Abstract
Honey bees forage for pollen and nectar. Sugar is an important stimulus for foraging and a major source of energy for honey bees. Any differential response of bees to different concentrations of sugary nectar can affect their foraging. The sugar responsiveness of Apis species (Apis dorsata, Apis florea, and Apis cerana) was determined in comparison to that of Apis mellifera by evaluating the proboscis extension response (PER) with eight serial concentrations (0.00001, 0.0001, 0.001, 0.01, 0.1, 0.5, 1.0, and 1.5 M) of sucrose, glucose and fructose. Nectar foragers of bee species (A. dorsata, A. florea, A. cerana, and A. mellifera) exhibited an equal response for sucrose, glucose, and fructose, with no significant differences in their PER at all tested concentrations of these sugars within the same species. The inter-species comparison between Apis species revealed the differential responsiveness to the different concentrations of sugars, and the lowest concentration at which a response occurs was considered as the response threshold of these bee species for sugar solutions. A. mellifera presented significantly higher responsiveness than A. dorsata to low concentrations (0.00001, 0.0001, 0.001, 0.01, and 0.1 M) of sucrose, glucose and fructose. A. mellifera displayed a significantly higher response to water than A. dorsata. A. florea and A. mellifera presented no significant difference in their responsiveness to sucrose, glucose, and fructose at all tested concentrations, and their water responsiveness was also significantly at par but relatively higher in A. mellifera than in A. florea. Likewise, the responsiveness of A. cerana and A. mellifera to different concentrations of sucrose, glucose and fructose was significantly at par with no difference in their water responsiveness. This study represents preliminary research comparing the response of different honey bee species to three sugar types at different concentrations. The results imply that the native species are all better adapted than A. mellifera under local climate conditions.
Keywords: Antennal response; Nectar sugars; Proboscis extension response; Apis dorsata; Apis cerana; Apis florea, Apis mellifera
1. Introduction
Honey bees are significant insects widely used in the pollination of different crops (Breed, 2010, Partap, 2011). They are frequent floral visitors, and collect nectar and pollen to yield honey (Hoover and Ovinge, 2018, Hung et al., 2018). In Pakistan, the species diversity of bees is very high, as in most of Southeast Asia (Waghchoure and Martin, 2008, Radloff et al., 2011). Three native and one exotic species of the genus Apis are commonly found in Pakistan (Iftikhar et al., 2011). Apis cerana (Asian honey bee), Apis florea (little honey bee) and Apis dorsata (giant honey bee or rock bee) are native species, whereas Apis mellifera (European honey bee) is an exotic species (Ascher and Rasmussen, 2010, Iftikhar et al., 2011). A. mellifera was introduced into Pakistan during the early 1980 s, and all of these bee species are now distributed in all parts of the country (Jasra et al., 2001). Prior to the invasion of A. mellifera, A. cerana was maintained and managed in hives for honey production in hilly regions of Pakistan (Waghchoure and Martin, 2008). A. dorsata and A. florea cannot be domesticated in hives, and traditional honey hunters harvest honey from wild colonies of these bee species (Bradbear, 2009). A. cerana and A. mellifera have now both been successfully domesticated in hives for pollination and honey production (Partap, 2011, Radloff et al., 2011).
A. dorsata is a giant honey bee that inhabits foothills, plains, forests, and semi desert areas up to an altitude of 1100 m in all provinces, whereas A. florea is an open-nesting dwarf honey bee that is mostly found up to an altitude of 600 m and is rarely found above 1500 m (Abrol, 2013). A. florea is an excellent colonizer even in less-favorable conditions, with expected effects on native biodiversity (Hepburn et al., 2005, Oldroyd and Wongsiri, 2006, Silva et al., 2020). A. dorsata and A. florea build their nests in open places and live wild in nature (Ahmad, 1988, Radloff et al., 2011).
A. cerana, the Asian honey bee, is better adapted to hilly areas of Pakistan, where it has been domesticated for decades at a considerably higher relative abundance than A. mellifera, A. florea, and A. dorsata (Khan et al., 2014). A. mellifera, the European honey bee, is mainly distributed in plane areas and some parts of hilly areas. A. cerana (a native honey bee) shows better performance than A. mellifera (an exotic honey bee) among scattered nectar sources (Zhou et al., 1991). A. mellifera produces more honey per colony than A. cerana, whereas more swarms have been recorded in A. cerana (Yang, 2005, Yu, 2010).
Honey bees collect nectar and pollen using the olfactory mechanism (Sandoz et al., 1995, Menzel and Müller, 1996, Menzel et al., 2005). Bees have the ability to associate floral signals with food rewards. Floral scent signals help bees to be specific in their foraging (Wright and Schiestl, 2009). The response of honey bees to different concentrations of sugary nectar can affect their foraging and nectar collection activities because sugar is an important stimulus for foraging and a major source of energy for honey bees (Scheiner et al., 2004). Nectar is a sugar solution that contains glucose, sucrose and fructose as major constituents (Corbet, 2003, Chalcoff et al., 2006). The sugar response of honeybees can be analyzed using the classical sophisticated phenomenon of the proboscis extension response (PER). The PER is a reflexive response of bees that involves the extension of the proboscis when the antennae come into contact with a sugar solution (Marshall, 1935, Iqbal and Mueller, 2007, Müller, 2013). The PER corresponds to the response of bees towards different sugar concentrations, and is referred as the sugar responsiveness (Pankiw et al., 2001, Scheiner et al., 2003, Scheiner et al., 2004). The lowest concentration at which a response occurs reflects the response threshold for sugar of that bee (Page et al., 1998). Likewise, a higher PER to lower concentrations reflects a lower threshold of bees to the sugar concentration and vice versa (Scheiner et al., 2001, Scheiner, 2004). High scoring individual have lower sugar response threshold and greater sugar sensitivity (Pankiw, 2004). It is also believed that these responses provide information about the nutritional status of individuals and the colony (Pankiw et al., 2001, Scheiner et al., 2003). Moreover, the sugar responsiveness threshold of honeybee is believed to be associated with the beginning of foraging, learning, and resource specialization (Scheiner et al., 2004, Yang et al., 2013, Metz et al., 2018).
