Version Changes
Revised. Amendments from Version 1
We have revised the conclusions of the abstract, expanded the reporting of the methods for risk of bias assessment and revised the discussion to clarify the disconnect between detection of SARS-CoV-RNA versus infectious SARS-CoV-2.
Abstract
Background: SARS-CoV-2 RNA has been detected in fomites which suggests the virus could be transmitted via inanimate objects. However, there is uncertainty about the mechanistic pathway for such transmissions. Our objective was to identify, appraise and summarise the evidence from primary studies and systematic reviews assessing the role of fomites in transmission.
Methods: This review is part of an Open Evidence Review on Transmission Dynamics of SARS-CoV-2. We conduct ongoing searches using WHO Covid-19 Database, LitCovid, medRxiv, and Google Scholar; assess study quality based on five criteria and report important findings on an ongoing basis.
Results: We found 64 studies: 63 primary studies and one systematic review (n=35). The settings for primary studies were predominantly in hospitals (69.8%) including general wards, ICU and SARS-CoV-2 isolation wards. There were variations in the study designs including timing of sample collection, hygiene procedures, ventilation settings and cycle threshold. The overall quality of reporting was low to moderate. The frequency of positive SARS-CoV-2 tests across 51 studies (using RT-PCR) ranged from 0.5% to 75%. Cycle threshold values ranged from 20.8 to 44.1. Viral concentrations were reported in 17 studies; however, discrepancies in the methods for estimation prevented comparison. Eleven studies (17.5%) attempted viral culture, but none found a cytopathic effect. Results of the systematic review showed that healthcare settings were most frequently tested (25/35, 71.4%), but laboratories reported the highest frequency of contaminated surfaces (20.5%, 17/83).
Conclusions: The majority of studies report identification of SARS-CoV-2 RNA on inanimate surfaces; however, there is a lack of evidence demonstrating the recovery of viable virus. Lack of positive viral cultures suggests that the risk of transmission of SARS-CoV-2 through fomites is low. Heterogeneity in study designs and methodology prevents comparisons of findings across studies. Standardized guidelines for conducting and reporting research on fomite transmission is warranted.
Keywords: Fomites, transmission, COVID-19, systematic review
Introduction
The SARS-CoV-2 (COVID-19) pandemic is a major public health concern. According to WHO statistics, there have been over 90 million confirmed cases and over two million deaths globally as of 18th January 2021 1 . Although many national governments have implemented control measures and vaccines are now being approved and administered, the rate of infection has not subsided as anticipated. Understanding the modes of transmission of SARS-CoV-2 is critical to developing effective public health and infection prevention measures to interrupt the chains of transmission 2 . Current evidence suggests SARS-CoV-2 is primarily transmitted via respiratory droplets and direct contact 3 , but other transmission routes have been suggested – aerosol and fomites.
While the respiratory, airborne, and direct contact modes of transmission have been investigated in detail, the role of fomites in the transmission of SARS-CoV-2 is less clear. Findings from previous systematic reviews have shown that viruses from the respiratory tract, such as coronaviridae, can persist on inanimate surfaces for some days 4 , and it has been suggested that SARS-CoV-2 can be transmitted indirectly through fomites or surfaces 5 . However, some authors have reported that there is a low risk of transmission of SARS-CoV-2 through fomites 6, 7 and others have reported that the risk of such transmission is exaggerated 8 .
Several studies investigating the role of fomites in SARS-CoV-2 are now being published but the evidence from such studies has not been systematically evaluated. The objective of this review was to identify, appraise and summarize the evidence from primary studies and systematic reviews investigating the role of fomites in the transmission of SARS-CoV-2. Terminology for this article can be found in Box 1.
Box 1. Terminology.
| Fomite: Object or surface contaminated by infected droplets. The contamination can occur through sneezing, coughing on, or touching surfaces 1 |
| Viral load: A measure of the number of viral particles present in an individual 2 |
| Cycle threshold: The number of cycles required for the fluorescent signal to cross the threshold. Ct levels are inversely proportional to the amount of target nucleic acid in the sample 3 |
1World Health Organization. Q&A: How is COVID-19 transmitted? https://www.who.int/vietnam/news/detail/14-07-2020-q-a-how-is-covid-19-transmitted
2 https://www.cebm.net/covid-19/sars-cov-2-viral-load-and-the-severity-of-covid-19/
Methods
We are undertaking an open evidence review investigating factors and circumstances that impact on the transmission of SARS-CoV-2, based on our published protocol last updated on the 1 December 2020 (archived protocol: Extended data: Appendix 1 9 ; original protocol: https://www.cebm.net/evidence-synthesis/transmission-dynamics-of-covid-19/). Briefly, this review aims to identify, appraise, and summarize the evidence (from studies peer-reviewed or awaiting peer review) relating to the role of fomites in the transmission of SARS-CoV-2 and the factors influencing transmissibility. We conducted an ongoing search in WHO Covid-19 Database, LitCovid, medRxiv, and Google Scholar for SARS-CoV-2 for keywords and associated synonyms. The searches for this update were conducted up to 20th December 2020. No language restrictions were imposed (see Extended data: Appendix 2 for the search strategies 9 ).
