Abstract
In the era of emerging options for mitral valvular intervention, we sought to characterize the relative utilization, outcomes, and posthospital dispositions of patients referred for transcatheter mitral valve repair (TMVRepair) and surgical mitral valve procedures (SMVP), by cancer-status. Leveraging the National Inpatient Sample, a representative national dataset, ICD-9 codes for all adults >18 years with co-morbid mitral regurgitation, and cancer without metastatic disease admitted from 2003 to 2015 were queried. TMVRepair was performed in 700 hospitalizations from 2012 to 2015, whereas SMVP was utilized during 12,863 hospitalizations from 2003 to 2015. During follow-up, we observed a proportional increase in TMVRepair utilization among cancer patients (vs noncancer), particularly in 2015 (14.2% vs 8.2%, p <0.0001). There was no difference in in-hospital mortality (1.4% vs 1.8%, p = 0.71), ischemic stroke (0.7% vs 0.6%, p = 0.97), major bleeding (8.6% vs 10.9%, p = 0.36), and home discharge (62.1% vs 65.7%, p = 0.45) by cancer-status among patients who underwent TMVRepair; but, cost of care was increased ($52,325 vs $48,832, p <0.0001). Similarly, there was no difference in in-hospital mortality (3.1% vs 3.4%, p = 0.36), ischemic stroke (2.6% vs 3.1%, p = 0.16) as well as the cost-of-care ($58,106 vs $58,844, p = 0.49) among those who underwent SMVP across the same period; but, cancer was associated with increased major bleeding (34.9% vs 30.5%, p <0.0001), and lower likelihood of home discharge (32.8% vs 38.6%, p <0.0001). In conclusion, TMVRepair and SMVP were associated with comparable in-hospital mortality and outcomes in cancer versus noncancer patients. However, cancer patients treated with SMVP experienced more frequent bleeding related complications compared with noncancer patients.
Cancer is an increasingly common co-morbid consideration among those presenting with valvular heart disease.1,2 Mitral valve disease remains the most common form of valvular heart disease, with a reported prevalence of mitral regurgitation nearing 2% in the United States alone.3 Traditional surgical mitral valve procedures (SMVP) are currently the only first line of treatment known to improve hemodynamic and functional outcomes as well as survival in those afflicted with limiting mitral regurgition.4 Recent breakthroughs have allowed for the expanded use of less invasive transcatheter mitral valve repair (TMVRepair; although MitraClip is currently the only TMVRepair device approved by the US Food and Drug Administration in patients with mitral regurgitant disease) techniques.5 This has represented a major advance in the care of patients previously thought to be at higher surgical risk and has been linked with comparable safety and outcomes.5 Moreover, it is plausible that TMVRepair could provide an alternative therapeutic option in cancer, given its superior safety profile. Yet, to date, there remain only isolated reports among those with cancer. Accordingly, we sought to evaluate the utilization patterns, outcomes, and posthospital disposition of cancer patients who underwent either TMVRepair or SMVP in nationally representative dataset.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. The National Inpatient Sample (NIS)6 is an inpatient database in the United States developed by the Agency for Healthcare Research and Quality (AHRQ). NIS is a self-weighted, stratified, systematic, random sample of 20% discharges from all hospitals (100%) in the sampling frame, after sorting discharges by diagnosis-related group, hospital, and admission month. The sample is stratified on hospital characteristics. This form of clustering tends to induce dependence among discharges within hospitals, hence variance analysis of subsets was performed in lines with prior validated NIS methodology.6,7 The data is publicly available and deidentified and hence exempt from IRB.
The NIS was queried using ICD-9 codes for adults >18 years and cancer (Supplementary Table 1).3,5 In the present study, we used data from January 1, 2003, through September 30, 2015 for SMVP. All TMVRepair specific analysis utilized data from January 1, 2012, through September 30, 2015. We first identified those inpatient admissions that had a primary diagnosis (DX1 of NIS) of mitral valve disease (ICD-9-CM 424.1). To maintain a homogenous study population and to limit confounding, those with mitral stenosis, rheumatic disease, infective endocarditis, aortic valve disease, or those who underwent any other vascular or cardiac surgery in the same admission were excluded from the analysis. The procedures of interest were TMVRepair (35.97) or SMVP (35.23, 35.24, and 35.12). We a priori decided to exclude metastatic disease, as cancer patients with metastatic progression are in a much higher risk category when compared to their nonmetastatic cancer counterparts.8 Further, expected survival varies significantly between metastatic cancers.9 Patients with an identified co-morbidity of metastasis were identified based on ICD-9 codes for metastatic disease. Since the baseline population who underwent SMVP is quite different from TMVRepair, parallel analyses for outcomes and dispositions were presented, but no direct statistical comparisons due to these inherent differences.