Some studies have tested comparative sucrose responsiveness between Apis species (A. mellifera vs A. cerana) in differential experimental setups in other parts of the world (Yang et al., 2013, Wang and Tan, 2014, Raza et al., 2019). Nevertheless, very little is known regarding the sugar responses of A. dorsata, A. florea, A. cerana and A. mellifera in the geographical regions of Pakistan. Agriculture is very important for Pakistan economy and almost 60% of industrial establishments are based on agriculture (Irshad and Stephen, 2014). With an economic value of 1.59 billion US$ for pollination services, the use of honeybee colonies is greatly recommended to maximize the benefits of pollination and agricultural production (Irshad and Stephen, 2013). Pakistan is a potential beekeeping region with quite a wide range of geographical and climatic conditions. Therefore, the present study aimed to investigate the responsiveness of four species belonging to the genus Apis to the serial concentrations of three common sugar types (fructose, glucose and sucrose) that are naturally present in nectar. We also tested whether the response of each Apis species to three common sugars also differed within each species. The collective outcomes will be helpful for understanding the response threshold of Apis species to different sugar types and concentrations. Furthermore, the data of cavity nesting bees and open nesting bees will be helpful to understand the adaptation of the different Apis species in the same habitats in Pakistan, and utilization of this potential information in bee foraging for effective crop pollination, and honey production.
2. Materials and methods
Sugar responsiveness in foragers of four honey bee species (Apis mellifera, Apis cerana, Apis florea, and Apis dorsata) was determined using the proboscis extension response (PER). Different concentrations of glucose, fructose and sucrose were used to elicit the response of the forager bees, which was recorded as the PER to express their sugar response (Scheiner et al., 2004).
2.1. Experimental location and source of honey bees
The experiments were conducted during July-August at two locations in Khyber Pakhtunkhwa (KPK) Province, Pakistan because all four bee species were not available at the same location at one time. Therefore, the trials on A. mellifera, A. florea and A. dorsata were performed at Ismaila Swabi, KPK (34°13′53.3″N 72°14′34.5″E), and experiments on A. mellifera and A. cerana were conducted at the Agriculture Research Institute Tarnab, Peshawar, KPK (34°00′35.4″N 71°42′10.1″E). All trials were carried out at room temperature (25 ± 5 °C).
The colonies of A. cerana and A. mellifera were obtained from the Forest Department of Peshawar, KPK and the Entomology Section of the Agriculture Research Institute Tarnab, Peshawar, KPK, respectively. The wild hives of A. florea and A. dorsata were found on trees in Ismaila Swabi, KPK, Pakistan.
2.2. Collection and preparation of bees
The outgoing nectar foragers of A. cerana and A. mellifera were collected from the entrance of hives using glass vials. A. dorsata was collected from watermelons covered with muslin cloth, and A. florea was collected from wild flowers using a hand net. A. dorsata and A. florea are open nesting bees and don’t make proper hives, whereas A. cerana and A. mellifera are cavity nesting bees (Partap, 2011). Therefore, the bees of these species were collected from their respective available habitats. Adult nectar foragers of each bee species were collected in the early morning (9:00 am) and harnessed according to the prescribed protocol (Iqbal and Mueller, 2007, Iqbal, 2009, Smith and Burden, 2014, Iqbal et al., 2019b). The captured foragers were immobilized on ice for 3–5 min (Iqbal et al., 2019a) and restrained in small harnessing tubes made from plastic bottle straws. The bees were harnessed with tape, allowing the free movement of their antenna and mouthparts (Hesselbach and Scheiner, 2018). All bees were allowed to acclimatize for at least 10 min, fed a small droplet of 0.5 M sucrose solution and left for 2 h on a bench top at room temperature before the behavioral tests (Fig. 1) to normalize all bees.
Fig. 1.
Harnessed honey bees (left to right) (A) A. dorsata, A. mellifera and A. cerana, (B) A. mellifera and A. florea, (C) A. dorsata, (D) A. mellifera and A. cerana.
2.3. Responsiveness test
The harnessed bees were tested sequentially with an ascending series of concentrations (0.00001, 0.0001, 0.001, 0.01, 0.1, 0.5, 1.0, and 1.5 M) of fructose, glucose and sucrose. Each bee was tested with only one type of sugar (glucose/ fructose/ sucrose), and individual experiments were conducted for all sugar types. The antenna was stimulated by touching a toothpick immersed in a sugar solution without feeding. The bees showed a response in the form of elicited proboscis extension, and the response was recorded as one (for active response) and zero (for no response). For each concentration, PER score corresponds to the proportion of bees who have elicited a response at this concentration. The PER corresponds to the sugar responsiveness of each bee (Scheiner, 2004, Iqbal and Mueller, 2007). The lowest sugar concentration that each bee species can distinguish from water was considered as sugar response threshold as described by Page et al. (1998). High scoring individual have lower sugar response threshold and greater sugar sensitivity (Pankiw, 2004).
Prior to each sugar concentration test, each bee was tested for its responsiveness towards water. Thus, the response of the bees to each sugar concentration could be compared to their response to water in the preceding trial. There was an interval of 3 min between each water and sugar concentration test. Forty bees of each species were tested for their responsiveness to each sugar concentration by evaluating five bees of each species/day/sugar type over a period of eight consecutive days.
To calculate the response of the bees exclusively to sugars, any response of bees to water (prior to a sugar test) was subtracted from the subsequent sugar response of the bees, which was referred to as the specificity index (SI). The SI reflects the sugar response of bees without their response to water. The SI is potentially a more sensitive parameter for analyzing any difference in the PER of bees in response to different sugar types. SI values for proportional PER of all bees in a group were calculated for the ascending concentrations of sugars.
