is considered a neutrophil infiltration index and indicates the formation of potent cytotoxic oxidants, such as H2O2, hydrochloric acid and chloride ions (Costello et al., 2020). The disturbed redox system can also be reflected in the increased levels of the protein carbonyls PCO and LPO (Garcia et al., 2019). Acute administration of AA through the rectal route increased the MPO and PCO activities, which were reduced after fava bean dietary supplementation in colitic rats. The modelling effect of fava beans on inflammatory markers was linked to the high content of powerful phenolic compounds and other bioactive compounds that could protect the mucosal tissue by increasing its immunity (Esmaillzadeh and Azadbakht, 2012). In this work, AA was adversely affected by inflammatory reaction after colonic injury, leading to a massive intracellular acidification that caused immense epithelial damage (Hall et al., 2020). Cytokines mediate immune responses after AA administration and manage the expansion of colonic mucosal damage (Pearl et al., 2013).
UC was related to an impaired immune response followed by the release of inflammatory molecule, IL-1β, which is expressed as inactive pro-IL-1β and is activated under caspase-1 control (Opipari and Franchi, 2015). Of note, Lazaridis et al. (2017) reported that, in the inflamed colonic mucosa, IL-1β was obviously enhanced and correlated with the severity of the disease. TNF-α is an important pro-inflammatory cytokine that in early inflammation is secreted from lymphocytes and macrophages. It was reported that inflammatory bowel disease plays an integral role in the pathogenesis of UC (Sheng et al., 2020). Earlier human studies (Dai et al., 2020, Zhang et al., 2020) indicated that TNF-α and IL-6 were increased in inflammatory bowel conditions and suggested that these factors play an integral role in their pathogenesis. In this study, the obtained histological findings and oedema formation caused by the elevated cytokines and epithelial cell necrosis were as reported previously by clinical studies (Tahan et al., 2011).
The results of elevated colonic PGE2 levels in colitic rats obtained here were consistent with those of Yu et al. (2019). Furthermore, many pro-inflammatory cytokines induce apoptosis in intestinal epithelia during the intestinal inflammation process. In colonic tissues, pro-inflammatory cytokines are involved in T-cell growth and B-cell proliferation (Santos et al., 2020). However, Bai et al., 2020, Cibor et al., 2020 reported that IL-6 serum levels were significantly high and proportional to the intensity of colonic mucosal injury.
Fava bean is rich in pro-anthocyanidins, which have anti-oxidant and anti-inflammatory properties. Several studies have demonstrated that pro-anthocyanidins can have a beneficial effect on endothelium injury, platelet aggregation and inflammation caused by systemic disease pathogenesis (Huang et al., 2014). In this study, the histological examinations confirmed the biochemical measurements, i.e., that AA caused marked mucosal necrosis and inflammatory cell infiltration. The study reported by Pastrelo et al. (2017) demonstrated that acetic-acid-induced colitis leads to different lesions in the colonic mucosa and necrosis associated with neutrophils. Evidence suggests that peroxides and hydroxyl groups destabilize cell-membrane, cause damage to DNA and cell death due lipid peroxidation (Krzystek-Korpacka et al., 2020).
Regarding the results of colonic mucosal DNA, the supplementation of the diet with sucrose led to changes in the colonic mucosal DNA. According to Guercio et al. (2018) dietary sucrose played a role of promoter or co-initiator of colon tumour formation. Genotoxic and mutagenic potential of sucrose in colonic tissues was attributed to oxidative stress induced DNA damage or derailed DNA repair mechanism (Van Hecke et al., 2019). Notably, Yu et al. (2018) concluded that a diet rich in sucrose doe dependently contributed to increased mutation rate in rat colon directly or indirectly, and lowers DNA levels. In addition, Heredia-García et al. (2019) found that sucrose promoted DNA damage in the colon, indicating pro-carcinogenic nature of sucrose. In contrast, fava beans have anti-mutagenic effects because of their high phenolic and flavonoid content, thus exerting a protective effect against DNA damage (Siah et al., 2012).
