Abstract
Acalabrutinib is a selective irreversible Bruton tyrosine kinase (BTK) inhibitor that does not affect IL2-associated tyrosine kinase or antibody-dependent cellular cytotoxicity, making it an attractive candidate for combination therapy with anti-CD20 antibodies. We investigated acalabrutinib plus obinutuzumab in a phase Ib/II study (NCT02296918) of patients with treatment-naïve or relapsed/refractory chronic lymphocytic leukemia (CLL). Nineteen treatment-naïve and 26 relapsed/refractory patients were treated with acalabrutinib (100 mg twice daily) until progression and obinutuzumab (cycle 1: 100 mg day 1, 900 mg day 2, 1000 mg days 8 and 15; cycles 2–6: 1,000 mg day 1). Grade 3/4 adverse events occurred in 71% of patients. Overall response rates were 95% (treatment-naïve) and 92% (relapsed/refractory). Thirty-two percent of treatment-naïve and 8% of relapsed/refractory patients achieved complete remission. At 36 months, 94% (treatment-naïve) and 88% (relapsed/refractory) were progression free. Acalabrutinib plus obinutuzumab was well tolerated, producing high and durable responses in treatment-naïve and relapsed/refractory CLL.
SIGNIFICANCE:
Rituximab plus the less selective BTK inhibitor ibrutinib has not shown benefit in CLL; however, the selective BTK inhibitor acalabrutinib plus the antibody-dependent cellular cytotoxicity–enhanced antibody obinutuzumab yielded durable responses that deepened over time in treatment naïve and relapsed/refractory CLL, supporting the evaluation of this approach in larger, comparative studies in CLL.
INTRODUCTION
Treatment of chronic lymphocytic leukemia (CLL) has been transformed by the introduction of targeted agents that inhibit the B-cell receptor signaling pathway. Bruton tyrosine kinase (BTK), an essential component of B-cell receptor signaling, is constitutively active in CLL and is essential for multiple survival pathways that are important in CLL (1). Inhibition of BTK by the oral agent ibrutinib has been shown to lead to abrogation of CLL cell signaling, proliferation, homing, and adhesion in vitro and in patients (1–7). In patients, this has translated to high response rates and durable remissions. Clinical trials have shown improved overall response rates (ORR), progression-free survival (PFS), and overall survival (OS) with ibrutinib compared with standard therapy in the relapsed (8) and first-line (9–11) settings.
Despite these promising results, real-world data suggest that many patients discontinue ibrutinib due to toxicity (12). Common causes for discontinuation are arthralgias, atrial fibrillation, and bleeding. These adverse effects are hypothesized to be the result of alternative irreversible and reversible targets of ibrutinib because they are not reported with high frequency in patients with X-linked agammaglobulinemia (13, 14). In addition to their potential roles in toxicity, alternative targets of ibrutinib may diminish the drug’s ability to be successfully combined with anti-CD20 antibodies, a mainstay of CLL therapy (15).
Acalabrutinib is a highly selective, potent, covalent inhibitor of BTK with minimal off-target activity (16, 17). Because of its short half-life, the drug can safely be dosed twice daily, resulting in sustained high occupancy of BTK that is hypothesized to lead to longer remission durations (16). The reported outcomes in patients with CLL are promising, with an ORR of 93% [including partial response with lymphocytosis (PRL)] and an 18-month PFS rate of 90% (95% CI, 83%–94%; ref. 18). The high selectivity of acalabrutinib may reduce the likelihood of off-target effects leading to toxicity. Although the results of a comparative trial between acalabrutinib and ibrutinib are not yet available, single-agent studies of acalabrutinib have shown lower rates of atrial fibrillation, hypertension, and arthralgias than similar studies with ibrutinib (18, 19). In addition, the greater selectivity of acalabrutinib leads to less interference with antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC), as IL2-associated tyrosine kinase (ITK) is not inhibited. This may allow for more effective combination with anti-CD20 antibodies (17, 20).
Given the promising clinical data with acalabrutinib monotherapy, we conducted the phase Ib/II ACE-CL-003 trial of acalabrutinib in combination with obinutuzumab in patients with relapsed/refractory and treatment-naïve CLL. Here we present the results of this study at a median follow-up of 3.5 years.
RESULTS
Patients and Baseline Characteristics
A total of 45 patients were enrolled and treated at a single center, including 19 patients with treatment-naïve CLL (1 patient had small lymphocytic lymphoma) and 26 patients with relapsed/refractory CLL. Full clinical characteristics can be found in Table 1. Among patients with treatment-naïve CLL, the median age was 61 years (range, 42–75 years). Fifty-three percent of patients were advanced Rai stages, and 53% (9/17) had unmutated immunoglobulin heavy-chain variable region gene (IGHV). Twenty-two percent (4/18) of patients had del(17)(p13.1) and 28% (5/18) had del(11) (q22.3) without or missing del(17)(p13.1). Baseline p53 gene (TP53) mutation was seen in 28% (5/18) of patients, and 21% (4/19) of patients had del(17)(p13.1) and TP53 mutation. Forty-two percent (8/19) had a complex karyotype, defined as three or more independent abnormalities. CLL International Prognostic Index (CLL-IPI) scores were low or intermediate risk in 37% of patients, high risk in 21%, and very high risk in 21%.
Table 1.
Demographic and baseline characteristics
| Characteristic | Treatment-naïve (n = 19) |
Relapsed/refractory (n = 26) |
|---|---|---|
| Median age (range), y | 61 (42–75) | 63 (42–76) |
| Male sex, n (%) | 11 (58) | 21 (81) |
| ECOG PS ≤1, n (%) | 19 (100) | 26 (100) |
| Bulky disease ≥5 cm, n (%) | 10 (53) | 13 (50) |
| Rai stage III–IV, n (%) | 10 (53) | 7 (27) |
| CLL-IPI score, n (%) | ||
| 0–3 (Low/intermediate risk) | 7 (37) | 11 (42) |
| 4–6 (High risk) | 4 (21) | 6 (23) |
| 7–10 (Very high risk) | 4 (21) | 3 (12) |
| β2-microglobulin >3 mg/L, n (%) | 13 (68) | 17 (65) |
| No. prior therapies, median (range) | 0 | 1 (1–9) |
| Histology, n (%) | ||
| CLL | 18 (95) | 26 (100) |
| SLL | 1 (5) | 0 |
| Genomic status, n/n (%) | ||
| Del(17)(p13.1) | 4/18 (22) | 5/25 (20) |
| Del(11)(q22.3) without or missing del(17)(p13.1) | 5/18 (28) | 9/26 (35) |
| Unmutated IGHV | 9/17 (53) | 17/26 (65) |
| TP53 mutation | 5/18 (28) | 6/24 (25) |
| TP53 mutation + del(17)(p13.1) | 4/19 (21) | 3/26 (12) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IGHV, immunoglobulin heavy-chain variable region gene; IPI, International Prognostic Index; SLL, small lymphocytic lymphoma.