2.4. Meteorological data
Temperature and humidity were recorded throughout the experimental duration (July-August). The outdoor temperature exhibited an average low of 26 °C and a high of 39 °C, and the humidity level was 53–66% in July. During August, the temperature presented an average low of 23 °C and high of 37 °C, and the humidity level was 58–70%. Visibility was clear during the trials, with occasional rains at night. The surrounding plants in the trial area were maize, cucurbits, okra, sunflower, weeds and ornamental plants in the flowering stage.
2.5. Statistical data analysis
The PER indicated the proportion of individuals responding to the concentrations of sugar and water. The data of proportional responding individuals were analyzed with Kruskal-Wallis (one-way analysis of variance) non-parametric test and Dunn's multiple comparisons test at p < 0.05 using GraphPad Prism 7 statistical program to find the difference in sugar responses of different bee species according to the sugar types among different species and within the same species. The water responsiveness of bees (comparison between two species) were analyzed using Mann-Whitney test at p < 0.05. To compare the sugar and water responses at each signal concentration, the data were analyzed using Pearson’s non-parametric (Chi-Square or Fisher's exact test of proportions *p < 0.05) (Van Nest, 2018, Hostachy et al., 2019).
3. Results
The responsiveness of the honeybee species was measured with a series of increasing concentrations of three common sugar types (fructose, glucose, and sucrose). The proboscis extension response (PER) of Apis dorsata, Apis florea and Apis cerana were recorded in comparison with that of Apis mellifera in individual experiments. The data is presented as the PER of responding individuals to indicate the sugar response at the antennal level in the nectar foragers of different bee species. The lowest concentration at which a response occurs was considered as the response threshold of these bee species for sugar solutions.
3.1. PER of Apis mellifera and Apis dorsata
Apis mellifera exhibited significantly higher response (in terms of higher PER) to lower concentrations (0.00001, 0.0001, 0.001, 0.01 and 0.1 M) of fructose, glucose and sucrose than did Apis dorsata, which showed lower response to these lower sugar concentrations (Fig. 2A & B). The PER of A. dorsata gradually increased beginning at a 0.5 M concentration of all three tested sugars, reaching its peak at 1.0 M to 1.5 M concentrations (Fig. 2B), showing equally high PERs to those observed in A. mellifera against these two concentrations (Fig. 2A). A. mellifera and A. dorsata foragers differed in their responses to water in the tests performed prior to each sugar concentration. A. mellifera showed a significantly higher PER towards water than did A. dorsata (Fig. 2C). This indicated that the sugar response of the bees presented in Fig. 2A & B also included the water response and may not reflect the correct threshold of bee response towards sugar concentrations. Therefore, the specificity index (SI) was calculated by subtracting the water response from the sugar response for each concentration. SI values indicate the actual responses of bees to sugars. The data revealed that A. mellifera were more responsive to lower sugar concentrations than did A. dorsata. A. mellifera exhibited a significantly high response to lower concentrations (0.0001, 0.001, 0.01 and 0.1 M) than did A. dorsata (Fig. 3A & B). The A. mellifera and A. dorsata were equally responsive for either type of sugar (fructose, glucose and sucrose) within the same species (Fig. 3A & B).
Fig. 2.
Responsiveness of Apis mellifera and Apis dorsata. The proportion proboscis extension response (PER) indicates the responsiveness level of the honey bee species to fructose, glucose, and sucrose at a series of concentrations (A & B) and to water (C): the water responsiveness of honey bees was tested prior to each sugar test, and the water response of bees in all trials is presented in Figure C. Asterisks indicate a significant difference (χ2 or Fisher's exact test *p < 0.05) between responses of A. mellifera (AM) and A. dorsata (AD) for the respective sugar types (A & B) and water (C). A. mellifera and A. dorsata were highly sensitive to lower concentrations of sugars. The response of the bees towards water (C) indicated that the sugar response of the bees (A & B) also included water response and may not represent the correct threshold due to discharging sensitization of the bees towards water. (A & B: A. mellifera: NFructose = 40, NGlucose = 40, NSucrose = 40; A. dorsata: NFructose = 40, NGlucose = 40, NSucrose = 40); (C & D: A. mellifera: N = 120; A. dorsata: N = 120).
Fig. 3.

Specificity index for the responsiveness of (A) Apis mellifera and (B) Apis dorsata to fructose, glucose and sucrose. SI was calculated by subtracting the response to water from the subsequent sugar response to determine the sugar response of the bees. Asterisks indicate the significant difference (χ2 or Fisher's exact test *p < 0.05) between responses of A. mellifera (AM) and A. dorsata (AD) for the respective sugar types. (A & B: A. mellifera: NFructose = 40, NGlucose = 40, NSucrose = 40; A. dorsata: NFructose = 40, NGlucose = 40, NSucrose = 40).