We included studies of any design that investigated fomite transmission. Predictive or modelling studies were excluded. Results were reviewed for relevance and for articles that looked particularly relevant, forward citation matching was undertaken and relevant results were identified. We assessed the risk of bias using five domains from the QUADAS-2 criteria 10 ; we adapted this tool because the included studies were not designed as diagnostic accuracy studies. The domains assessed were: (i) study description - was there sufficient description of methods to enable replication of the study? (ii) sample sources – was there a clear description of sample sources? (iii) description of results - was the reporting of study results and analysis appropriate? (iv) risk of bias - did the authors acknowledge any potential biases, if yes were any attempts made to address these biases? (v) applicability – is there any concern that the interpretation of test results differs from the study question? For each bias domain, the risk was judged as “low”, “unclear” or “high”. We extracted the following information from included studies: study characteristics, population, main methods, and associated outcomes including the number of swab samples taken with frequency and timing of samples, and cycle thresholds and samples concentrations where reported. We also extracted information on viral cultures including the methods. One reviewer (IJO) assessed the risk of bias and extracted data from the included studies, and these were independently checked by a second reviewer (EAS). We presented the results in tabular format, and bar charts used to present the frequency of positive tests. Because of substantial heterogeneity across the included studies, we did not perform a meta-analysis.
Results
We identified 709 non-duplicate citations of which 91 were considered eligible ( Figure 1). We excluded 27 full-text studies because they did not meet our inclusion criteria (see Extended data: Appendix 3 9 for the list of excluded studies and reasons for exclusion). Finally, we included 64 studies: 63 primary studies and one systematic review (see Extended data: Appendix 4; characteristics of studies in Table 1 and Table 2 9 ).
Figure 1. Flow diagram showing the process for inclusion of studies assessing fomites transmission in SARS-CoV-2.
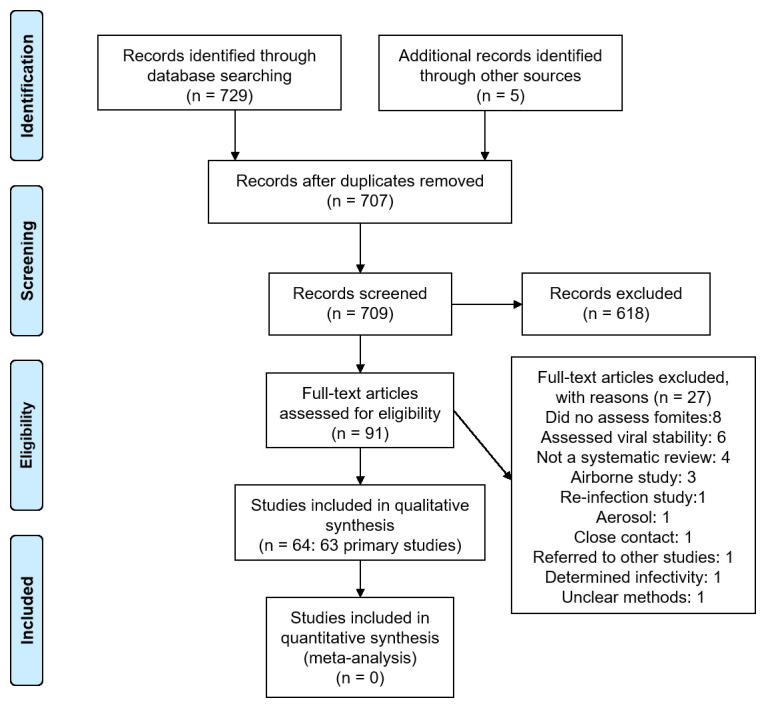
Table 1. Primary studies characteristics.