5. Conclusions
Increased levels of inflammatory mediators in the rat colon indicated that acetic acid triggered an inflammatory state in colon tissues. In addition, high-sucrose diets increase colonic inflammation and induce histological and mucosal DNA alterations. Dietary supplementation with dried ground fava bean significantly corrected the impaired oxidative and inflammatory biomarker levels and modulated histological features and DNA alterations. Finally, fava bean attenuated the oxidative damage and colonic injury induced by acetic acid, which confirmed its high anti-oxidant and anti-incendiary properties.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for profit sectors.
Declaration of Competing Interest
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdin A.A. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur. J. Pharmacol. 2013;718:145–153. doi: 10.1016/j.ejphar.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Abraham B.P., Ahmed T., Ali T. Inflammatory bowel disease: pathophysiology and current therapeutic approaches. Gastrointest. Pharmacol. 2017;239:115–146. doi: 10.1007/164_2016_122. [DOI] [PubMed] [Google Scholar]
- Ağış E.R., Savaş B., Melli M. Impact of colonic mucosal lipoxin A4 synthesis capacity on healing in rats with dextran sodium sulfate-induced colitis. Prostag. Oth. Lipid M. 2015;121:63–69. doi: 10.1016/j.prostaglandins.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Alcázar-Valle M., Lugo-Cervantes E., Mojica L., Morales-Hernández N., Reyes-Ramírez H., Enríquez-Vara J.N., García-Morales S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules. 2020;25:3528–3536. doi: 10.3390/molecules25153528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC (Association of Official Analytical Chemists), 2000. Official methods of Analysis of Association of Official Analytical Chemists, edited B, Kenesseth Helrick. Fifteenth Edition.
- Ashry E.E., Abdellatief R.B., Mohamed A.E., Kotb H.I. Protective Effect of Ketamine against Acetic Acid-Induced Ulcerative Colitis in Rats. Pharmacol. Pharm. 2016;7:9–18. [Google Scholar]
- Bai X., Bai G., Tang L., Liu L., Li Y., Jiang W. Changes in MMP-2, MMP-9, inflammation, blood coagulation and intestinal mucosal permeability in patients with active ulcerative colitis. Exp. Ther. Med. 2020;20:269–274. doi: 10.3892/etm.2020.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., Gou X., Cai P., Xu C., Cao L., Zhao Z., Huang M., Jin J. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxid. Med. Cell. Longev. 2019:1–20. doi: 10.1155/2019/2432416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer-Salimia R., Camilli B., Scacchi S., Noli E., Awad M. Genetic Diversity among Palestinian Faba Bean (Vicia faba L.) Ecotypes based on single nucleotide polymorphisms. Eur. J. Hortic. Sci. 2014;79:300–305. [Google Scholar]
- Bitiren M., Karakilcik A.Z., Zerin M., Ozardalı I., Selek S., Nazlıgül Y., Ozgonul A., Musa D., Uzunkoy A. Protective effects of selenium and vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol. Trace. Elem. Res. 2020;136:87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- Bouchenak M., Lamri-Senhadji M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J. Med. Food. 2013;16:185–198. doi: 10.1089/jmf.2011.0238. [DOI] [PubMed] [Google Scholar]
- Cagin Y.F., Parlakpinar H., Vardi N., Polat A., Atayan Y., Erdogan M.A., Tanbek K. Effects of dexpanthenol on acetic acid-induced colitis in rats. Exper. Ther. Med. 2016;12:2958–2964. doi: 10.3892/etm.2016.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casassa L.F., Larsen R.C., Beaver C.W., Mireles M.S., Keller M., Riley W.R., Smithyman R., Harbertson J.F. Impact of extended maceration and regulated deficit irrigation (RDI) in Cabernet Sauvignon wines: characterization of proanthocyanidin distribution, anthocyanin extraction, and chromatic properties. J. Agric. Food Chem. 2013;61:6446–6457. doi: 10.1021/jf400733u. [DOI] [PubMed] [Google Scholar]