Among patients with relapsed/refractory CLL, the median age was 63 years (range, 42–76 years). The median number of prior therapies was 1 (range, 1–9). No patients had previously received a BTK inhibitor or a BCL2 inhibitor; 1 patient had received a PI3K inhibitor (Supplementary Table S1). Forty-six percent of patients had previously received a nucleoside analogue, and 1 patient had a previous allogeneic stem-cell transplant. Sixty-five percent (17/26) had unmutated IGHV, 20% (5/25) had del(17)(p13.1), and 35% (9/26) had del(11)(q22.3) without or missing del(17)(p13.1). TP53 mutation was observed in 25% (6/24) of patients and 12% (3/26) of patients had del(17)(p13.1) and TP53 mutation. Complex karyotype was observed in 56% (14/25) of patients. CLL-IPI scores were low or intermediate risk in 42% of patients, high risk in 23%, and very high risk in 12%.
Patient Disposition
In patients with treatment-naïve CLL, the median follow-up was 39 months (range, 1–45 months). Eighty-nine percent of patients in this cohort completed all planned doses of obinutuzumab, and 89% remain on acalabrutinib therapy, with 58% requiring at least 1 dose interruption of acalabrutinib for an adverse event (AE). Two patients required dose deescalation for grade 3 toxicity (grade 3 neutropenia, n = 1; and grade 3 peripheral edema, n = 1). Two patients discontinued acalabrutinib, with 1 discontinuation due to Richter transformation and 1 due to an AE.
In patients with relapsed/refractory CLL, the median follow-up was 42 months (range, 20–49 months). All patients completed all doses of obinutuzumab, and 69% remain on acalabrutinib. Sixty-two percent of relapsed/refractory patients required a dose interruption of acalabrutinib due to an AE. One patient required dose deescalation due to grade 2 neutropenia. Eight patients discontinued acalabrutinib, with 2 discontinuations due to Richter transformation, 4 due to AEs, 1 due to progressive disease, and 1 due to death.
Richter transformation occurred in 1 treatment-naïve patient at 15.8 months and in 2 relapsed/refractory patients at 18.4 and 24 months. Two of 3 patients (1 treatment-naïve and 1 relapsed/refractory) had del(17)(p13.1) and unmutated IGHV. All 3 patients had complex karyotype, and none had del(11)(q22.3).
Pharmacodynamics
Dosing with 100 mg acalabrutinib twice daily provided greater target coverage overall (96%–99%) compared with 200 mg once daily (87%–95%; P < 0.05 for all timepoints compared; Fig. 1A). Acalabrutinib treatment resulted in pronounced inhibition of BTK phosphorylation and a reduction of total BTK protein levels in the tumor CD19+CD5+ cell subset (Fig. 1B and C). Obinutuzumab did not affect acalabrutinib binding to BTK, downstream kinase function, or protein levels. No clinically significant changes in T-cell (CD4+, CD8+), natural killer (NK)–cell (CD16+CD56+), or monocyte (CD14+) numbers were observed in any treated patients (Fig. 2A–D); similar results were noted for the treatment-naïve and relapsed/refractory cohorts. Immunoglobulin A and G levels did not change appreciably over time, whereas immunoglobulin M levels gradually decreased (Supplementary Fig. S1A–S1C).
Figure 1.
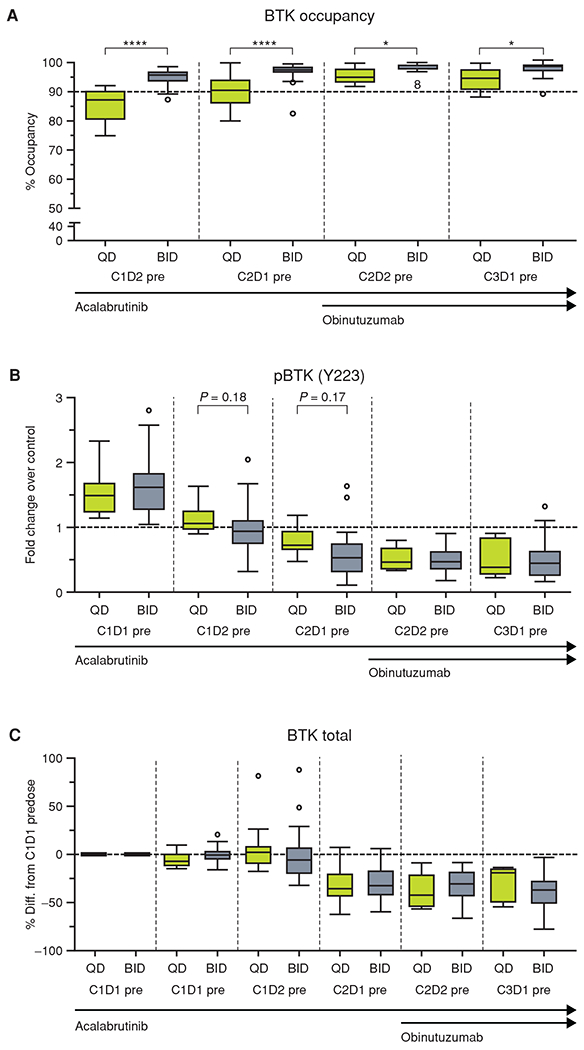
Pharmacodynamics of acalabrutinib. A, The percentage of BTK occupancy by acalabrutinib at each timepoint for patients with a signal to noise ≥5 for the day 1 presample. B, The pBTK fold change over control (C1D1 pre + 1 μmol/L exogenous acalabrutinib) for each timepoint. C, The BTK fold change over control (C1D1 pre + 1 μmol/L exogenous acalabrutinib) for each timepoint. For each figure, the horizontal line in the center of the box represents the median. Significance was determined using a paired two-tailed parametric t test: *, P < 0.5; ****, P < 0.0001. BID, twice a day; C, cycle; D, day; pBTK, phosphorylated BTK; pre, predose; QD, once a day.
Figure 2.