| Study ID
(n=63) |
Setting | Sources of fomites | Number of swab samples
taken |
Viral culture | Notes |
|---|---|---|---|---|---|
| Abrahão 2020 | Public places in urban area
Brazil April 2020 |
15 bus stations, front-door sidewalk of 8
hospitals, 4 bus terminals, 3 benches and tables in public squares |
101 | No | Densely populated area. Ct<40
considered positive |
| Akter 2020 | 2 southern districts of
Bangladesh over a 3- month period |
Banknotes in circulation
6 non-issuable banknotes spiked with SARS-CoV-2 positive nasopharyngeal samples |
850: both sides of each
banknote from circulation swabbed |
No | Circulating banknotes of varying
denominations were collected from retail shops, ticket vendors and auto rickshaw drivers. intercity transport authority were regulated to ensure wearing masks, maintain social distancing (carrying 50% of total capacity) with personal hygiene. |
| Amoah 2020 | 2 peri-urban informal
settlements in South Africa September 2020 |
Cistern handle, toilet seat, floor surface
in front of the toilet, internal pull latch of cubicle door and tap in wash hand basin |
68 | No | Sampling was done twice in September
2020 when the reported active clinical cases were low in South Africa. |
|
Ben-Shmuel
2020 |
COVID-19 isolation units
in two hospitals and one quarantine facility in Israel |
Mild COVID-19:
Floor, bed rails, bedside
table, faucet handle, mobile phones, eyeglasses, patient's walker, air sampling filter Severe COVID-19: Bed rails, faucet handle, ventilator, staff computer mouse, staff mobile phone, bedside table, trash bin top, bench top, air sampling filter Patient's toilets: Toilet seat, handle grip, door handle Nurse station: Floor, bench top, computer mouse, staff mobile phone, glucometer, electric thermometer, BP cuff, air sampling filter Doffing area: Floor, door handle, trash bin top, air sampling filter |
Smaller objects were
swabbed entirely: 2 wet swabs plus 1 dry swab. COVID-19 isolation units of Hospitals Patient rooms (1–3 patients in mild condition): 21 samples Ventilated patients' rooms (invasive and non-invasive: 13 samples Patient's toilets: 4 samples Nurse station: 8 samples Doffing area: 4 samples Quarantine hotel for asymptomatic and mild COVID-19 patients Hotel room: 21 samples Public spaces: 21 samples |
Yes | Patients stayed in private rooms either
alone or as a family, but were free to move around the hotel and socialize in public spaces. Viral culture method: Vero E6 cells. CPE observed after 5 days. |
| Bloise 2020 | Laboratory
Spain |
High-touch surfaces: Landline, barcode
scanner, mobile phone, mouse, keyboard, environmental |
22 | No | |
| Cheng 2020 | Environmental surveillance
in hospital, Hong Kong |
Bench, bedside rail, locker, bed table,
alcohol dispenser, and window bench |
Not reported | No | Close contact referred to those with
unprotected exposure, defined as HCWs who had provided care for a case patient with inappropriate PPE and patients who had stayed within the same cubicle of the index case regardless of the duration of exposure. |
| Cheng 2020a | Hospital AIIRs in China
February-March 2020 |
Bed rail, locker, bed table, toilet door
handle, and the patient’s mobile phone |
377 | No | 21 patients. 12 air changes per hour.
Samples were collected before daily environmental disinfection. |
| Chia 2020 | Hospital rooms of infected
patients Singapore |
Floor, bedrail, locker handle, cardiac table,
electric switch, chair, toilet seat and flush, air exhaust vent |
245 | No | 12 air changes per hour. |
| Colaneri 2020 | Referral hospital in
Northern Italy 21 to 29 February 2020 |
Buffer zone of patients' rooms: Door
handles, waste container covers, sink handles, wall surfaces Doctors' and nurses' lounge: Kitchen table and sink, desks, computer keyboards, medical charts and parameters, tabs, door handles, therapy trolleys Staff personal belongings: Mobile phones |
16 | No | HCWs involved in the direct care of
patients used PPE. Standard cleaning procedures were in place. |
|
Colaneri
2020a |
Infectious Disease
Emergency Unit of a hospital in Italy |
Rooms of patients with CPAP helmet, room
of patient in high-flow oxygen therapy, PPE, staff equipment |
26 | Yes | HCWs involved in the direct care of
patients used PPE. Standard cleaning procedures were in place. Air change in our wards is typically 7 volumes per hour. Swabs were performed around 12 noon, approximately 4 hours after cleaning. Viral culture method: Vero E6 cell line. CPE observed at 7 days |
| D'Accolti 2020 | Acute COVID-19 ward of an
Italian hospital |
Inside: Floor, bedside table, bathroom sink,
and bed headboard Outside: Ward corridor, nurse area and door, and warehouse shelves |
22 | No | Standard cleaning procedures twice
daily in the morning and afternoon. Sampling was performed seven hours after cleaning. All staff wore PPE. |
|
Declementi
2020 |
COVID-19 non-Intensive
Care Unit Italy May 2020 |
Bed rail, sheets and pillow, floor and wall