3.2. PER of Apis mellifera and Apis florea
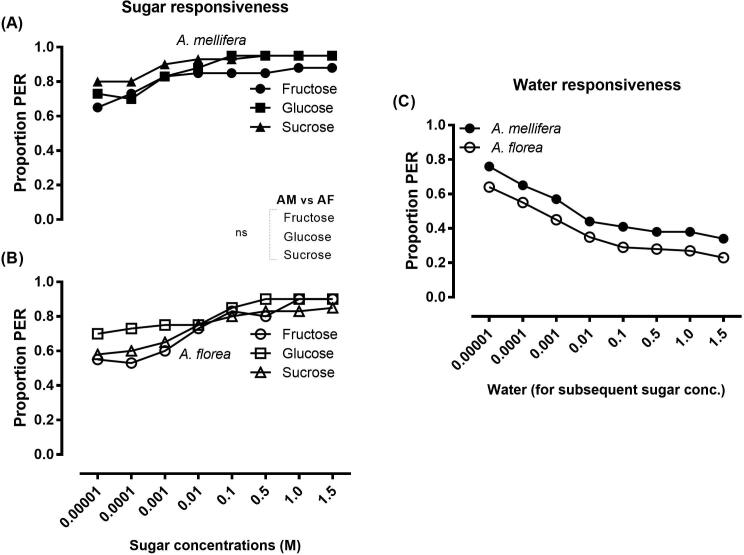
The responsiveness of A. mellifera and A. florea to the sugars (fructose, glucose and sucrose) was not significantly different across all tested concentrations (Fig. 4A & B). A. mellifera and A. florea were responsive to water in the tests performed prior to presentation of each sugar concentration with relatively higher PER in A. mellifera towards water than did A. florea, but the responses of these two species were significantly at par (Fig. 4C). The data in Fig. 4C disclosed that the sugar response illustrated in Fig. 4A & B comprised the water response of the bees and may not reflect the factual bee response towards sugar concentrations. Thus, the water response was subtracted from the sugar response for each concentration to calculate the SI values. The SI values indicated the responses of the bees to the sugars alone, and the data showed that the responsiveness of A. mellifera and A. florea was significantly at par for all concentrations of fructose, glucose and sucrose (Fig. 5A & B). A. mellifera and A. florea displayed the comparable response to lower sugar concentrations and the data indicated that the response threshold to lower sugar concentrations was equivalent in both species. The A. mellifera and A. florea were equally responsive for either type of sugar (fructose, glucose and sucrose) within the same species (Fig. 5A & B).
Fig. 4.
Responsiveness of Apis mellifera and Apis florea. The proportion proboscis extension response (PER) indicates the responsiveness level of honey bee species to fructose, glucose, and sucrose at a series of concentrations (A & B) and to water (C): the water responsiveness of honey bees was tested prior to each sugar test, and the water response of bees in all trials is presented in Figure C. A. mellifera and A. florea were equally sensitive to lower concentrations of sugars. No significant difference (χ2 or Fisher's exact test *p < 0.05) was found between responses of A. mellifera (AM) and A. florea (AF) for the respective sugar types (A & B) and water (C). The response of the bees towards water (C) indicated that the sugar response of the bees (A & B) also included water response and may not represent the correct threshold due to discharging sensitization of the bees towards water. (A & B: A. mellifera: NFructose = 40, NGlucose = 40, NSucrose = 40; A. florea: NFructose = 40, NGlucose = 40, NSucrose = 40); (C & D: A. mellifera: N = 120; A. florea: N = 120).
Fig. 5.

Specificity index for the responsiveness of (A) Apis mellifera and (B) Apis florea to fructose, glucose and sucrose. SI was calculated by subtracting the response to water from the subsequent sugar response to determine the sugar response of the bees. No significant difference (χ2 or Fisher's exact test *p < 0.05) was found between responses of A. mellifera (AM) and A. florea (AF) for the respective sugar types. (A & B: A. mellifera: NFructose = 40, NGlucose = 40, NSucrose = 40; A. dorsata: NFructose = 40, NGlucose = 40, NSucrose = 40).
3.3. PER of Apis mellifera and Apis cerana
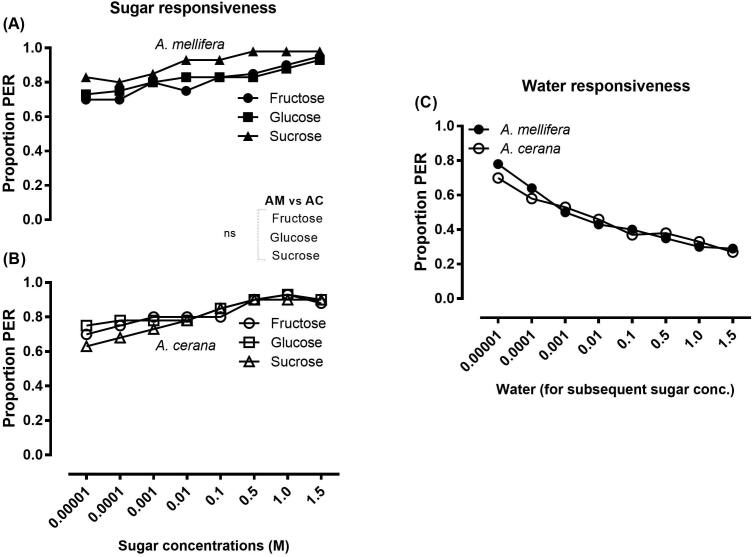
A. mellifera and A. cerana exhibited no significant difference in their PER to all concentrations of fructose, glucose and sucrose (Fig. 6A & B). A. mellifera and A. cerana were responsive to water in the tests performed prior to the presentation of each sugar concentration, but the water responses of these two bee species were significantly at par (Fig. 6C). The data in Fig. 6C unveiled that the values of response to sugar presented in Fig. 6A & B also included the water response of the bees and may not reflect the realistic response of bees towards different sugar concentrations. Thus, SI values were calculated by subtracting the water response from the sugar response to each concentration. The SI values revealed that the elicited PER of A. mellifera and A. cerana were significantly similar for all tested concentrations of fructose, glucose and sucrose (Fig. 7A & B). The data indicate that the response threshold to lower sugar concentrations was equivalent in A. mellifera and A. cerana. A. mellifera and A. cerana were equally responsive for fructose, glucose and sucrose within the same species (Fig. 7A & B).
Fig. 6.
Responsiveness of Apis mellifera and Apis cerana. The proportion proboscis extension response (PER) score indicates the responsiveness level of honeybee species to fructose, glucose, and sucrose at a series of concentrations (A & B) and to water (C). The water responsiveness of honeybees was tested prior to each sugar test, and the water response of bees in all trials is presented in the Figure C. A. mellifera and A. cerana were equally sensitive to lower concentrations of sugars. No significant difference (χ2 or Fisher's exact test *p < 0.05) was found between responses of A. mellifera (AM) and A. florea (AF) for the respective sugar types (A & B) and water (C). The response of the bees towards water (C) indicated that the sugar response of the bees (A & B) also included water response and may not represent the correct threshold due to discharging sensitization of the bees towards water. (A): A. mellifera: NFructose = 40, NGlucose = 40, NSucrose = 40; (B): A. cerana: NFructose = 40, NGlucose = 40, NSucrose = 40); (C): A. mellifera: N = 120; (D): A. cerana: N = 120).