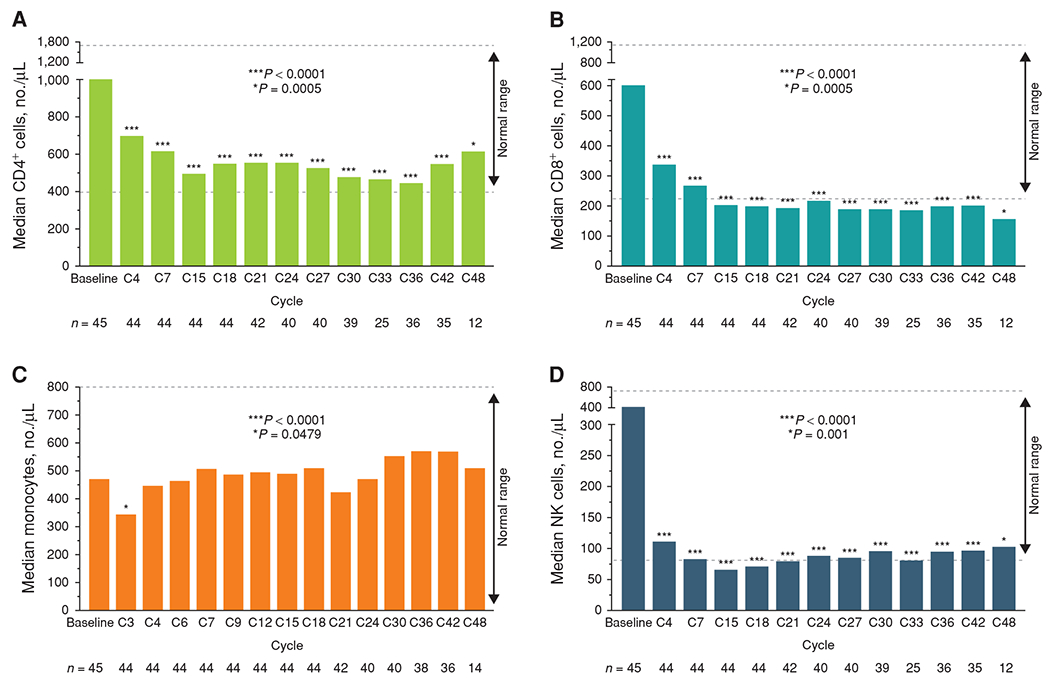
T cells, monocytes, and NK cell counts in peripheral blood from all acalabrutinib-treated patients over time. Absolute counts of CD4+ (A) and CD8+ (B) T cells, CD14+ monocytes (C), and NK cells (CD16+CD56+; D). Cells were measured by flow cytometry. The normal reference range for each subset is shown with dashed lines. All plots include patients being assessed at baseline assessment and the respective visit. P values are from the Wilcoxon signed-rank test. NK, natural killer.
Efficacy
Using International Workshop on CLL (iwCLL) 2008 guidelines, the ORR was 95% (95% CI, 74%–100%) for patients with treatment-naïve CLL and 92% (95% CI, 75%–99%) for those with relapsed/refractory CLL. ORR including PRL was 100% in relapsed/refractory CLL, and no PRLs were observed in treatment-naïve CLL. Complete responses (CR) were achieved by 32% of patients with treatment-naïve CLL and 8% of patients with relapsed/refractory CLL. All responding treatment-naïve patients (18/19) achieved at least partial response (PR) by the first disease assessment (2.8 months). The median time to ≥PR in relapsed/refractory patients was also 2.8 months (range, 2.7–21.4). The median time to ≥CR was 18.4 months (range, 5.6–32.3) in treatment-naïve patients and 12.9 months (range, 10.2–15.7) in relapsed/refractory patients.
Bone marrow biopsies were performed at 12 months and repeated thereafter only for patients with minimal residual disease (MRD)–negative peripheral blood assessed by flow cytometry with evidence of CR on CT scan. At 12 months, MRD negativity in the bone marrow was attained by 3 of the 6 patients (50%) in the treatment-naïve group who had a CR and by none of the 2 patients in the relapsed/refractory group who had a CR. Interestingly, 2 of the 12 treatment-naïve patients who achieved PR (17%) and 4 of the 22 relapsed/refractory patients who achieved PR (18%) achieved bone marrow MRD negativity at 12 months. CR rates may have been underestimated, as MRD was monitored in peripheral blood every 3 cycles, and some patients with low-level blood disease (>0.01% to <1% CLL cells) would likely have had an MRD-positive CR rather than a PR if MRD had been evaluated using bone marrow. At the time of last evaluation, 69% of patients in the total population (31/45) had lymph nodes >1.5 cm on CT scan; however, 47% (21/45) had lymph nodes <2 cm.
Median PFS (Fig. 3A) and median OS (Fig. 3B) have not been reached for either cohort. For patients with treatment-naïve CLL, PFS was 94.4% (95% CI, 66.6%–99.2%) and OS was 100% (95% CI, 100%–100%) at 39 months. For patients with relapsed/refractory CLL, PFS was 72.7% (95% CI, 43.8%–88.4%) and OS was 82% (95% CI, 57%–93%) at 42 months. PFS by CLL-IPI risk score and complex karyotype status are presented in Supplementary Fig. S2A and S2B.
Figure 3.