within 1m of bed, surgical mask, disposable gowns |
24 | No | Sampling: 1st day - 18 hrs after
disinfection; 2nd day - 24 hrs. 12 samples were collected before extra- ordinary sanitization procedures and 12 after extra-ordinary sanitization procedures. |
| Ding 2020 | The Second Hospital of
Nanjing, China February 2020 |
Four isolation rooms, a nursing station, a
corridor, an air-conditioning system, and other spaces in the airborne infectious- disease |
107 | No | 10 patients. A sample was defined as
positive at a Ct ≤38, and weakly positive at a Ct of 37–38. All HCWs used PPE. Sampling done before disinfection. Cleaning and disinfection of these rooms was conducted twice daily. |
| Döhla 2020 | High-prevalence
community setting with Germany's first largest high-prevalence cluster with regard to COVID-19 known at that point of time in March 2020 |
Electronic devices, Knobs and handles,
Plants and animals, Furniture, Food and drinks, Clothing |
119 | Yes | Quarantined households. No
standardised environmental sampling was carried out. No characterization of cleaning methods or materials was performed. Viral culture method: Vero E6 cells. CPE observed after "several days". |
|
Escudero
2020 |
Multipurpose ICU and a
cardiac ICU in Spain All patients had high level of disease severity 16 to 27 April 2020 |
Door knob, chair telephone, computer
keyboard, computer mouse, sink faucet, perfusion pump, cart, door handle, ICU workers’ shoe sole, table bench, bed, bed rail, mattress, ventilator, bag valve mask, BP cuff, ECG electrodes, oxygen supply system, sling, waste container, tracheal tube |
102 | No | All the ICU units were equipped with
negative pressure of –10 Pa and an air flow circuit with circulation from the central area to the boxes with an air change rate of 20 cycles/hour. All staff used PPE. Standard cleaning was performed twice daily (morning and afternoon). |
| Feng 2020 | Frequently touched
surfaces in hospital isolation wards China 13/02/2020 to 05/03/2020 |
Public surfaces in the isolation room:
Door handles, window handle, lavatory door handle, lavatory floor, lavatory floor drain, toilet seat, toilet flush button, and faucet Private surfaces in the isolation room: Patient’s toothbrush, mouthwash cup, towel, pillow, bed sheet, bedrails, bedside table, bedside wall above the patient’s head, bedside floor, kettle handle, and cup |
202
Private surfaces: 132 Public surfaces: 70 |
Yes | Viral culture method: Not specified |
|
Fernández-
de-Mera 2020 |
Isolated rural community
in Spain with a high COVID‐19 prevalence 13/05/2020 and 05/06/2020 |
Households:
Toothpaste tubes, fridge and
oven handles, and the main door handle Public service areas: Keyboards, tables, chairs, refrigerators and entry door handles |
55 | No | |
| Ge 2020 | Hospital wards in 3
different hospitals (ICU plus hospital ward) February 2020 |
Door handle, computer keyboard, nurses'
station , urinal, bedhead, passage way, weighing scale, handrail, medical record rack |
112 | No | The 3 hospitals with different protection
levels. Ct value <40 was considered as positive. Routine disinfection was acted every 4 h in ICU. Samples collected 1–3 times in surfaces across sites |
| Guo 2020 | Hospital Wards, Wuhan,
China February 19 through March 2, 2020 |
Floors, computer mice, trash cans,
sickbed handrails, patient masks, personal protective equipment, and air outlets |
105 | No | |
| Harvey 2020 | Public locations and
essential businesses Massachusetts, USA March-June 2020 |
High-touch nonporous surfaces likely to be
contaminated with SARS-CoV-2 during an outbreak: a trash can, liquor store, bank, metro entrance, grocery store, gas station, laundromat, restaurant, convenience store, post office box, and crosswalks. |
348 | No | Observed a total of 1815 people and 781
bare-hand touches across all sites from April 23 to June 23. Mean temperature on sampling days was 17°C, the mean relative humidity 61% |
| Hu 2020 | Hospitals with COVID-19
patients in Wuhan, China 16 February to 14 March, 2020 |
Cabinet, patient's bedrail, door handle and
patient monitor |
24 | No | |
| Hu 2020a | Quarantine room,
Qingdao, China Before and after study March 2020 |
Corridor, bathroom, bedroom, living room
- high-frequency touch surfaces |
46 | No | All sites were sampled 3 times - 1st
sample 4 h after case confirmation; subsequent samples were taken within 24 h after every disinfection. A Ct value <37 was defined as positive, Ct value of ≥40 was defined as a negative. |
| Jerry 2020 | ED, ICU, HDU, 6 medical
wards Dublin, Ireland 5th May and 15th May 2020 |
Patient room housing a laboratory-
confirmed COVID-19 patient; empty patient room following terminal cleaning and UVC decontamination carried out after the discharge of a laboratory-confirmed COVID-19 case; and the nurses' station of each of the wards with COVID-19 patients. |
81 | No | Timing of surface swab samples was