NIS provided data on specific outcomes of interest, including hospitalization charges, length of stay, in-hospital mortality, and discharge disposition, by cancer status. The actual cost of hospitalization was obtained by multiplying each hospital’s charges with their cost-to-charge ratios and wage index for a given year. The wage index helps correct for geographic variations in costs among hospitals. Charges and costs were inflation-adjusted to 2015. In 2015, the Healthcare Cost and Utilization Project (HCUP) State Inpatient Database was used to create an index based on 29 co-morbidity measures designed to predict in-hospital mortality.10 In addition to NIS provided co-morbidities other co-morbidities and outcomes which have been highlighted in other TMVR studies including ischemic stroke, major bleeding and acute kidney injury were recoded using ICD-9-CM and DXCCS fields (Supplementary Table 1).
Annual variance analysis for NIS datasets was performed using the DOMAIN method for all years. We followed the recommendations from AHRQ for analysis using survey data. Survey-specific statements with hospital and patient-level weights were used to obtain national estimates.8 Survey-specific statements (SURVEYMEANS, SURVEYFREQ) with hospital and patient-level weights were used to obtain national estimates. The Rao-Scott Chi-Square test was used to compare categorical variables, and a survey specific t-test (SURVEYREG) was used for continuous variables. We used the Cochran Armitage test of trend for categorical variables and survey-specific linear regression for continuous variables. The Cochran Armitage trend test tends to have higher power than the chi-squared test, but when the suspected trend is correct. This particular trend test leverages the suspected effect direction to increase power, but in doing so does not affect the sampling distribution under the null hypothesis. Hospital charges and length of stay were log-transformed because they were not normally distributed, and the geometric mean was presented.11,12 For a length of stay of 0 days, a value of 0.0001 was imputed to avoid negative log values. To assess for differences in clinical outcomes, propensity score matching was performed. A nonparsimonious multivariate logistic regression was performed using gender, age, race/ethnicity, primary insurance type, income, presence of CHF, CAD, prior MI, prior CABG, AF, HTN, diabetes, renal disease, chronic lung disease, coagulopathy, smoking, Elixhauser’s co-morbidity (0, 1, 2, >2), hospital region, hospital type, hospital size, discharge weight as variables, to generate the propensity score. NIS weights were used in the propensity estimation. The propensity score was then used to standardize outcomes across cancer and noncancer cohort using inverse probability of treatment weight algorithm13 to avoid loss of sample size given overall less number of patients who underwent TMVR Finally, the 2015 data was stratified by cancer type which included breast cancer, lung cancer, colon cancer, prostate cancer, leukemia & lymphoma, and other cancer types. Due to insufficient hospitalizations in specific cancer groups that is empty clusters, NIS methodology is suggestive of inaccurate weighted estimates.14 All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
A total of 8,878,537 patients with mitral regurgitation admissions were identified between 2003 and s2015 from NIS. Of these, 1,211,716 had nonmetastatic cancer (13.7%) and concomitant mitral valve disease. Among those with co-morbid cancer, TMVRepair utilization was performed in 700 hospitalizations from 2012 to 2015. Cancer patients who underwent TMVRepair from 2012 to 2015 were older (70 vs 64 years, p = 0.03), with no difference in gender (41% females), and socioeconomic profile when compared with noncancer patients. Cancer patients also had higher rates of traditional CVD risk factors, including hypertension (82% vs 72%, p = 0.008), similar healthcare cost and utilization project (HCUP) mortality score (4.8 ± 1.1 vs 5.2 ± 0.5, p = 0.69), as well as similar rates of diabetes (27%).