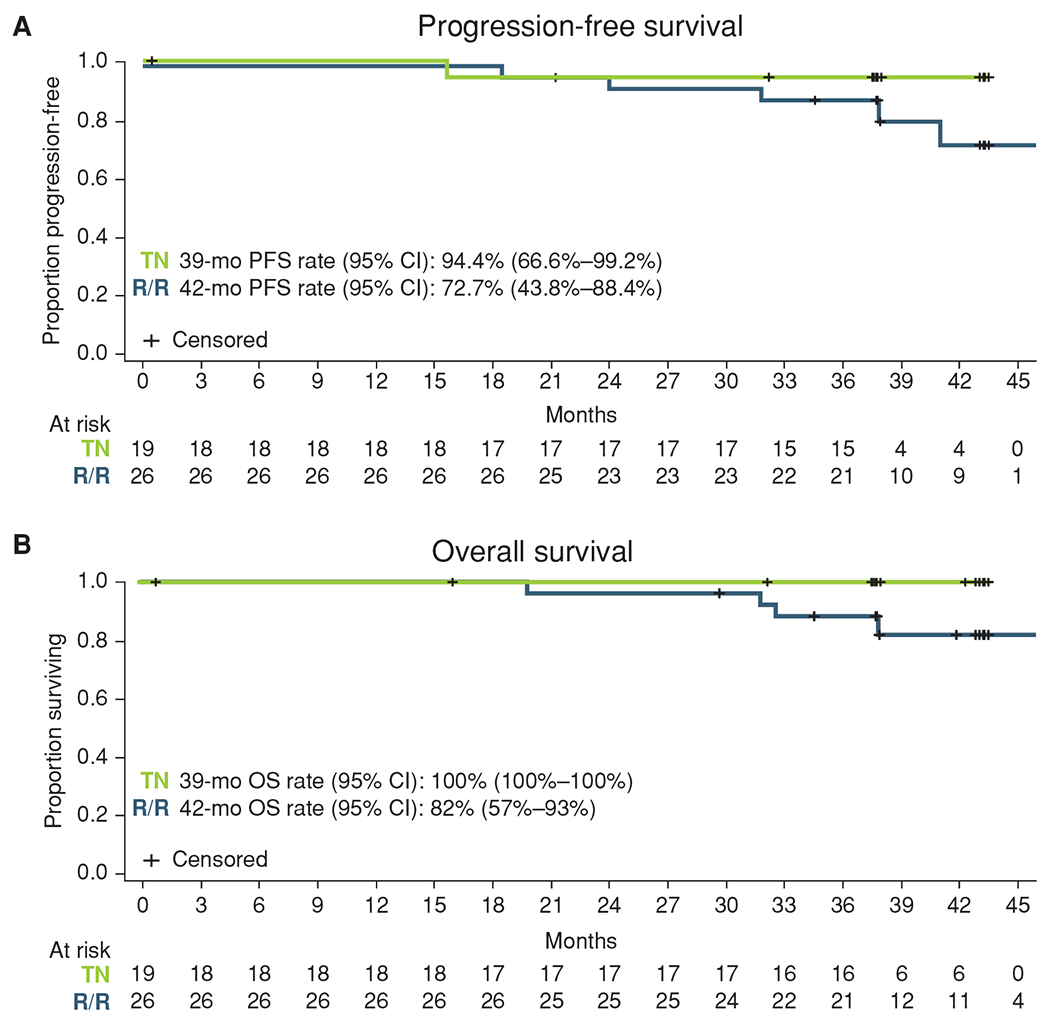
Kaplan–Meier curves for progression-free survival (A) and overall survival (B). mo, months; OS, overall survival; PFS, progression-free survival; R/R, relapsed/refractory; TN, treatment-naïve.
TP53 mutation, TP53 loss of copy number, or del(17)(p13.1) was present at baseline in 5 of 19 treatment-naïve patients (26%; 1/6 CRs, 3/11 PRs, and 1 patient whose responses were not assessed due to early discontinuation) and 8 of 26 relapsed/refractory patients (31%; 1/2 CRs and 7/22 PR/PRLs; Fig. 4A). No enrichment of TP53 loss (mutation or chromosome deletion) was observed in any response category or with any MRD status.
Figure 4.
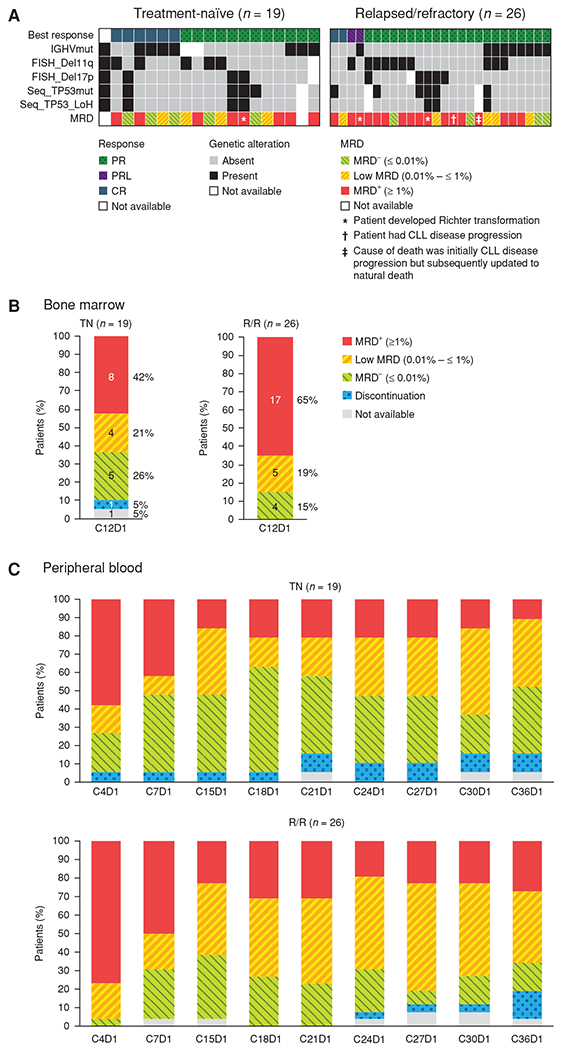
A, Response and MRD status by baseline genomic alterations. B and C, MRD in bone marrow (B) and peripheral blood (C). LOH, loss of heterozygosity; mut, mutated; TN, treatment-naïve; R/R, relapsed/refractory.
MRD
At 12 months, 5 of 19 patients (26%) in the treatment-naïve cohort and 4 of 26 patients (15%) in the relapsed/refractory cohort had achieved MRD negativity in bone marrow assessed by 10-color flow cytometry using 10−4 CLL cells/leukocyte as cutoff (Fig. 4B). Peripheral blood MRD was measured longitudinally every 3 cycles, and MRD-based responses in the peripheral blood generally deepened over time. A trend toward deeper MRD negativity in treatment-naïve patients was observed (Fig. 4C), although it appears that the discontinuation of obinutuzumab temporarily increased peripheral blood tumor burden.