determined by passage of time from most recent clean. COVID-19 patient rooms were cleaned once daily and nurses' station areas twice. For swabs of these areas, a minimum time of 4 h was allowed to elapse before samples were taken |
| Jiang 2020 | 2 isolation areas at the
First Hospital of Jilin University, China |
Door handle, general surface, consulting
rooms, observation rooms, laboratory, buffer room, keyboard, thermometers, window frames, PPE |
130 | No | 15 patients. |
| Jiang 2020a | 2 rooms of a quarantine
hotel China March 2020 |
Door handle, light switch, faucet, bathroom
door handle, toilet seat, flush handle, thermometer, TV remote, pillow cover, duvet cover, sheet, towel |
22 | No | 2 patients. Ct <40 was considered
positive. |
| Jin 2020 | ICU in hospital, China
March 11, 2020 |
Armrests on the patient’s bed, desk surface
of patient’s ward area, door handles, desk surface of the nurse's station, computer keyboard at the nurse's station |
5 | No | The ICU was routinely cleaned three
times daily. All staff wore PPE. Sampling done 2 hours after the completion of routine cleaning |
| Kim 2020 | Hospitalised patients with
COVID-19, South Korea March 25 to April 8, 2020 |
Bed rails, medical carts, the floor, door
handles, the bathroom sink, the toilet, and other fomites (e.g., cell phones, intercoms, and TV remote controllers) |
220 | No | Medical staff used PPE and everyone
in the hospital was encouraged to wear masks and follow hand hygiene practices. |
| Lee 2020 | 6 hospitals and 2 mass
facilities in South Korea February-March 2020 |
Frequently touched surfaces in wards
(telephones, bedrails, chairs, and door handle) and communal facilities of COVID- 19 patients in the hospital. |
80 | No | Disinfection and cleaning had been
performed by the local health centers before samples were collected from hospitals. No prior disinfection and cleaning procedures in mass facilities. Ct<35 was considered positive |
| Lei 2020 | ICU and an isolation ward
for COVID‐19 patients, China |
Patient areas:
Floor, bedrail, bedside table,
patient clothing, bedsheet, control panel of ventilator, ventilator outdoor valve, mobile phone, toilet, bathroom door handle, sink faucet handles Healthcare workers area: Changing room door handle, floor, sink faucets, keyboard mouse of mobile computer, handle of mop used by the cleaning staff |
182 | No | Two samples collected in the morning.
Average air changes per hour were 240–360. The floor of the ICU was cleaned twice a day, at 11 am and 3 pm The furniture and equipment in the ward are also cleaned once a day at 11 am. CT<40 was considered positive. |
| Lui 2020 | Hospital in Hong Kong | Disposable chopsticks | 14 | No | 5 consecutive asymptomatic and
postsymptomatic patients. |
| Lv 2020 | Laboratory, China, Feb and
Mar 2020 |
Door handle, elevator buttons, handles of
sample transport boxes, surfaces of lab testing equipments, PPE, lab floor |
61 | No | |
| Ma 2020 | COVID-19 patients in ICU
and hospital wards in China |
COVID-19 patients:
Toilet seat and handle,
patient transport cart, floor, pillowcase, corridor handrail, seat pedal, hands, ventilation duct, computer keyboard, faucet handle, toilet flush button, remote control, table top, door handle Control group: Table top, pillow towel, mobile phone, toilet pit, toilet exhaust fan |
242 | No | |
| Maestre 2020 | 2 home-quarantined
subjects in the USA |
Floors, toilet door handle, AC filter, sink
handle, toilet seat, door knob, refrigerator handle, high chair, phone screen, couch TV top surface, dining table |
22 | No | Home was naturally ventilated one
hour per day, in the early morning; HVAC temperature setting was kept at 23.9°C day and night with the air conditioner; average relative humidity 56.6%. 1 home was cleaned daily; other home was cleaned 2–3 times/week. Samples collected 2 months after onset of symptoms (one month after COVID-19 symptoms had resolved in the household) |
|
Marshall
2020 |
9 workplace locations in
Europe and the USA |
24 high-frequency-touch point surfaces:
Office desks, door handles, entrance push button, faucet handles, log book, control panels, file drawer handle, mouse, keyboard, elevator button, refrigerator handle, work bench, plastic bin |
Locations with positive employees: 2400 Locations without positive employees: 3000 |
No | Sampling occurred near the end of
work shifts and before surfaces were cleaned and disinfected. Five surfaces were swabbed daily during the study and were considered the 5 greatest-risk sentinel surfaces. Ten surfaces were swabbed daily and were rotated among the remaining locations and were considered systematic surfaces. Ct≤38 was considered positive. Both RT-PCR and serology. |
| Moore 2020 | Hospitalised patient in
the UK 3rd March 2020 to 12th May 2020 |
Toilet door handle, door handle, nurse call
button, portable vital signs monitor, bed rail, bed control, monitor, syringe driver, bedside computer, chair arm, curtain, window sill, air vent, trolley drawer |
336 | Yes | 11 negative pressure isolation rooms.