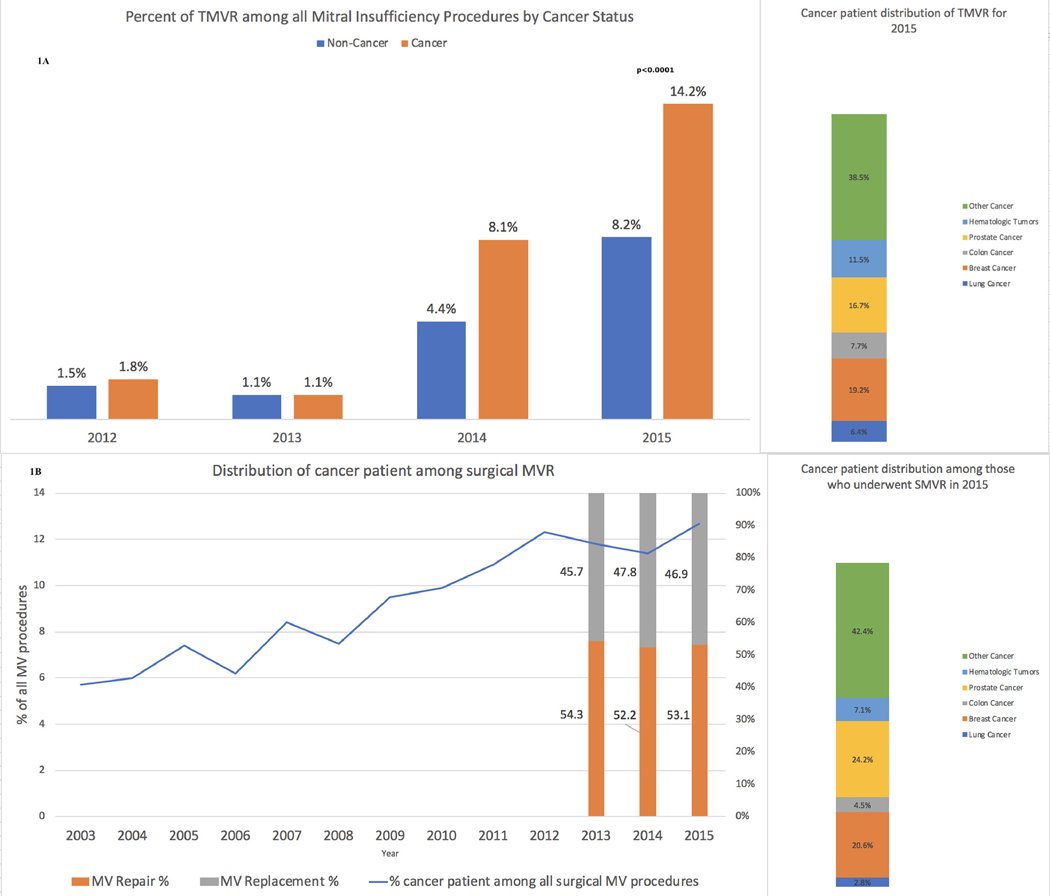
Over the study period, we saw an increase in the proportion of patients who underwent TMVRepair among all patients who underwent either percutaneous (TMVRepair) or open (SMVP) mitral valve procedure for mitral regurgitation (p <0.0001) (Figure 1A). Moreover, upon comparing the cancer and noncancer groups especially in the year 2015, the increase was higher in cancer patients when compared with noncancer (14.2% vs 8.2%, p <0.0001). Further breakdown according to different type of cancers for the year 2015 is shown in Figure 1. TMVRepair utilization was the highest in breast cancer (19.2%) followed by prostate (16.7%) and hematologic tumors (11.5%). Additional crude outcomes specific to TMVRepair in different cancer types are shown in Supplementary Table 2. TMVRepair was associated with comparable in-hospital mortality (1.4% vs 1.8%, p = 0.71), ischemic stroke (0.7% vs 0.6%, p = 0.97), major bleeding (8.6% vs 10.9%, p = 0.36), and home discharge (62.1% vs 65.7%, p = 0.45), but increased cost of care (p <0.0001) among cancer versus noncancer patients, respectively (Table 1A).
Figure 1.
Transcatheter mitral valve replacement (A), and surgical mitral valve (B) procedures, by cancer status. Breakdown by cancer type (far right) is also shown. (A) Transcatheter mitral valve replacement utilizations in cancer and noncancer patients, respectively, from 2012 to 2015 (p <0.0001 for all years). (B) Trends in surgical mitral valve use among cancer patients from 2003 to 2015 (p <0.0001 for all years).
Table 1A.