Safety
Supplementary Table S2 shows all treatment-emergent AEs occurring in ≥20% of patients. Eight patients (42%) in the treatment-naïve group and 17 patients (65%) in the relapsed/refractory group experienced headache of any grade. Infusion-related reactions of any grade occurred in 6 patients (32%) in the treatment-naïve group and 13 patients (50%) in the relapsed/refractory group, and all of these events were grade 1 or 2. There was 1 event of tumor lysis syndrome (grade 3), which occurred in the relapsed/refractory group. Grade ≥ 3 AEs were observed in 63% of treatment-naïve and 77% of relapsed/refractory patients (Table 2). Grade ≥3 AEs observed in >5% of patients included decreased neutrophil count [n = 11 (24%)], syncope [n = 5 (11%)], decreased platelet count [n = 4 (9%)], cellulitis [n = 4 (9%)], increased weight [n = 4 (9%)], hypertension [n = 3 (7%)], and hypophosphatemia [n = 3 (7%)]. Serious AEs (SAE) occurred in 16% of treatment-naïve and 46% of relapsed/refractory patients. The most commonly reported SAEs (≥2 patients) were cellulitis [n = 4 (9%)], diarrhea [n = 2 (4%)], dyspnea [n = 2 (4%)], pneumonia [n = 2 (4%)], pyrexia [n = 2 (4%)], and syncope [n = 2 (4%)]. One patient in the treatment-naïve cohort discontinued acalabrutinib and obinutuzumab due to an AE of metastatic squamous cell carcinoma during the first week of therapy, and 4 patients discontinued acalabrutinib in the relapsed/refractory cohort due to AEs that included grade 1 vomiting (n = 1), grade 3 maculopapular rash (n = 1), lung adenocarcinoma (n = 1), and grade 3 diarrhea (n = 1). No grade 5 AEs were reported. Four patient deaths occurred from Richter transformation (n = 2), lung adenocarcinoma (n = 1), or disease progression (n = 1).
Table 2.
Grade ≥3 AEs observed in >2 patients
| All patients (N = 45) | ||
|---|---|---|
| Grade 3/4 AE, n(%) | Grade 3 | Grade 4 |
| Neutrophil count decreased | 5 (11) | 6 (13) |
| Syncope | 5 (11) | 0 (0) |
| Platelet count decreased | 3 (7) | 1 (2) |
| Weight increased | 4 (9) | 0 (0) |
| Cellulitis | 4 (9) | 0 (0) |
| Hypophosphatemia | 3 (7) | 0 (0) |
| Hypertension | 3 (7) | 0 (0) |
| Pneumonia | 2 (4) | 0 (0) |
| Anemia | 2 (4) | 0 (0) |
| Diarrhea | 2 (4) | 0 (0) |
| Fall | 2 (4) | 0 (0) |
| Dyspnea | 2 (4) | 0 (0) |
Of particular interest were toxicities commonly associated with the BTK inhibitor ibrutinib, including atrial fibrillation and bleeding (12). Atrial fibrillation was observed in 1 patient (2%; grade 3). No serious or severe ventricular arrhythmias or sudden deaths have been reported. Hypertension of any grade (either new or worsening from baseline) was seen in 40% of patients, including 3 patients (7%) with grade ≥3 events. Bleeding events (any grade) occurred in 71% of patients, most commonly (≥20% of patients) contusion [n = 19 (42%)] and petechiae [n = 11 (24%)]. Two relapsed/refractory patients (4%) had grade 3 bleeding events [hematuria (n = 1) and muscle hemorrhage (n = 1)].
Incidence of BTKC481S Mutations
One treatment-naïve patient developed a BTKC481S mutation (0.2% variant allele frequency) 48 months after the initiation of therapy but has no sign of clinical progression. In the relapsed/refractory cohort, 1 patient who progressed with CLL developed a BTKC481S mutation 3 months before clinical progression, and 1 patient who developed Richter transformation developed a BTKC481S mutation at progression. Phospholipase Cγ2 mutations were not assessed in this study.
Predictors of Outcome
We used baseline genomic mutation profiles and cytogenetic status to explore predictors of clinical response or molecular MRD responses. Although there are no predictors of clinical/molecular outcome, of the 3 patients with Richter transformation, 2 had mutated TP53 and 2 had unmutated IGHV (Fig. 4A).
Quality of Life
The European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life questionnaire (QLQ) and the Short Form-36 Health Survey (SF-36) were used to assess patient-reported quality of life. There were no significant effects of time or cohort, or significant interactions between time and cohort in quality-of-life measures between baseline and 12 months (Supplementary Tables S3–S5). Across the study period, from baseline to 24 months, there were no significant differences between relapsed/refractory and treatment-naïve patients (Table 3). Overall, the analyses suggested patients generally perceived improvement with time, with significant gains on the physical component scale of the SF-36 (P = 0.01). Patients reported a 7-point improvement on the 0 to 100 global health scale of the EORTC (P = 0.03). For the 5 functional items, patients reported improvements in physical functioning (P = 0.01), which may have resulted from gains reported by relapsed/refractory patients by 24 months (93.33) compared with their baseline score (83.81). Improvements were found for 3 of the 9 symptom items, including reductions in appetite loss (P = 0.04), diarrhea (P = 0.01), and dyspnea (P = 0.01). No significant change from baseline was observed for the remaining functional and symptom scores.
Table 3.
SF-36 and EORTC scores at baseline and cycle 24
| Treatment-naïve patients |
Relapsed/refractory patients |
Repeated measures ANOVA |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Cycle 24 | Baseline | Cycle 24 | Time | Cohort | |||
| Outcome | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | F | P | F | P |
| SF-36 | ||||||||
| PCS | 43.73 (2.39) | 48.70 (1.83) | 42.16 (2.85) | 46.98 (2.18) | 8.01 | 0.01 | 0.34 | 0.56 |
| MCS | 52.61 (2.21) | 53.02 (2.15) | 49.02 (2.64) | 50.37 (2.57) | 0.80 | 0.38 | 0.93 | 0.34 |
| EORTC | ||||||||
| Global health | 71.67 (4.73) | 78.33 (4.30) | 70.24 (5.65) | 77.98 (5.13) | 5.04 | 0.03 | 0.02 | 0.88 |
| EORTC symptom domainsa | ||||||||
| Financial difficulties | 10.00 (5.17) | 6.67 (4.35) | 11.91 (6.18) | 4.76 (5.20) | 0.86 | 0.36 | 0.00 | 1.00 |
| Diarrhea | 10.00 (4.49) | 5.00 (3.30) | 19.05 (5.37) | 4.76 (3.94) | 7.36 | 0.01 | 0.77 | 0.39 |
| Constipation | 3.33 (3.27) | 3.33 (4.43) | 7.14 (3.91) | 16.67 (5.29) | 1.67 | 0.21 | 3.16 | 0.09 |
| Appetite loss | 5.00 (3.22) | 5.00 (2.72) | 14.29 (3.85) | 4.76 (3.25) | 4.43 | 0.04 | 1.24 | 0.27 |
| Insomnia | 31.58 (7.18) | 19.30 (6.78) | 26.19 (8.36) | 28.57 (7.90) | 0.70 | 0.41 | 0.05 | 0.83 |
| Dyspnea | 8.33 (3.99) | 3.33 (2.69) | 19.05 (4.77) | 7.14 (3.21) | 6.94 | 0.01 | 2.96 | 0.10 |
| Pain | 22.50 (5.69) | 20.00 (5.23) | 16.67 (6.80) | 19.05 (6.27) | 0.00 | 0.99 | 0.20 | 0.66 |
| Nausea/vomiting | 1.67 (1.70) | 3.33 (1.55) | 4.76 (2.03) | 3.57 (1.86) | 0.05 | 0.83 | 0.54 | 0.47 |
| Fatigue | 26.67 (5.27) | 25.00 (4.19) | 32.54 (6.29) | 26.19 (5.01) | 0.82 | 0.37 | 0.35 | 0.56 |
| EORTC functional domainsb | ||||||||
| Social functioning | 90.00 (3.73) | 92.50 (2.97) | 89.29 (4.46) | 92.86 (3.56) | 0.95 | 0.34 | 0.00 | 0.97 |
| Cognitive functioning | 86.67 (3.78) | 83.33 (5.15) | 80.95 (4.52) | 77.38 (6.16) | 1.64 | 0.21 | 0.80 | 0.38 |
| Emotional functioning | 87.92 (3.86) | 87.92 (3.74) | 85.12 (4.61) | 83.33 (4.47) | 0.15 | 0.70 | 0.46 | 0.50 |
| Role functioning | 85.00 (5.18) | 87.50 (4.23) | 83.33 (6.19) | 91.67 (5.06) | 3.09 | 0.09 | 0.04 | 0.85 |
| Physical functioning | 92.83 (3.47) | 96.67 (2.13) | 83.81 (4.15) | 93.33 (2.54) | 7.50 | 0.01 | 2.69 | 0.11 |
Note: Significant time effects are noted in bold text.