- Chen Z., Wang P.P., Woodrow J., Zhu Y., Roebothan B., Mclaughlin J.R., Parfrey P.S. Dietary patterns and colorectal cancer: results from a Canadian population-based study. Nutr. J. 2015;14:8–15. doi: 10.1186/1475-2891-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibor D., Szczeklik K., Kozioł K., Pocztar H., Mach T., Owczarek D. Serum concentration of selected biochemical markers of endothelial dysfunction and inflammation in patients with the varying activity of inflammatory bowel disease. Pol. Arch. Intern. Med. 2020;130:598–606. doi: 10.20452/pamw.15463. [DOI] [PubMed] [Google Scholar]
- Costello S.P., Day A., Yao C.K., Bryant R.V. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2019-233135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L., Perera D.S., Burcher E., Liu L. Hemokinin-1 and substance P stimulate production of inflammatory cytokines and chemokines in human colonic mucosa via both NK (1) and NK (2) tachykinin receptors. Neuropeptides. 2020;82 doi: 10.1016/j.npep.2020.102061. [DOI] [PubMed] [Google Scholar]
- Dhingra D., Michael M., Rajput H., Patil R.T. Dietary fibre in foods: a review. J. Food Sci. Technol. 2012;49(3):255–266. doi: 10.1007/s13197-011-0365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distrutti E., Mencarelli A., Renga B., Caliendo G., Santagada V., Severino B., Fiorucci S. A nitro-arginine derivative of trimebutine (NO2-Arg-Trim) attenuates pain induced by colorectal distension in conscious rats. Pharmacol. Res. 2009;59:319–329. doi: 10.1016/j.phrs.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Dorai D.T., Bachhawat B.K. Purification and properties of brain alkaline phosphatase. J. Neurochem. 1977;29:503–512. doi: 10.1111/j.1471-4159.1977.tb10699.x. [DOI] [PubMed] [Google Scholar]
- Dothel G., Vasina V., Barbara G., De Ponti F. Animal models of chemically induced intestinal inflammation: predictivity and ethical issues. Pharmacol. Ther. 2013;139:71–86. doi: 10.1016/j.pharmthera.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Esmaillzadeh A., Azadbakht L. Legume consumption is inversely associated with serum concentrations of adhesion molecules and inflammatory biomarkers among Iranian women. J. Nutr. 2012;142:334–339. doi: 10.3945/jn.111.146167. [DOI] [PubMed] [Google Scholar]
- Ganesan K., Xu B. Polyphenol-Rich Lentils and Their Health Promoting Effects. Int. J. Mol. Sci. 2017;18:2390. doi: 10.3390/ijms18112390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia I.J.P., Kinoshita P.F., Silva L.N.D.E., De Souza Busch M., Atella G.C., Scavone C., Cortes V.F., Barbosa L.A., De Lima Santos H. Ouabain attenuates oxidative stress and modulates lipid composition in hippocampus of rats in lipopolysaccharide-induced hypocampal neuroinflammation in rats. J. Cell. Biochem. 2019;120:4081–4091. doi: 10.1002/jcb.27693. [DOI] [PubMed] [Google Scholar]
- Gillberg L., Varsanyi M., Sjostrom M., Lordal M., Lindholm J., Hellstrom P.M. Nitric Oxide Pathway- Related Gene Alterations in Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2012;47:1283–1298. doi: 10.3109/00365521.2012.706830. [DOI] [PubMed] [Google Scholar]