Propensity matched outcomes, complications, and discharge dispositions of transcatheter mitral valve replacement (TMVR) and surgical mitral valve procedures (SMVP) from 2012 to 2015, by cancer status
| Variable | TMVR |
SMVP |
||||
|---|---|---|---|---|---|---|
| Cancer (N = 700) | Non-Cancer (N = 2,905) | p | Cancer (N = 5,685) | Non-Cancer (N = 35,795) | p | |
| In-hospital Mortality | 1.4 % | 1.8 % | 0.71 | 3.1 % | 3.4 % | 0.36 |
| Ischemic Stroke | 0.7 % | 0.6 % | 0.97 | 2.6 % | 3.1 % | 0.16 |
| Major Bleeding | 8.6 % | 10.9 % | 0.36 | 34.9 % | 30.5 % | <0.0001 |
| Acute Kidney Injury | 14.3 % | 15.7 % | 0.71 | 19.5 % | 19.6 % | 0.92 |
| Home Discharge | 62.1 % | 65.7 % | 0.45 | 32.8 % | 38.6 % | <0.0001 |
| Nursing Home Use | 13.6 % | 11.7 % | 0.48 | 26.9 % | 22.8 % | <0.0001 |
| Home Health Care Use | 22.9 % | 20.3 % | 0.51 | 37.2 % | 35 % | 0.05 |
| Length of Stay, days (SE) | 1.1 (0.09) | 1.2 (0.05) | 0.60 | 2.1 (0.02) | 2.1 (0.01) | 0.39 |
| Cost of Care, $ (SE) | 52,325 (3525) | 48,832 (2154) | <0.0001 | 58,106 (1225) | 58,844 (672) | 0.49 |
SE = standard error.
SMVP utilization occurred in 12,863 hospitalizations among those with co-morbid cancer from 2003 to 2015. Cancer patients who underwent SMVP during the contemporary years or the same time period as TMVRepair (2012 to 2015) were older (77 vs. 75 years, p <0.0001), and with no difference in gender (44% females) when compared with noncancer group. Further, cancer patients had higher rates of traditional CVD risk factors, including hypertension (63% vs 56%, p <0.0001), lower HCUP mortality score (6.8 ± 0.3 vs 7.6 ± 0.2, p = 0.004), but similar rates of diabetes (27%).
Over time, the proportion of cancer patients who underwent SMVP for mitral valve regurgitation also increased (p <0.0001), with greater use of mitral valve repair compared with mitral valve replacement (in 2015 repair vs replacement was 53.1% vs 46.9%, p <0.0001) (Figure 1). SMVP utilization was the highest in prostate cancer (24.2%) followed by breast (20.6%) and hematologic tumors (7.1%). Additional crude outcomes specific to surgical mitral valve repair and replacement in different cancer types are shown in Supplementary Table 3 and 4, respectively.
Among patients who underwent SMVP in the contemporary years (2012 to 2015), cancer was associated with comparable in-hospital mortality (3.1% vs 3.4%, p = 0.36), ischemic stroke (2.6% vs 3.1%, p = 0.16), increased episodes of major bleeding (34.9% vs 30.5%, p <0.0001), but decreased tendency for home discharge (32.8% vs 38.6%, p <0.0001) when compared with noncancer patients, respectively (Table 1A). However, cost of care did not differ between cancer and noncancer patients (p = 0.49).
SMVP outcomes remained stable or slightly worsened in cancer but improved significantly in noncancer in the year 2003 to 2011 compared with 2012 to 2015. The in-hospital mortality remained fairly stable in noncancer patients over the year from 6.1% to 4.1% to 3.4% in the years 2003 to 2007, 2008 to 2011 and 2012 to 2015, respectively. Similarly, the in-hospital mortality in cancer patients also remained stable during the same time period (3%, 2.9%, and 3.1% for 2003 to 2007, 2008 to 2011 and 2012 to 2015, respectively). Additional outcomes associated with SMVP over the time period 2003 to 2015 are mentioned in Table 1A and B.
Table 1B.