Abbreviations: F, variation between sample means divided by the variation within samples; MCS, mental component score; PCS, physical component score.
Score range 0–100, with a higher score indicative of a higher level of symptoms. Correction for 9 multiple comparisons is P = 0.006.
Score range 0–100, with a higher score indicative of a higher level of functioning. Correction for 5 multiple comparisons is P = 0.01.
DISCUSSION
Here we show for the first time that the combination of acalabrutinib with obinutuzumab resulted in high response rates and durable remissions in patients with relapsed/refractory or treatment-naïve CLL. These results were observed in patients with poor risk factors, and although 3 patients experienced Richter transformation, only 1 patient had CLL progression with the current follow-up of 3.5 years. This regimen was also well tolerated. AEs were generally manageable, with few patients discontinuing the drug due to toxicity (11%).
Acalabrutinib monotherapy can produce high response rates and durable remissions in patients with CLL; obinutuzumab was added to produce deeper responses and to potentially prolong PFS. Although the combination of a CD20 antibody with ibrutinib does not improve outcomes over ibrutinib monotherapy in randomized studies (10), this question has not been addressed with BTK inhibitors optimized for selectivity or CD20 antibodies engineered to optimize ADCC. Ibrutinib, through inhibition of ITK and other non-BTK kinases, abrogates NK-cell ADCC and ADCP in vitro (20–22) and may reduce the effectiveness of the CD20 antibody in patients. Acalabrutinib is highly selective for BTK and is not a potent inhibitor of most other kinases (15, 23), suggesting that this BTK inhibitor might be more appropriate for combination therapy with antibodies. Similarly, several groups have demonstrated that the engineered CD20 antibody obinutuzumab mediates improved ADCC over rituximab (24, 25). Indeed, although our study was small, the results suggest that this combination of BTK inhibitor plus immune therapy may have improved outcomes compared with previously published data for acalabrutinib alone (16). Additional data will come from the ongoing phase III study (NCT02475681) comparing chlorambucil plus obinutuzumab, acalabrutinib alone, and acalabrutinib plus obinutuzumab in previously untreated CLL.
Despite the addition of obinutuzumab, CR rates with the combination remained relatively low, although responses tended to deepen over time. Lower MRD negativity rates were seen in bone marrow due to MRD testing in bone marrow being conducted only on cycle 12 day 1. Although patients are currently treated to progression, it is unclear whether continuous treatment is necessary or whether treatment for specific patients could be discontinued; these issues must be addressed in prospective clinical trials.
This regimen appears quite tolerable, with a low rate of discontinuation due to AEs, despite the long follow-up. Real-world studies of BTK inhibitors have highlighted that a favorable safety profile in clinical trials does not guarantee that patients outside of trials will tolerate drugs well (12); however, toxicities that generally led to discontinuation of ibrutinib, such as atrial fibrillation and arthralgias, did not lead to acalabrutinib discontinuation in this study. Moreover, the overall rate of atrial fibrillation in this study was low, with only 1 patient experiencing an atrial fibrillation event. This also suggests that patients who develop or are at high risk for developing these specific adverse effects might benefit from acalabrutinib if BTK inhibitor treatment is indicated.
Historically, chemotherapy treatment for hematologic malignancies was associated with significant numbers or severity of AEs and quality-of-life burdens (26, 27). Targeted therapies like acalabrutinib have the potential for more favorable AE profiles and are less likely to be associated with quality-of-life decrements (12, 28). Our data show that the combination of acalabrutinib plus obinutuzumab did not decrease patients’ quality of life. Instead, some improvements were found, with patients viewing their physical health as generally improved, and physical symptoms were less disruptive to their quality of life. The source for the latter may derive from improvements in 3 common symptoms: diarrhea, dyspnea, and appetite loss. During the course of treatment, all 3 common symptoms were of sufficient frequency/intensity to rise to the level of AE ratings (any grade: 64%, 24%, and 36%, respectively). But despite this, patients reported symptoms as having improved by cycle 24. This affirms that this regimen is tolerable and can be given safely without impairing patients’ overall quality of life.
In conclusion, the combination of acalabrutinib and obinutuzumab leads to high response rates and prolonged remission duration. In this study, acalabrutinib plus obinutuzumab was tolerable, with low rates of discontinuation due to AEs. Further study of this combination is warranted in CLL and potentially in other lymphoid malignancies.