Fig. 7.

Specificity index for the responsiveness of (A) Apis mellifera and (B) Apis cerana to fructose, glucose and sucrose. SI was calculated by subtracting the response to water from the subsequent sugar response to determine the sugar response of the bees. No significant difference (χ2 or Fisher's exact test *p < 0.05) was found between responses of A. mellifera (AM) and A. cerana (AC) for the respective sugar types. (A): A. mellifera: NFructose = 40, NGlucose = 40, NSucrose = 40; (B): A. cerana: NFructose = 40, NGlucose = 40, NSucrose = 40).
4. Discussion
This study investigated and compared the response of four species of honeybee (A. mellifera, A. florea, A. cerana, and A. dorsata) to a series of concentrations of three nectar sugars (fructose, glucose, and sucrose). The response of bees was assessed by touching the antenna of the bees with a particular sugar concentration, and the responses of the bees were recorded as the proboscis extension response (PER). The responsiveness of the bees towards water was recorded prior to the presentation of each sugar concentration and was later excluded from the sugar response of the bees to calculate the specificity index (SI), which corresponds to the precise response of bees for sugar concentrations. With a series of PER essay, we showed that each of four Apis species showed an equal response to fructose, glucose and sucrose within the same species. A. mellifera showed higher responsiveness to lower concentrations of sugar than A. dorsata. No differences in responsiveness of A. florea vs A. mellifera, and A. cerana vs A. mellifera were found for all tested concentrations of furctose, glucose and sucrose.
4.1. Responsiveness of Apis dorsata and Apis mellifera
A. mellifera exhibited significantly higher responsiveness to lower concentrations of fructose, glucose and sucrose than did A. dorsata. The high response of A. mellifera to lower concentrations of sugars may aid the bees in detecting the less concentrated nectars in the flowers visited during foraging. These findings of high response of A. mellifera for less-concentrated sugars (fructose, glucose and sucrose) is partially in accordance with the findings of Yang et al. (2013), who tested only sucrose and reported that A. mellifera was more responsive to less-concentrated sucrose in their PER analysis. The differential response to less-concentrated sugar between A. mellifera and A. dorsata could be due to the difference in their body size. A. dorsata, which is considered a giant honey bee (Somanathan et al., 2009), may require more-concentrated sugars to meet the energy requirements of its large body size (Abrol, 2016). The difference in floral species and the nectar it contains can influence the pollinator behavior and could also affect the sugar responsiveness of bees (Rathcke, 1992, Nicolson et al., 2007).
A. dorsata and A. mellifera did not show any differences in responsiveness for any of the three tested sugars (fructose, glucose and sucrose) within the same species. This means that the two species of bees exhibit an equal response for fructose, glucose and sucrose. This distinctive behavior may be beneficial in the collection of plentiful nectar from a variety of flowers regardless of the nature of the sugar present in the flower. In contrast, Simcock et al. (2018) reported the highest antennal sensitivity to sucrose, followed by glucose and fructose, in A. mellifera. A field study with different experimental conditions documented that A. dorsata was more attracted to sucrose- than to glucose- and fructose-dominated flowers (Abrol, 2016). However, A. dorsata showed equal responses to fructose, glucose and sucrose according to our dataset.
Both bee species were significantly responsive to water, but A. dorsata showed a relatively lower PER to water than did A. mellifera. A. dorsata usually builds nests in the wild and prefers brackish water over fresh water. The presence of diverse micronutrients in brackish water fulfills some of the nutritional requirements of A. dorsata (Abrol et al., 2012). This preference could explain the observed higher water response of A. dorsata compared to A. mellifera because the PER was tested in the present data with regular water.
4.2. Responsiveness of Apis florea and Apis mellifera
A. florea and A. mellifera showed no significant differences in their responsiveness towards all concentrations of fructose, glucose and sucrose. This means that the two species exhibit equal response for sugars at low and high concentrations. The water responsiveness of the two species was significantly at par but relatively higher in A. mellifera than in A. florea. A. florea is a widely dominant honey bee in southeast Asia (Oldroyd and Wongsiri, 2006) and is a well-adapted native honey bee species in Pakistan (Waghchoure and Martin, 2008, Iftikhar et al., 2011). A. florea shows foraging preferences for flowers based on their nectar sugar concentration (Abrol, 2010), which could be the reason for the difference in its PER to water compared to A. mellifera. Pankiw (2003) documented that A. mellifera prefers low concentrations of sugar, while A. florea prefers high concentrations, which differs from our findings showing that the two species did not show any preference for sugars with high or low concentrations in the designed PER experiment.
4.3. Responsiveness of Apis cerana and Apis mellifera
A. cerana and A. mellifera possess equal response to low and high concentrations of sugars. The two species showed a similar PER to all concentrations of fructose, glucose and sucrose. The two species also exhibited analogous responsiveness to water. These findings indicate that A. mellifera and A. cerana may show identical response to different sugar types present in flower nectar. Previous studies have reported a higher responsiveness of A. mellifera than A. cerana to various sucrose concentrations (Yang et al., 2013, Wang and Tan, 2014, Raza et al., 2019), but the mean sugar concentrations in the nectar collected by nectar foragers of both species have been found to be identical (Yang et al., 2013). The Asian honey bee, A. cerana, is a dynamic, outstanding pollinator of various crops, similar to the European honey bee, A. mellifera, and the two species coexist in Asia (Partap, 2011). A. cerana is recognized as being highly equivalent to A. mellifera, as these two nesting bees exhibit identical life cycles, metabolic rates and nectar collection duties (Ruttner, 1988). These two species can be domesticated over a vast range of ecological conditions (Koetz, 2013). These similar features could explain the identical response of the two species towards different sugars and water; which could be a reflection of their foraging behavior, as the two species are well adapted to scattered nectar sources (Dyer and Seeley, 1987, Miao et al., 2001). The comparable response of the two species towards water and sugars could explain the potential utility of A. cerana, which is as productive as A. mellifera for honey production and pollination (Partap, 2011).