Propensity matched outcomes, complications, and discharge dispositions of surgical mitral valve procedures (SMVP) from 2003 to 2007 and 2008 to 2011, by cancer status
| Variable | SMVP (2003–2007) |
SMVP (2008–2011) |
||||
|---|---|---|---|---|---|---|
| Cancer (N = 3,215) | Non-Cancer (N = 32,184) | p | Cancer (N = 3,962) | Non-Cancer (N = 31,017) | p | |
| HCUP Mortality Score | 3.2±0.3 | 3.6±0.2 | 0.22 | 5.9±0.3 | 5.9±0.3 | 0.94 |
| In-hospital Mortality | 3 % | 6.1 % | <0.0001 | 2.9 % | 4.1 % | 0.02 |
| Ischemic Stroke | 1.2 % | 2.1 % | 0.009 | 2.6 % | 3.2 % | 0.11 |
| Major Bleeding | 32.5 % | 26.2 % | <0.0001 | 39.9 % | 32.6 % | <0.0001 |
| Acute Kidney Injury | 7.4 % | 12.9 % | <0.0001 | 15.3 % | 16.8 % | 0.11 |
| Home Discharge | 38.4 % | 43.1 % | 0.0003 | 35.7 % | 39.4 % | 0.005 |
| Nursing Home Use | 20.8 % | 20.4 % | 0.71 | 24.8 % | 21.8 % | 0.02 |
| Home Health Care Use | 37.4 % | 30.1 % | <0.0001 | 36.5 % | 34.6 % | 0.14 |
| Length of Stay, days (SE) | 2.2 (0.02) | 2.3 (0.01) | <0.0001 | 2.1 (0.02) | 2.2 (0.12) | 0.01 |
| Cost of Care, $ (SE) | 57,804 (2599) | 65,620 (1540) | <0.0001 | 55,987 (1717) | 59,023 (1307) | 0.007 |
HCUP = healthcare cost and utilization project; SE = standard error.
Discussion
In this contemporary study of the impacts of a cancer diagnosis on the growing use and outcomes of mitral valve interventions, we found that the proportions of patients who underwent TMVRepair for mitral regurgitation increased over time. This increase was more evident among cancer patients when compared with those without cancer across the available time period considered (2012 to 2015). Over time, the proportion of cancer patients who underwent SMVP for mitral valve disease also increased, primarily driven by the use in mitral valve repair rather than mitral valve replacement. Moreover, there was no difference in in-hospital mortality or safety profile with the use of either TMVRepair or SMVP, based on the presence or absence of cancer. These observations are of particular importance given the growing number of cancer patients affected by mitral regurgitation, and the lack of available noninvasive options to manage this condition.
The observation of clinical benefit to early mitral regurgitant intervention has long been established.15 However, over the last decade TMVRepair has increasingly emerged as viable therapeutic option, particularly in those considered to be at higher traditional surgical risk. In a recent trial of heart failure patients with secondary mitral regurgitation, TMVRepair was associated with early, substantial, and sustained improvement in health status compared with standard care, suggesting that TMVRepair might improve patients’ symptoms and quality of life.16,17 And although in a more board study of patients with severe secondary mitral regurgitation, TMVRepair was not associated with a reduction in the composite of death or hospitalization for heart failure, more recently available data continue to suggest benefit among higher risk patients.15,18 Notably, in a previous evaluation, TMVRepair was considered to be comparable to SMVP among patients with severe mitral regurgitation.19 However, none of these studies explored the efficacy of TMVRepair and SMVP in cancer patients.
Our study provides novel insights into current practice patterns associated with treating mitral valve disease in cancer. Concurrent with SMVP, TMVRepair use has been on the rise in cancer patients. This can be attributed to the fact that TMVRepair has been shown to have a superior safety profile in terms of indicators, such as discharged disposition and length of inpatient stay, when compared with SMVP.5 Our study highlighted that when appropriate either TMVRepair or SMVP is a reasonable option among cancer patients. However, TMVRepair may be associated with an even greater safety profile compared with SMVP, because of increased episodes of major bleeding associated with SMVP in cancer. This may be explained by the observation that cancer patients usually tend towards increased bleeding following a major surgical procedure owing to their inflamed and hypercoagulable nature, as well as persistent thrombocytopenia.20
There are several limitations of this study that warrant consideration. Because of reliance on ICD-9-CM codes, we were unable to determine the physician-perceived indication for hospital admission by specific cancer type. We also could not determine the clinical severity, etiology and different pathologies of mitral valve disease, or other concomitant disease like chronic kidney disease, pulmonary hypertension, coronary artery disease, duration of a cancer diagnosis, cancer stage (active vs prior), life expectancy, or concomitant cancer treatments (radiation vs chemotherapy vs recent cancer surgery). We also acknowledge that grouping repair for degenerative mitral valve and replacement for functional mitral pathologies is not ideal as they are associated with different outcomes. However, individual outcomes related to percutaneous mitral valve repair, surgical mitral valve repair, and surgical mitral valve replacement in different cancer types are presented in the supplement. Moreover, we were also not able to determine if this study includes repeated observations from patients who underwent >1 MVR (TMVRepair or SMVP) during the study period. The size of the cohort exploring outcomes specific to TMVRepair use in different cancer types was small and thus may represent wrong estimate due to anomalous variance analysis.