METHODS
Patients
Eligible patients had a diagnosis of intermediate or high-risk Rai stage CLL or small lymphocytic lymphoma by iwCLL 2008 criteria (29); patients in the previously untreated cohort were ≥65 years old or <65 and refused or were ineligible for chemoimmunotherapy, and patients in the relapsed/refractory cohort had ≥1 prior therapy for CLL. All patients were required to be ≥18 years of age with an Eastern Cooperative Oncology Group performance status of ≤2 and active disease requiring therapy per iwCLL 2008 guidelines (29). Patients were required to have measurable nodal disease, and adequate organ function defined as creatinine clearance ≤2.5 × the upper limit of normal (ULN), alanine aminotransferase and aspartate aminotransferase ≤3.0 × ULN, and bilirubin ≤2.5 × ULN. Platelets had to be ≥50 × 109/L and absolute neutrophil count (ANC) had to be ≥750/mm3; for patients with bone marrow involvement by CLL, platelets had to be ≥30 × 109/L and ANC ≥ 500/mm3. Prior BTK inhibitor treatment was permitted if the reason for discontinuation was not disease progression during treatment.
Study Design
In this phase Ib study, both cohorts received the same regimen of acalabrutinib plus obinutuzumab. Acalabrutinib was started on cycle 1 day 1 and administered in 28-day cycles until disease progression or unacceptable toxicity. In the first 15 patients, the dose of acalabrutinib was 200 mg once daily; however, upon observing that BTK occupancy was higher with twice-daily dosing (16), all patients subsequently received 100 mg twice daily. Obinutuzumab was given by intravenous infusion starting with cycle 2: 100 mg on day 1, 900 mg on day 2, and then 1,000 mg on days 8 and 15 of cycle 2, and 1,000 mg on day 1 of cycles 3–7.
This was a single-center phase Ib/II study conducted at The Ohio State University Comprehensive Cancer Center (NCT02296918). All patients provided written informed consent. The study was coordinated by Acerta Pharma, a member of the AstraZeneca Group, and was approved by the Institutional Review Board of The Ohio State University (Columbus, OH). The study was conducted in accordance with the principles of the Declaration of Helsinki, and safety was monitored every 3 months by The Ohio State University Data Safety Monitoring Board.
Endpoints and Assessments
The primary endpoints were ORR (CR + PR) and safety with acalabrutinib plus obinutuzumab. Secondary endpoints included, but were not limited to, analysis of MRD negativity, PFS, OS, time to next treatment (data not shown), pharmacodynamics, and correlative assessments of mutational data and efficacy endpoints. Patients underwent bone marrow biopsy at screening, on day 1 of cycle 12, at the time of presumed CR defined by CT scans and MRD-negative peripheral blood, and at relapse. Disease assessments were conducted every 3 cycles for the first 24 cycles, and then every 6 cycles thereafter. Evaluations included toxicity assessment, physical exam, laboratory studies, and CT scans. Bone marrow biopsy was performed at day 1 of cycle 12 and thereafter only if criteria for CR were met and peripheral blood was MRD negative as measured by flow cytometry. Toxicities were evaluated using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 or higher for nonhematologic toxicities and iwCLL 2008 guidelines (29) for hematologic toxicities.
Responses were determined according to iwCLL 2008 guidelines. Patients were screened for BTKC481S mutations using droplet digital PCR every visit starting 12 cycles after treatment began; all patients had >1 year of screening. MRD was assessed using flow cytometry for the peripheral blood every 3 cycles, and for the bone marrow at cycle 12 and any time CR criteria were met by CT scans and when peripheral blood MRD as measured by flow cytometry was negative. A cutoff of <1 cell in 10,000 lymphocytes was used to define MRD negativity, in accordance with standard definitions.
Correlative Analyses
Baseline TP53 mutation analysis was performed in the Experimental Hematology Laboratory at The Ohio State University as described previously (10).
MRD analysis was performed using 10-color flow cytometry in the Clinical Flow Cytometry laboratory at The Ohio State University as described previously (ref. 10; combination of antibodies listed in Supplementary Table S3). The method allows reliable and reproducible detection of CLL MRD at the level of 0.01% (10−4) of all events, which corresponds with 1 CLL cell in 10,000 total cells analyzed. Criteria used for CLL MRD status determination for each sample were described in detail previously (30, 31).
Pharmacodynamics
Occupancy of BTK by acalabrntinib (using a validated ELISA) was measured in peripheral blood mononuclear cells (PBMC) with the aid of a drug-analogue probe at predose on day 1 of cycles 1–3 and predose on day 2 of cycles 1 and 2. PBMCs were also used for flow cytometry–based signaling assays to measure BTK phosphorylated at Y223 and total BTK protein levels. Additional details on the methods for pharmacodynamic analyses were reported previously (16).
Quality of Life
A repeated measures design was used to obtain quality-of-life data. Quality-of-life assessments were obtained from patients on screening/day 1 (cycle 1) and day 1 of cycles, 5, 9, and 12, and then on day 1 of cycles 18 and 24. Excepting individuals leaving the trial, there were no missing data at each timepoint.
With the aid of an assistant, patients completed self-report measures of quality of life on TeleForms that were subsequently scanned; there was no hand entry of data. Two measures were used: the SF-36 Health Survey and the EORTC-QLQ. The SF-36 is a 36-item questionnaire that uses 2 summary scales—the physical component and mental component scales (32). The EORTC-QLQ is a 30-item questionnaire designed to assess health-related quality of life in patients with cancer participating in clinical trials.
Cohort, time, and cohort × time interactions were assessed. Prior to analysis, Spearman correlations between SF-36 and EORTC-QLQ scores at baseline, as well as sex and age were evaluated for significant associations (P < 0.05) to determine the need for inclusion as covariates in the analyses. Linear mixed effects models were used for primary analyses and fitting was performed using an autoregressive covariance structure to account for correlations between proximate timepoints. A repeated measures ANOVA was used to assess quality of life across the study period, using baseline and 24-month outcomes. Cohort (treatment-naïve and relapsed/refractory) was the factor used among patients to assess time-related outcomes and to determine any cohort, time, and interaction effects for individual patients.
Statistical Considerations
Data are presented as of February 12, 2019. Efficacy and safety analyses were performed on all enrolled patients who received ≥1 dose of acalabrutinib. Safety analyses include both cohorts combined except as indicated and include all treatment-emergent AEs regardless of attribution to study drugs. Time-to-event endpoints, including duration of response (DOR), PFS, and OS were estimated using the Kaplan–Meier method. DOR and time to response analyses were conducted on patients who achieved CR or PR as their best overall response. Descriptive statistics were used to summarize the findings.