The sugar responses of honey bees are very important for honey productivity and pollination because foragers are known to respond to any changes in the floral characteristics, nectar quality and concentrations, and size of collected load (Seeley, 1995, Page et al., 1998). The foragers select the most suitable food source relative to their abundance, and illustrate the adjustable acceptance threshold according to the availability of food resources (Seeley, 1995, Spivak, 1996). The availability of quality nectar production corresponds to a high level of pollination (Silva and Dean, 2000, Abou-Shaara, 2014).The main difference of ecological adaptation between cavity nesting bees (A. cerana and A. mellifera) and open nesting bees (A. dorsata and A. florea) in Pakistan could be even more developed by further investigations.
5. Conclusion
Our results demonstrated that four honeybee Apis species (A. dorsata, A. mellifera, A. florea and A. cerana) displayed differential responses among each other for fructose, glucose, and sucrose at different concentrations. A. mellifera was highly responsive to low concentrations of sugars and water than did A. dorsata. No differences were found between the sugar responsiveness of A. cerana vs A. mellifera and A. florea vs A. mellifera. It is concluded that the native (A. dorsata, A. florea and A. cerana) and exotic (A. mellifera) bee species are equally well adapted under local climatic conditions of Pakistan. These findings will help to understand the adaptation and sugar responses of the different Apis species in the local habitats of Pakistan. The collective outcomes will also be helpful to enhance the bee foraging for crop pollination and honey production by understanding the pattern of their feeding responses to common nectar sugars.
Authors contributions
“Conceptualization, A.S.A., J.I. and H.A.; methodology, H.A., J.I., H.S.R. and A.S.A.; software, H.A. and J.I.; validation, A.S.A. and J.I.; formal analysis, H.A. and J.I.; investigation, H.A., H.S.R., J.I. and A.S.A; resources, A.S.A.; data curation, H.A. and J.I.; writing-original draft preparation, J.I. and H.A.; writing-review and editing, A.S.A and J.I.; visualization, A.S.A; supervision, A.S.A.; project administration, A.S.A; funding acquisition, A.S.A. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia, through research group project no. RGP-189.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at the King Saud University for funding the work through the research group project No. RGP-189-1441.
ORCID
Abdulaziz S. Alqarni https://orcid.org/0000-0003-1324-332X.
Javaid Iqbal https://orcid.org/0000-0002-8298-129X
Footnotes
Peer review under responsibility of King Saud University.
References
- Abou-Shaara H.F. The foraging behaviour of honey bees Apis mellifera: A review. Vet. Med.-Czech. 2014;59(1):1–10. doi: 10.17221/7240-VETMED. [DOI] [Google Scholar]
- Abrol D.P. Foraging behaviour of Apis florea F., an important pollinator of Allium cepa L. J. Apic. Res. 2010;49(4):318–325. doi: 10.3896/IBRA.1.49.4.04. [DOI] [Google Scholar]
- Abrol D.P. Scientific Publishers; 2013. Beekeeping : A compressive guide to bees and beekeeping. [Google Scholar]
- Abrol D.P. Foraging strategies in honeybees, Apis dorsata F. and Apis florea F. in relation to availability of energy rewards. J. Apic. 2016;31(1):9–18. doi: 10.17519/apiculture.2016.04.31.1.9. [DOI] [Google Scholar]
- Abrol D.P., Shankar U., Chatterjee D., Kotwal S., Sharma D., Bhau B. Why does Apis dorsata F. forage on brackish water and other non-conventional sources? Curr. Sci. 2012;102(10):1350–1351. [Google Scholar]
- Ahmad R. Honeybee parasitic mites and their control in Pakistan. Prog Farm. 1988;8(1):34–36. [Google Scholar]
- Ascher J., Rasmussen C. Food and Agriculture Organization of the United Nations; Rome, Italy: 2010. Report on the bee fauna and pollination in Pakistan; pp. 1–52. [Google Scholar]
- Bradbear N. Food and Agriculture Organization of the United Nations; Room, Italy: 2009. Bees and their role in forest livelihoods, A guide to the services provided by bees and the sustainable harvesting, processing and marketing of their products. [Google Scholar]
- Breed M.D. Honeybees. In: Breed M.D., Moore J., editors. Encyclopedia of Animal Behavior. Academic Press; Oxford: 2010. pp. 89–96. [Google Scholar]
- Chalcoff V.R., Aizen M.A., Galetto L. Nectar concentration and composition of 26 species from the temperate forest of South America. Ann. Bot. 2006;97(3):413–421. doi: 10.1093/aob/mcj043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet S.A. Nectar sugar content: estimating standing crop and secretion rate in the field. Apidologie. 2003;34(1):1–10. doi: 10.1051/apido:2002049. [DOI] [Google Scholar]
- Dyer F.C., Seeley T.D. Interspecific comparisons of endothermy in honey-bees (APIS): Deviations from the expected size-related patterns. J. Exp. Biol. 1987;127(1):1–26. [Google Scholar]
- Hepburn H.R., Radloff S.E., Otis G.W., Fuchs S., Verma L.R., Ken T., Chaiyawong T., Tahmasebi G., Ebadi R., Wongsiri S. Apis florea: morphometrics, classification and biogeography. Apidologie. 2005;36(3):359–376. doi: 10.1051/apido:2005023. [DOI] [Google Scholar]