Viral culture method: Vero E6 cells. CPE observed at 7 days. |
|
Nakamura
2020 |
Hospitalised COVID-
positive patients in Japan January 29th to February 29th, 2020 |
Ventilation exits, phones, tablets, masks,
PPE, stethoscopes, blood pressure cuffs, intubation tubes, infusion pump, pillows, TV remote controls, bed remote controls, syringes, patient clothes, personal data assistants, personal computers, computer mouse, consent form paper, patient palm, pulse oximeter probe, door knobs, bed guardrails, over tables, touch screen of ventilator, monitor, nurse call buttons, TV, curtains, toilet seats, hand soap dispensers, window sill, exhaust port, door sensor |
141 | No | Environmental samples from all rooms
(except Room 2) were collected after 6–8 hours of daily room cleaning and disinfection. Room 2 was cleaned and items were disinfected at least once a day. |
| Nelson 2020 | Long-term care facilities
undergoing COVID-19 outbreaks, Canada |
High-touch surfaces, communal sites, and
mobile medical equipment |
89 | No | |
| Ong 2020 | ICU ward of hospital in
Singapore |
Bedrail, floor, stethoscope, surgical
pendant, ventilators, air outlet vents, infusion pumps, glass window, cardiac table |
200 | Yes | Routine twice-daily environmental
cleaning. All sampling was conducted in the morning before the scheduled environmental cleaning (ie, the last cleaning time was the afternoon prior to environmental sampling). Viral culture method: Vero C1008 cells. CPE observed at 7 days. |
| Ong 2020a | Dedicated SARS-CoV-2
outbreak center (isolation rooms) in Singapore Jan-Feb 2020 |
Infection isolation rooms (12 air exchanges
per hour) with anterooms and bathrooms, PPE |
38 | No | One patient’s room was sampled
before routine cleaning and 2 patients’ rooms after routine cleaning. Twice- daily cleaning of high-touch areas was done using 5000 ppm of sodium dichloroisocyanurate. The floor was cleaned daily. |
| Ong 2020b | HCWs caring for confirmed
COVID-19 patients in a hospital in Singapore |
PPE | 90 | No | 15 patients. The median time spent by
HCWs in the patient’s room overall was 6 minutes (IQR, 5–10). Activities ranged from casual contact (eg, administering medications or cleaning) to closer contact (eg, physical examination or collection of respiratory samples).Gloves and gowns were not swabbed because these are disposed after each use. |
|
Pasquarella
2020 |
Single hospital room with
elderly COVID-19 patient Italy |
Right bed rail, the call button, the bed
trapeze bar, the stethoscope; moreover, the patient’s inner surgical mask |
15 | No | Surfaces sampling was carried out two
days after the patient’s second positive swab (Ct 24), 7 days after hospitalization. The surfaces sampling was carried out 2 hours after cleaning and disinfection procedures. |
| Peyrony 2020 | ED at a university hospital,
France April 1 to April 8, 2020 |
Patient care area: Stretchers, cuffs for
arterial blood pressure measurement, pulse oximeter clips, stethoscopes, ECG or ultrasound (US) devices, trolleys, monitor screens, benches, inside door handle, oxygen delivery manometer, plastic screen between patients, and floor. Non-patient care area: Patients waiting room, corridor with personal protective equipment (PPE) storage, staff working rooms, refreshment room, toilets, changing room, research office and medical equipment stockroom |
192 | Yes | Air exchange rate in the different rooms
where the samples were made ranged from 1 to 7 m3/h and room sizes from 30 to 60 m3, thus the entire air renewal duration of these rooms could range from 4 to more than 24 h. Monitoring room and staff working rooms were regularly decontaminated every 2 or 3 h. HCWs wore PPEs. Viral culture method: Not specified |
| Piana 2020 | Hospital in Italy
May-June 2020 |
Indoor surfaces from three COVID-
reference hospitals, buildings open to public use (1 office, 1 fast food, 1 church), outdoor areas, used handkerchiefs with nasopharyngeal secretions. |
92 | No | CT values ≤40 were considered positive. |
| Razzini 2020 | COVID-19 ward of hospital
in Italy May 12, 2020 |
Corridor for patients, ICU, undressing
room, locker/passage for medical staff, dressing room |
37 | No | Negative airflow system. Sampling
was carried out before daily cleaning operations. Temperature was 20° to 22 °C and relative humidity 40 to 60%. Medical and paramedical staff used PPE. Ct value was ≤40 was considered positive. |
| Ryu 2020 | 2 different hospital settings
in South Korea March 2020 |
Patient monitor, ventilator monitor, HFNC,