Supplementary Material
Acknowledgments
This study was sponsored by Acerta Pharma, a member of the AstraZeneca Group. We thank the patients who participated in this trial and their families and caregivers; the investigators and coordinators at each study site; and the study team of Acerta Pharma, a member of the AstraZeneca Group. Medical writing assistance was provided by Stephanie Morgan of Team 9 Science and funded by Acerta Pharma, a member of the AstraZeneca Group. The authors thank Kathleen Burke, Oncology R&D, and AstraZeneca Boston, for bioinformatic support. The CLL team at The Ohio State University was supported by the The Ohio State University Leukemia Tissue Bank supported by a grant from the NIH; P30 CA016058, R01 CA197870 (to J.A. Woyach), R35 CA198183 (to J.C. Byrd), and further research support from the Four Winds Foundation and the D. Warren Brown Foundation. K.A. Rogers is a Scholar in Clinical Research of The Leukemia & Lymphoma Society. D.M. Weiss is a Pelotonia Fellow at The Ohio State University Comprehensive Cancer Center.
Disclosure of Potential Conflicts of Interest
J.A. Woyach is a consultant for Janssen Oncology, Pharmacyclics, AstraZeneca, and ArQule, reports receiving commercial research grants from Loxo and AbbVie, and has received other commercial research support from Pharmacyclics, Janssen Oncology, Karyopharm, MorphoSys, and Verastem. J.S. Blachly is an advisory board member for AbbVie, AstraZeneca, Innate Pharma, and KITE Pharma. K.A. Rogers has participated in advisory boards at Acerta Pharma, AstraZeneca, and Pharmacyclics. S.A. Bhat is a consultant/advisory board member for Pharmacyclics and Janssen. M. Gulrajani is a senior scientist at Acerta Pharma and has ownership interest in the same. M.M. Frigault is the head of translational science at AstraZeneca and has ownership interest in the same. A. Hamdy is VP, clinical development, at Acerta and has ownership interest in the same. R. Izumi is an EVP at Acerta Pharma and has ownership interest in the same. V. Munugalavadla is director, clinical biomarkers, at Acerta Pharma, a member of the AstraZeneca Group; and has a family member association with Gilead Sciences C. Quah is senior medical director at Acerta Pharma. M.-H. Wang is a statistician at Acerta Pharma. J.C. Byrd is an advisor at Acerta, AstraZeneca, Jazz, and Pharmacyclics and has ownership interest in a patent on a small molecule unrelated to this study. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Supplementary Material Access the most recent supplemental material at: http://cancerdiscovery.aacrjournals.org/content/suppl/2020/01/07/2159-8290.CD-19-1130.DC1
REFERENCES
- 1.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014;123:3286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman SE, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with ibrutinib inhibits BTK- and VLA-4-dependent adhesion of chronic lymphocytic leukemia cells in vivo. Clin Cancer Res 2015;21:4642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woyach JA, Smucker K, Smith LL, Lozanski A, Zhong Y, Ruppert AS, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood 2014;123:1810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW, et al. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity 2007;26:93–104. [DOI] [PubMed] [Google Scholar]
- 6.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor-and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012;119:2590–4. [DOI] [PubMed] [Google Scholar]
- 7.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012;119:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med 2018;379:2517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med 2019;381:432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018;103:874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine 2006;85:193–202. [DOI] [PubMed] [Google Scholar]
- 14.Howard V, Greene JM, Pahwa S, Winkelstein JA, Boyle JM, Kocak M, et al. The health status and quality of life of adults with X-linked agammaglobulinemia. Clin Immunol 2006;118:201–8. [DOI] [PubMed] [Google Scholar]
- 15.Golay J, Ubiali G, Introna M. The specific Bruton tyrosine kinase inhibitor acalabrutinib (ACP-196) shows favorable in vitro activity against chronic lymphocytic leukemia B cells with CD20 antibodies. Haematologica 2017;102:e400–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther 2017;363:240–52. [DOI] [PubMed] [Google Scholar]
- 18.Byrd JC, Wierda W, Schuh A, Devereaux S, Chaves J, Brown JR, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated results from the phase 1/2 ACE-CL-001 Study. Blood 2017;130:498. [Google Scholar]
- 19.O’Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood 2018;131:1910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanDerMeid KR, Elliott MR, Baran AM, Barr PM, Chu CC, Zent CS. Cellular cytotoxicity of next-generation CD20 monoclonal antibodies. Cancer Immunol Res 2018;6:1150–60. [DOI] [PubMed] [Google Scholar]
- 21.Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SE, Butchar JP, Cheney C, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood 2014;123:1957–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da Roit F, Engelberts PJ, Taylor RP, Breij EC, Gritti G, Rambaldi A, et al. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica 2015;100:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hassenruck F, Knodgen E, Gockeritz E, Midda SH, Vondey V, Neumann L, et al. Sensitive detection of the natural killer cell-mediated cytotoxicity of anti-CD20 antibodies and its impairment by B-cell receptor pathway inhibitors. Biomed Res Int 2018;2018:1023490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bologna L, Gotti E, Manganini M, Rambaldi A, Intermesoli T, Introna M, et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol 2011;186:3762–9. [DOI] [PubMed] [Google Scholar]
- 25.Rafiq S, Butchar JP, Cheney C, Mo X, Trotta R, Caligiuri M, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol 2013;190: 2702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichhorst BF, Busch R, Obwandner T, Kuhn-Hallek I, Herschbach P, Hallek M, et al. Health-related quality of life in younger patients with chronic lymphocytic leukemia treated with fludarabine plus cyclophosphamide or fludarabine alone for first-line therapy: a study by the German CLL Study Group. J Clin Oncol 2007;25:1722–31. [DOI] [PubMed] [Google Scholar]
- 27.Holzner B, Kemmler G, Kopp M, Nguyen-Van-Tam D, Sperner-Unterweger B, Greil R. Quality of life of patients with chronic lymphocytic leukemia: results of a longitudinal investigation over 1 yr. Eur J Haematol 2004;72:381–9. [DOI] [PubMed] [Google Scholar]
- 28.Barrientos JC, O’Brien S, Brown JR, Kay NE, Reddy NM, Coutre S, et al. Improvement in parameters of hematologic and immunologic function and patient well-being in the phase III RESONATE Study of ibrutinib versus ofatumumab in patients with previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma. Clin Lymphoma Myeloma Leuk 2018;18:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rawstron AC, Villamor N, Ritgen M, Bottcher S, Ghia P, Zehnder JL, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 2007;21:956–64. [DOI] [PubMed] [Google Scholar]
- 31.Rawstron AC, Fazi C, Agathangelidis A, Villamor N, Letestu R, Nomdedeu J, et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia 2016;30:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware JE, Gandek B, Group IP. The SF-36 Health Survey: development and use in Mental Health Research and the IQOLA Project. Int J Mental Health 1994;23:49–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