- Hesselbach H., Scheiner R. Effects of the novel pesticide flupyradifurone (Sivanto) on honeybee taste and cognition. Sci. Rep. 2018;8(1):4954. doi: 10.1038/s41598-018-23200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover S.E., Ovinge L.P. Pollen Collection, Honey Production, and Pollination Services: Managing Honey Bees in an Agricultural Setting. J. Econ. Entomol. 2018;111(4):1509–1516. doi: 10.1093/jee/toy125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostachy C., Couzi P., Hanafi-Portier M., Portemer G., Halleguen A., Murmu M., Deisig N., Dacher M. Responsiveness to Sugar Solutions in the Moth Agrotis ipsilon: Parameters Affecting Proboscis Extension. Front. Physiol. 2019;101423–1423 doi: 10.3389/fphys.2019.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung K.-L.J., Kingston J.M., Albrecht M., Holway D.A., Kohn J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. Lond. B. Biol. Sci. 2018;285(1870):20172140. doi: 10.1098/rspb.2017.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikhar F., Masood M.A., Waghchoure E.S. Comparison of Apis cerana, Apis dorsata, Apis florea and Apis mellifera: Honey from different areas of Pakistan. Asian J. Exp. Biol. Sci. 2011;2(3):399–403. [Google Scholar]
- Iqbal, J., 2009. Stress activated protein kinase: Central mediator of stress-and infection- induced changes in sensory processing, learning and memory in honeybee (Apis mellifera) (PhD Thesis). Universitat des Saarlandes, Saarbrucken, Germany. doi:10.22028/D291-22586
- Iqbal J., Ali H., Owayss A.A., Raweh H.S.A., Engel M.S., Alqarni A.S., Smith B.H. Olfactory associative behavioral differences in three honey bee Apis mellifera L. races under the arid zone ecosystem of central Saudi Arabia. Saudi. J. Biol. Sci. 2019;26(3):563–568. doi: 10.1016/j.sjbs.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Alqarni A.S., Raweh H.S.A. Effect of sub-lethal doses of imidacloprid on learning and memory formation of indigenous Arabian bee (Apis mellifera jemenitica Ruttner) adult foragers. Neotrop. Entomol. 2019;48(3):373–380. doi: 10.1007/s13744-018-0651-2. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Mueller U. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. Lond. B. Biol. Sci. 2007;274(1617):1517–1521. doi: 10.1098/rspb.2007.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad M., Stephen E. Value of insect pollinators to agriculture of Pakistan. Int. J. Agronomy Agri. Res. 2013;3(5):14–21. doi: 10.6084/m9.figshare.1384947.v1. [DOI] [Google Scholar]
- Irshad M., Stephen E. Review: Pollination, pollinated and pollinators interaction in Pakistan. J. Bioresour. Manage. 2014;1(1):19–25. doi: 10.35691/JBM.4102.0003. [DOI] [Google Scholar]
- Jasra A.W., Ashfaq S., Kasi M.A. Apple Pollination Problems in Balochistan Pakistan. Int. J. Agri. Biol. 2001;3(2):210–213. [Google Scholar]
- Khan K.A., Ansari M.J., Al-Ghamdi A., Sharma D., Ali H. Biodiversity and relative abundance of different honeybee species (Hymenoptera: Apidae) in Murree-Punjab. Pakistan. J. Ento Zoo Stud. 2014;2(4):324–327. [Google Scholar]
- Koetz A.H. Ecology, behaviour and control of Apis cerana with a focus on relevance to the Australian Incursion. Insects. 2013;4(4):558–592. doi: 10.3390/insects4040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. On the Sensitivity of the Chemoreceptors on the Antenna and Fore-tarsus of the Honey-Bee Apis mellifica L. J. Exp. Biol. 1935;12(1):17. [Google Scholar]
- Menzel R., Greggers U., Smith A., Berger S., Brandt R., Brunke S., Bundrock G., Watzl S. Honey bees navigate according to a map-like spatial memory. PNAS. 2005;102(8) doi: 10.1073/pnas.0408550102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Müller U. Learning and memory in honeybees: from behavior to neural substrates. Annu. Rev. Neurosci. 1996:19379–19404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- Metz B.N., Lucas H.M., Sagili R.R. Sucrose response thresholds of honey bee (Apis mellifera) foragers are not modulated by brood ester pheromone. J. Asia-Pac. Entomol. 2018;21(2):592–597. doi: 10.1016/j.aspen.2018.03.015. [DOI] [Google Scholar]
- Miao X.Q., Zhang Q.K., Wu Z.H., Fu Z.M., Wang J.D. Comparative respiratory metabolisms in worker pupa of Apis cerana and Apis mellifera. J. Fujian Agri. Forestry. 2001:30524–30527. [Google Scholar]
- Müller U. Chapter 31 - Memory Phases and Signaling Cascades in Honeybees. In: Menzel R., Benjamin P.R., editors. Handbook of Behavioral Neuroscience. Elsevier; 2013. pp. 433–441. [Google Scholar]
- Nicolson S.W., Nepi M., Pacini E. Springer; Netherlands: 2007. Nectaries and Nectar. [Google Scholar]
- Oldroyd B.P., Wongsiri S. Harvard University Press; Cambridge: 2006. Asian honey bees : biology, conservation, and human interaction. [Google Scholar]
- Page R.E., Jr., Erber J., Fondrk M.K. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) J. Comp. Physiol. A. 1998;182(4):489–500. doi: 10.1007/s003590050196. [DOI] [PubMed] [Google Scholar]
- Pankiw T. Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.) Behav. Ecol. Sociobiol. 2003;54(5):458–464. [Google Scholar]
- Pankiw T. Brood pheromone regulates foraging activity of honey bees (Hymenoptera: Apidae) J. Econ. Entomol. 2004;97(3):748–751. doi: 10.1093/jee/97.3.748. [DOI] [PubMed] [Google Scholar]