- Gu J., Xiao Y., Shu D., Liang X., Hu X., Xie Y., Lin D., Li H. Metabolomics Analysis in Serum from Patients with Colorectal Polyp and Colorectal Cancer by 1H-NMR Spectrometry. Dis. Markers. 2019:1–14. doi: 10.1155/2019/3491852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guercio B.J., Zhang S., Niedzwiecki D., Li Y., Babic A., Morales-Oyarvide V., Saltz L.B., Mayer R.J., Mowat R.B., Whittom R., Hantel A., Benson A., Atienza D., Messino M., Kindler H., Venook A., Ogino S., Zoltick E.S., Stampfer M., Ng K., Wu K., Willett W.C., Giovannucci E.L., Meyerhardt J.A., Fuchs C.S. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: Results from CALGB 89803 (Alliance) PLoS One. 2018;13 doi: 10.1371/journal.pone.0199244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.H.T., Lee S., Murphy E.M., Gerich M.E., Dran R., Glover L.E., Abdulla Z.I., Skelton M.R., Colgan S.P. Creatine Transporter, Reduced in Colon Tissues From Patients With Inflammatory Bowel Diseases, Regulates Energy Balance in Intestinal Epithelial Cells, Epithelial Integrity, and Barrier Function. Gastroenterology. 2020;159:984–998. doi: 10.1053/j.gastro.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia-García G., Gómez-Oliván L.M., Orozco-Hernández J.M., Luja-Mondragón M., Islas-Flores H., SanJuan-Reyes N., Galar-Martínez M., García-Medina S., Dublán-García O. Alterations to DNA, apoptosis and oxidative damage induced by sucralose in blood cells of Cyprinus carpio. Sci. Total Environ. 2019;692:411–442. doi: 10.1016/j.scitotenv.2019.07.165. [DOI] [PubMed] [Google Scholar]
- Huang Y., Liu W., Liu H., Yang Y., Cui J., Zhang P., Zhao H., He F., Cheng Y., Ni J., Cai J., Li B., Gao F. Grape seed pro-anthocyanidins ameliorates radiation-induced lung injury. J. Cell. Mol. Med. 2014;18:1267–1277. doi: 10.1111/jcmm.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena G., Trivedi P.P., Sandala B. Oxidative Stress in Ulcerative Colitis: An Old Concept but a New Concern. Free Radic. Res. 2012;46:1339–1345. doi: 10.3109/10715762.2012.717692. [DOI] [PubMed] [Google Scholar]
- Jiao Y.F., Lu M., Zhao Y.P., Liu N., Niu Y.T., Niu Y., Zhao R., Yu J.Q. N-Methylcytisine Ameliorates Dextran-Sulfate-Sodium-Induced Colitis in Mice by Inhibiting the Inflammatory Response. Molecules. 2018;23:510–521. doi: 10.3390/molecules23030510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz J.E., Sharon P., Stenson W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Krishnan M., Jayaraj R.L., Megala J., Elangovan N. Antioxidant mediated antiulcer effect of Eupatorium triplinerve Vahl against acetic acid induced ulcerative colitis in mice. Biomed. Aging Pathol. 2014;4:153–160. [Google Scholar]
- Krzystek-Korpacka M., Kempiński R., Bromke M.A., Neubauer K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics (Basel) 2020;10:601. doi: 10.3390/diagnostics10080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridis L.D., Pistiki A., Giamarellos-Bourboulis E.J., Georgitsi M., Damoraki G., Polymeros D., Dimitriadis G.D., Triantafyllou K. Activation of NLRP3 inflammasome in inflammatory bowel disease: differences between Crohn's disease and ulcerative colitis. Dig. Dis. Sci. 2017;62:2348–2356. doi: 10.1007/s10620-017-4609-8. [DOI] [PubMed] [Google Scholar]
- León-Espinosa E.B., Sánchez-Chino X., Garduño-Siciliano L., Álvarez-González R.I., Dávila-Ortiz G., Madrigal-Bujaidar E., Téllez-Medina D.I., Jiménez-Martínez C. Hypocholesterolemic and Anticarcinogenic Effect of Vicia faba Protein Hydrolyzates. Nutr. Cancer. 2016;68:856–864. doi: 10.1080/01635581.2016.1180406. [DOI] [PubMed] [Google Scholar]
- Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Meth. Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Lonnie M., Laurie I., Myers M., Horgan G., Russell W.R., Johnstone A.M. Exploring Health-Promoting Attributes of Plant Proteins as a Functional Ingredient for the Food Sector: A Systematic Review of Human Interventional Studies. Nutrients. 2020;12:2291. doi: 10.3390/nu12082291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molodecky N.A., Soon S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., Kaplan G.G. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Nguyen L.H., Örtqvist A.K., Cao Y., Simon T.G., Roelstraete B., Song M., Joshi A.D., Staller K., Chan A.T., Khalili H., Olén O., Ludvigsson J.F. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol. Hepatol. 2020;5:986–995. doi: 10.1016/S2468-1253(20)30267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opipari A., Franchi L. Role of inflammasomes in intestinal inflammation and Crohn’s disease. J. Inflamm. Bowel Dis. Disord. 2015;21:173–181. doi: 10.1097/MIB.0000000000000230. [DOI] [PubMed] [Google Scholar]
- Palla A.H., Iqbal N.T., Minhas K., Gilani A.H. Flaxseed extract exhibits mucosal protective effect in acetic acid induced colitis in mice by modulating cytokines, antioxidant and antiinflammatory mechanisms. Int. Immunopharmacol. 2016;38:153–166. doi: 10.1016/j.intimp.2016.04.043. [DOI] [PubMed] [Google Scholar]
- Pastrelo M.M., Ribeiro C.C.D., Duarte J.W., Gollücke A.P.B., Artigiani-Neto R., Ribeiro D.A., Miszputen S.J., Oshima C.T.F., Paiotti A.P.R. Effect of concentrated apple extract on experimental colitis induced by acetic acid. Int. J. Mol. Cell. Med. 2017;6:38–49. [PMC free article] [PubMed] [Google Scholar]
- Pearl D.S., Shah K., Whittaker M.A., Nitch-Smith H., Brown J.F., Shute J.K., Trebble T.M. Cytokine mucosal expression in ulcerative colitis, the relationship between cytokine release and disease activity. J. Crohns Colitis. 2013;7:481–489. doi: 10.1016/j.crohns.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Pettan-Brewer C., Morton J., Mangalindan R., Ladiges W. Curcumin suppresses intestinal polyps in APC Min mice fed a high fat diet. Pathobiol. Aging Age Relat. Dis. 2011;1:7013. doi: 10.3402/pba.v1i0.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puiggròs F., Salvadó M.J., Bladé C., Arola L. Differential modulation of apoptotic processes by proanthocyanidins as a dietary strategy for delaying chronic pathologies. Crit. Rev. Food Sci. Nutr. 2014;54:277–291. doi: 10.1080/10408398.2011.565456. [DOI] [PubMed] [Google Scholar]
- Ramya K.B., Thaakur S. Herbs containing L-Dopa: an update. Anc. Sci. Life. 2007;27:50–55. [PMC free article] [PubMed] [Google Scholar]
- Randhawa P.K., Singh K., Singh N., Jaggi A.S. A review on chemical-inducedinflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014;18:279–288. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawla P., Sunkara T., Barsouk A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przeglad gastroenterologiczny. 2019;14(2):89. doi: 10.5114/pg.2018.81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr M., Narasimhulu C.A., Sharma D., Doomra M., Riad A., Naser S., Parthasarathy S. Inflammatory diseases of the gut. J. Med. Food. 2018;21(2):113–126. doi: 10.1089/jmf.2017.0138. [DOI] [PubMed] [Google Scholar]
- Santos, J.D.M., Peña-Sánchez, J.N., Fowler, S.A., 2020. Patients' perspectives on medication for inflammatory bowel disease: a mixed-method systematic review. Eur. J. Gastroenterol. Hepatol. [DOI] [PubMed]
- Scioli M.G., Stasi M.A., Passeri D., Doldo E., Costanza G., Camerini R., Fociani P., Arcuri G., Lombardo K., Pace S., Borsini F., Orlandi A. Propionyl-L-Carnitine is Efficacious in Ulcerative Colitis Through its Action on the Immune Function and Microvasculature. Clin. Transl. Gastroenterol. 2014;5:55. doi: 10.1038/ctg.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Shanmugam S., Thangaraj P., Dos Santos Lima B., Trindade G.G.G., Narain N., Mara de Oliveira E., Silva A., Santin J.R., Broering M.F., Serafini M.R., Quintans-Júnior L.J., de Souza Antunes, Araújo A. Protective effects of flavonoid composition rich P. subpeltata Ortega. on indomethacin induced experimental ulcerative colitis in rat models of inflammatory bowel diseases. J. Ethnopharmacol. 2020;248 doi: 10.1016/j.jep.2019.112350. [DOI] [PubMed] [Google Scholar]