blood pressure cuff, pillow, suction bottle and line, Ambu bag, infusion pump, wall oxygen supply, fluid stand, door button or knob, bed side rail, head and foot of the bed, nurse call controller, lower part of the window frame, top of the television [TV], air exhaust damper, wall and floor of the room, toilet paper holder, and inside and seat of the toilet); the anteroom (ie, door button, keyboard, mouse, and floor); the floor of an adjacent common corridor; and the nursing station (ie, counter, interphone, keyboard, mouse, chair, and floor). |
No | No | Negative pressure rooms (A); 2
common 4-bed rooms without negative pressure and ventilation systems (B). Room cleaning, and disinfection were not performed every day due to the shortage of PPE and vague fears of cleaners. |
|
Santarpia
2020 |
Residential isolation rooms
housing individuals testing positive for SARS-CoV-2, USA |
Personal items, remote controls, toilets,
floor, bedside table, bedrail |
Non-specific (121 surface and
aerosol samples) |
Yes | Negative-pressure rooms (>12 ACH);
negative-pressure hallways; key-card access control; unit-specific infection prevention and control (IPC) protocols including hand hygiene and changing of gloves between rooms; and PPE for staff that included contact and aerosol protection. Viral culture method: Vero E6 cells. CPE observed 3–4 days |
|
Seyedmehdi
2020 |
Cross-sectional study
Covid-19 ICU ward, Iran April 29, 2020 |
Not specified | 10 | No | Surface disinfection was performed
three times a day. Air temperature 24°C, humidity 35%, air pressure 1005 mb and air velocity of 0.09 m/s. All the staff used conventional PPE. |
| Shin 2020 | Chungbuk National
University Hospital, South Korea April 2020 |
Bedside table, bed rail, mobile phone,
tablet, call bell attached to bed, floor, door handle, sink (bathroom), toilet bowel |
12 | No | Mother and daughter who were COVID-
positive. The most recent cleaning had occurred 4 days prior to environmental sample collection. A cycle threshold (Ct) value <40 is reported as positive. |
| Suzuki 2020 | Cross-sectional study
Cruise ship, Japan February 2020 |
Light switch, toilet seat, toilet floor, chair
arm, TV remote, phone, table, door knob, pillow |
601 | Yes | Median highest and lowest temperature
13.0°C (range 6.5-18.5) and 5.5°C (0.0-9.3); median highest and lowest humidity 73 (41–98) and 40 (17–76)%. Samples collected prior to disinfection of the vessel. Case-cabins disinfected prior to sampling. Air re-circulation turned off. Subjects confined to cabins but allowed 60 mins daily walk on the deck while wearing masks and 1m social distance. Viral culture method: VeroE6/ TMPRSS2. CPE observation time after 4 days. |
| Wang 2020 | Wuhan Leishenshan
Hospital in Wuhan, China March 2020 |
ICU, treatment room, laundry room,
handwashing sink, nurses' station, dialysis machine, PPE, air outlet, door handles, bed rails, dustbin, bedstand, infusion pump |
62 | No | 7 COVID-19 patients. Negative pressure
isolation ward for patients. Surfaces of objects were cleaned and disinfected 4 times/day. Diagnostic and treatment equipment were cleaned after each use. |
| Wang 2020a | Isolation wards in the
First Affiliated Hospital of Zhejiang University, China February 2020 |
Isolation ICU ward and Isolation wards,
including the clean area, the semi- contaminated area, and the contaminated area; front surface of N95 masks and gloves of staffs in isolation wards |
45 | Yes | 33 laboratory-confirmed COVID-19
patients. Surfaces of objects were disinfected every 4 h in Isolation ICU ward and every 8 h in general Isolation wards. The isolation rooms were not under negative pressure. A sample was considered positive when the qRT-PCR Ct value was ≤40. Viral culture method: Vero-E6 cells. CPE was observed after 96 h. |
| Wee 2020 | Dedicated isolation
wards at tertiary hospital, Singapore February-May 2020 |
High-touch areas in the patient's
immediate vicinity, toilet facilities |
445 | No | 28 patients. Sterile premoistened swab
sticks used to swab high-touch areas for 2–3 minutes over a large surface. Environmental sampling was done in the rooms to test for SARS-CoV-2 prior to terminal cleaning |
| Wei 2020 | Non-ICU rooms in a
designated isolation ward in Chengdu, China April 2020 |
Bedrails, room and toilet door handles,
light switches, foot flush buttons, sink rims, sink and toilet bowls and drains, bedside tables, bedsheets, pillows, equipment belts on walls, floors, and air exhaust outlets |
112 | No | 10 COVID-positive patients. Negative
air pressure rooms. Rooms and toilets were cleaned and disinfected twice daily. Samples collected 4 to 7 h after the first daily cleaning. |
| Wei 2020a | Non-ICU isolation ward
China March 2020 |
High-touch areas and floors in patient
rooms and toilets, HCWs PPE |
93 | No | Surfaces cleaned/disinfected twice daily.