In summary, among cancer patients, the use of either SMVP or TMVRepair appears to associate with comparable outcomes to those seen in similar cancer-free patients. Although, the use of SMVP among cancer patients appears to associate with higher rates of major bleeding and need for increased care after discharge, not seen with TMVRepair. In the era of rapidly improving cancer survival, further research into the modifiable factors related to these differences are needed.
Supplementary Material
Acknowledgment
The manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: Dr. Addison is supported by NIH grant number K12-CA133250.
Role of the Funder: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures
Authors declare no conflicts of interests in relation to the work in this manuscript.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2020.01.047.
References
- 1.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 2.Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, Kohler BA, Jemal A. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2018;124:2785–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta T, Khera S, Kolte D, Goel K, Villablanca PA, Abbott JD, Fonarow GC, Rihal CS, Garcia MJ, Bhatt DL, Latib A, Weisz G. Trends in utilization of surgical and transcatheter mitral valve repair in the United States(small star, filled). Am J Cardiol 2019;123: 1187–1189. [DOI] [PubMed] [Google Scholar]
- 4.Kilic A, Shah AS, Conte JV, Baumgartner WA, Yuh DD. Operative outcomes in mitral valve surgery: combined effect of surgeon and hospital volume in a population-based analysis. J Thorac Cardiovasc Surg 2013;146:638–646. [DOI] [PubMed] [Google Scholar]
- 5.Chikwe J, Chiang YP, Egorova NN, Itagaki S, Adams DH. Survival and outcomes following bioprosthetic vs mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA 2015;313: 1435–1442. [DOI] [PubMed] [Google Scholar]
- 6.Method Series Report 2015–09. Healthcare Cost and Utilization Project (HCUP). Rockville, MD:: Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 7.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the National Inpatient Sample. JAMA 2017;318: 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guha A, Dey AK, Armanious M, Dodd K, Bonsu J, Jneid H, Abraham W, Fradley MG, Addison D. Health care utilization and mortality associated with heart failure-related admissions among cancer patients. ESC Heart Failure 2019;6:733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dizon DS, Krilov L, Cohen E, Gangadhar T, Ganz PA, Hensing TA, Hunger S, Krishnamurthi SS, Lassman AB, Markham MJ, Mayer E, Neuss M, Pal SK, Richardson LC, Schilsky R, Schwartz GK, Spriggs DR, Villalona-Calero MA, Villani G, Masters G. Clinical cancer advances 2016: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 2016;34: 987–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and In-hospital mortality using hospital administrative data: the AHRQ elixhauser comorbidity index. Med Care 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 11.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ 1996;312:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert BD, Horstman JM. Captain’s LOG: Taking Command of SAS Logarithm Functions. 2014. [Google Scholar]
- 13.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf 2006;15:698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suri RM, Vanoverschelde J- L, Grigioni F, Schaff HV, Tribouilloy C, Avierinos J- F, Barbieri A, Pasquet A, Huebner M, Rusinaru D, Russo A, Michelena HI, Enriquez-Sarano M. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013;310:609–616. [DOI] [PubMed] [Google Scholar]
- 16.Arnold SV, Chinnakondepalli KM, Spertus JA, Magnuson EA, Baron SJ, Kar S, Lim DS, Mishell JM, Abraham WT, Lindenfeld JA, Mack MJ, Stone GW, Cohen DJ, Investigators C. Health status after transcatheter mitral-valve repair in heart failure and secondary mitral regurgitation: COAPT trial. J Am Coll Cardiol 2019;73:2123–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, Investigators C. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–2318. [DOI] [PubMed] [Google Scholar]
- 18.Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefevre T, Piot C, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, MaucortBoulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N, Investigators M- F. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–2306. [DOI] [PubMed] [Google Scholar]
- 19.Feldman T, Foster E, Qureshi M, Whisenant B, Williams J, Glower D, Mauri L. TCT-788 the EVEREST II Randomized Controlled Trial (RCT): three year outcomes. J Am Coll Cardiol 2012;60:B229–B230. [Google Scholar]
- 20.Habr B, Charpentier J, Champigneulle B, Dechartres A, Daviaud F, Geri G, Cariou A, Chiche JD, Mira JP, Pene F. Platelet transfusions in cancer patients with hypoproliferative thrombocytopenia in the intensive care unit. Ann Intensive Care 2015;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.