- Pankiw T., Waddington K.D., Page R.E., Jr. Modulation of sucrose response thresholds in honey bees (Apis mellifera L.): influence of genotype, feeding, and foraging experience. J. Comp. Physiol. A. 2001;187(4):293–301. doi: 10.1007/s003590100201. [DOI] [PubMed] [Google Scholar]
- Partap U. Honeybees of Asia; Springer, Berlin, Heidelberg: 2011. The pollination role of honeybees; pp. 227–255. [Google Scholar]
- Radloff E.S., Hepburn H.R., Engel M.S. The Asian species of Apis. In: Radloff E.S., Hepburn H.R., editors. Honeybees of Asia. Springer-Verlag, Berlin Heidelberg; Germany: 2011. pp. 1–22. [Google Scholar]
- Rathcke B.J. 5 - Nectar Distributions, Pollinator Behavior, and Plant Reproductive Success. In: Hunter M.D., Ohgushi T., Price P.W., editors. Effects of Resource Distribution on Animal-Plant Interactions. Academic Press; Boston: 1992. pp. 113–138. [Google Scholar]
- Raza M.F., Li Z., Rizwan M., Kalan H.A., Su S. Comparison of learning and memory of eastern (Apis cerana cerana) and western honey bees (Apis mellifera L.) Appl. Ecol. Environ. Res. 2019;17(2):4971–4984. doi: 10.15666/aeer/1702_49714984. [DOI] [Google Scholar]
- Ruttner F. Springer; Berlin, Heidelberg: 1988. Biogeography and taxonomy of honeybees. [Google Scholar]
- Sandoz J.C., Roger B., Pham-Delegue M.H. Olfactory learning and memory in the honeybee: comparison of different classical conditioning procedures of the proboscis extension response. C. R. Acad. Sci. III. 1995;318(7):749–755. [PubMed] [Google Scholar]
- Scheiner R. Responsiveness to sucrose and habituation of the proboscis extension response in honey bees. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2004;190(9):727–733. doi: 10.1007/s00359-004-0531-6. [DOI] [PubMed] [Google Scholar]
- Scheiner R., Barnert M., Erber J. Variation in water and sucrose responsiveness during the foraging season affects proboscis extension learning in honey bees. Apidologie. 2003:3467–3472. [Google Scholar]
- Scheiner R., Page R.E., Erber J. Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera) Apidologie. 2004;35(2):133–142. [Google Scholar]
- Scheiner R., Page R.E., Jr., Erber J. The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.) Neurobiol. Learn Mem. 2001;76(2):138–150. doi: 10.1006/nlme.2000.3996. [DOI] [PubMed] [Google Scholar]
- Seeley T.D. Harvard University Press; Cambridge: 1995. The wisdom of the hive: The social physiology of honey bee colonies. [Google Scholar]
- Silva D.P., Castro A.C.F., Vilela B., Ong X.R., Thomas J.C., Alqarni A.S., Engel M.S., Ascher J.S. Colonizing the east and the west: distribution and niche properties of a dwarf Asian honey bee invading Africa, the Middle East, the Malay Peninsula, and Taiwan. Apidologie. 2020;51(1):75–87. doi: 10.1007/s13592-019-00711-x. [DOI] [Google Scholar]
- Silva E.M., Dean B.B. Effect of nectar composition and nectar concentration on honey bee (Hymenoptera: Apidae) visitations to hybrid onion flowers. J. Econ. Entomol. 2000;93(4):1216–1221. doi: 10.1603/0022-0493-93.4.1216. [DOI] [PubMed] [Google Scholar]
- Simcock N.K., Gray H., Bouchebti S., Wright G.A. Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J. Insect Physiol. 2018:10671–10677. doi: 10.1016/j.jinsphys.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Smith B.H., Burden C.M. A proboscis extension response protocol for investigating behavioral plasticity in insects: Application to basic, biomedical, and agricultural research. Jove-J. Vis. Exp. 2014;91 doi: 10.3791/51057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanathan H., Warrant E.J., Borges R.M., Wallen R., Kelber A. Resolution and sensitivity of the eyes of the Asian honeybees Apis florea, Apis cerana and Apis dorsata. J. Exp. Biol. 2009;212(15):2448–2453. doi: 10.1242/jeb.031484. [DOI] [PubMed] [Google Scholar]
- Spivak M. The Wisdom of the Hive—the Social Physiology of Honey Bee Colonies. Ann Entomol Soc Am. 1996;89(6):907–908. doi: 10.1093/aesa/89.6.907. [DOI] [Google Scholar]
- Van Nest B.N. The Olfactory Proboscis Extension Response in the Honey Bee: A Laboratory Exercise in Classical Conditioning. J. Undergrad. Neurosci. Educ. 2018;16(2):A168–A176. [PMC free article] [PubMed] [Google Scholar]
- Waghchoure C., Martin S. Beekeeping in Pakistan - A bright future in a troubled land. Am. Bee J. 2008;148(8):726–728. [Google Scholar]
- Wang Z.W., Tan K. Comparative analysis of olfactory learning of Apis cerana and Apis mellifera. Apidologie. 2014;45(1):45–52. doi: 10.1007/s13592-013-0228-3. [DOI] [Google Scholar]
- Wright G.A., Schiestl F.P. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 2009;23(5):841–851. doi: 10.1111/j.1365-2435.2009.01627.x. [DOI] [Google Scholar]
- Yang G.H. Harm of introducing the western honeybee Apis mellifera L. to the Chinese honeybee Apis cerana F. and its ecological impact. Acta Entomol. Sinica. 2005;48(3):401–406. [Google Scholar]
- Yang W.C., Kuang H.O., Wang S.S., Wang J., Liu W., Wu Z.H., Tian Y.Y., Huang Z.Y., Miao X.Q. Comparative Sucrose Responsiveness in Apis mellifera and A. cerana Foragers. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0079026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.S. Causes of absconding and preventive measures in Apis cerana. Spec. Econ. Anim. Plants. 2010;28 [Google Scholar]
- Zhou B.F., Jiang W.J., Zhang L.C. Studies on the nectar foraging ability of individual workers of Apis cerana cerana. Apicult. China. 1991:43–46. (in Chinese) [Google Scholar]