- Sheng N., Ma Z., Zhou Y., Xu J., Gao Y., Fu X.Y. Cholesterol 25-hydroxylase protects against experimental colitis in mice by modulating epithelial gut barrier function. Sci. Rep. 2020;10:14246. doi: 10.1038/s41598-020-71198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siah S.D., Konczak I., Agboola S., Wood J.A., Blanchard C.L. In vitro investigations of the potential health benefits of Australian-grown faba beans (Vicia faba L.): chemopreventative capacity and inhibitory effects on the angiotensin-converting enzyme, α-glucosidase and lipase. Br. J. Nutr. 2012;108:S123–S134. doi: 10.1017/S0007114512000803. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Bharati R.C., Pedpati A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013;8:6634–6641. [Google Scholar]
- Tahan G., Aytac E., Aytekin H., Gunduz F., Dogusoy G., Aydin S., Tahan V., Uzun H. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid–induced ulcerative colitis in rats. Can. J. Surg. 2011;54:333–338. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke T., De Vrieze J., Boon N., De Vos W.H., Vossen E., De Smet S. Combined Consumption of Beef-Based Cooked Mince and Sucrose Stimulates Oxidative Stress, Cardiac Hypertrophy, and Colonic Outgrowth of Desulfovibrionaceae in Rats. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201800962. [DOI] [PubMed] [Google Scholar]
- Venkatachalam, K., Vinayagam, R., Arokia Vijaya Anand, M., Isa, N.M., 2020. Ponnaiyan R Biochemical and molecular aspects of 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis: a review. Toxicol. Res. (Camb) 9, 2-18. [DOI] [PMC free article] [PubMed]
- Wang B., Bobe G., LaPres J.J., Bourquin L.D. High sucrose diets promote intestinal epithelial cell proliferation and tumorigenesis in APC (Min) mice by increasing insulin and IGF-I levels. Nutr. Cancer. 2009;61:81–93. doi: 10.1080/01635580802372609. [DOI] [PubMed] [Google Scholar]
- Xu B., Chang S.K.C. Phenolic substance characterization and chemical and cell-based antioxidant activities of 11 lentils grown in the Northern United States. J. Agric. Food Chem. 2010;58:1509–1517. doi: 10.1021/jf903532y. [DOI] [PubMed] [Google Scholar]
- Yu Y., Cheng D., Parfrey P., Liu G., Savas S. Two functional indel polymorphisms in the promoter region of the Brahma gene (BRM) and disease risk and progression-free survival in colorectal cancer. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Yoo S.M., Park H.H., Baek S.Y., Kim Y.J., Lee S., Kim Y.L., Seo K.W., Kang K.S. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. Tissue Eng. Regen. Med. 2019;13:1792–1804. doi: 10.1002/term.2930. [DOI] [PubMed] [Google Scholar]
- Zhang T., Jiang J., Liu J., Xu L., Duan S., Sun L., Zhao W., Qian F. MK2 Is Required for Neutrophil-Derived ROS Production and Inflammatory Bowel Disease. Front. Med. (Lausanne) 2020;7:207. doi: 10.3389/fmed.2020.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zińczuk J., Maciejczyk M., Zaręba K., Pryczynicz A., Dymicka-Piekarska V., Kamińska J., Koper-Lenkiewicz O., Matowicka-Karna J., Kędra B., Zalewska A., Guzińska-Ustymowicz K. Pro-Oxidant Enzymes, Redox Balance and Oxidative Damage to Proteins, Lipids and DNA in Colorectal Cancer Tissue. Is Oxidative Stress Dependent on Tumour Budding and Inflammatory Infiltration? Cancers (Basel) 2020;12:1636.70. doi: 10.3390/cancers12061636. [DOI] [PMC free article] [PubMed] [Google Scholar]