Samples collected before the first daily cleaning. Patients had prolonged (> 30 day) SARS-CoV-2 PCR positive status for clinical samples |
| Wong 2020 | Non-healthcare settings in
Singapore February-March 2020 |
Accommodation rooms, toilets and
elevators that have been used by COVID-19 cases |
428 | No | All samples were taken after the infected
persons vacated the sites and have been isolated in healthcare facilities. Half of surface swabs were taken before the cleaning and disinfection and the other half was taken after the disinfection procedure. Mechanical ventilation, ambient temperature and fan-coil unit. |
| Wu 2020 | Wuhan Hospital, China
January 2020 |
Beeper, keyboard, computer mouse,
telephone, door handle, desktop, medical equipment, bedrail, bedside table, oxygen cylinder valve, elevator button, and others such as refrigerator, IV port, and sample transfer box. |
200 | No | All samples were collected around
8:00 AM before routine cleaning and disinfection. HCWs used PPE. A sample was considered positive when the Ct value was ≤43. |
| Ye 2020 | Zhongnan Medical Center
in Wuhan, China February 2020 |
Major hospital function zones, hospital
equipment/objects and medical supplies, PPE, administrative areas, and the parking lot. |
626 | No | Three sets of surface samples were
collected using dacron swabs across major hospital function zones, hospital equipment/objects and medical supplies, and HCW's used PPE. |
| Yuan 2020 | Hospital in Wuhan, China
March 11 to March 19, 2020 |
High-frequency contacted surfaces in the
contaminated area and the surfaces of medical staff's PPE |
38 | No | Samples collected 4 hours after morning
disinfection of the disease area. High- flow exhaust fans on their windows and at the end of the corridor of the contaminated area to discharge the air out to the open outdoor area; natural new air inlet, to ensure that the indoor air ventilation 18 to 20 times per hour. Use of PPE by HCWs |
| Yung 2020 | Hospital in Singapore | Bedding; the cot rail; a table situated 1
meter away from the infant's bed; and the HCW's face shield, N95 mask, and waterproof gown |
6 | No | Infant with COVID-19. 1 HCW. Ct values
<36 were considered positive. |
| Zhang 2020 | Hospital outdoor
environment China February-March 2020 |
Entrance, outdoor toilet, background, in-
and out-patient department |
13 | No | |
| Zhou 2020 | Hospital in London, UK
April 2 to 20, 2020 |
Bedrails, BP monitors, ward telephones,
computer keyboards, clinical equipment (syringe pumps, urinary catheters), hand- cleaning facilities (hand washing basins, alcohol gel dispensers, nonpatient care areas (i.e. nursing stations and staff rooms) |
218 | Yes | Sampling was conducted during three
tracheostomy procedures. High touch surfaces disinfected twice daily, other surfaces once daily. Viral culture method: Vero E6. CPE observed at 5–7 days. |
| Zhou 2020a | Hospital in Wuhan, China | Nosocomial surfaces, medical touching
surfaces, delivery window, shoe cabinet, patient touching surfaces, clean area surfaces, hospital floor |
318 | No | |
|
Zuckerman
2020 |
Virology Laboratory, Israel
March 15th 2020 |
Door knobs, the outer surface of all
equipment in the room, etc., with special attention to “high-touched areas |
6 | No |
Table 2. Systematic review characteristics.
| Study ID | Objective | Databases
searched |
Search
dates |
Assessment
of reporting quality |
No. of
included studies |
Main results | Key conclusions |
|---|---|---|---|---|---|---|---|
|
Bedrosian
2020 |
To assess the
effectiveness of hygiene interventions against SARS- CoV-2 |
1. NIH COVID-19 Portfolio; 2. CDC COVID- 19 Research Articles Downloadable Database |
22/01/2020
to 10/06/2020; 10/06/2020 to 10/07/2020 |
Not reported | 35 | No study assessed viral infectivity or
viability, but all tested the presence or absence of SARS-CoV-2 RNA. Healthcare settings were most frequently tested (25/35, 71.4%), with households being the least tested (2/35, 5.7%). Laboratories reported the highest frequency of contaminated surfaces (20.5%, 17/ 83), while households of COVID-19 patients had the lowest frequency (2.5%, 4/161). |
There is an inability to align SARS-CoV-2
contaminated surfaces with survivability data. There is a knowledge gap on fomite contribution to SARS-COV-2 transmission and a need for testing method standardization to ensure data comparability. There is a need for testing method standardization to ensure data comparability. |
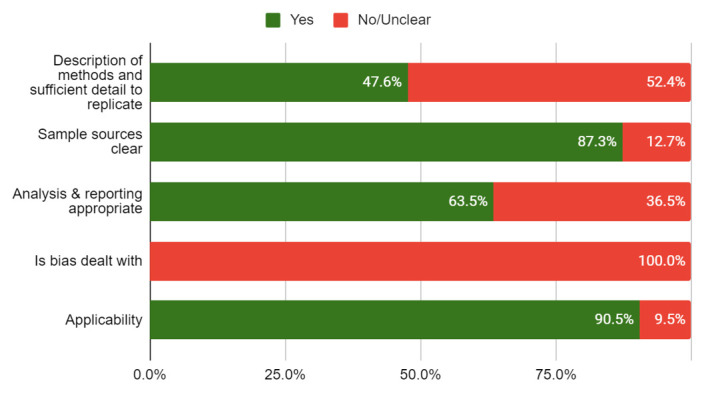
Quality of included studies
None of the included studies were linked to or mentioned a published protocol. The risk of bias of the included studies is shown in Table 4. Less than half of the studies (47.6%) adequately reported the methods used, and none used methods to minimise bias. The overall quality of the studies was rated low to moderate (see Figure 2).
Figure 2. Risk of bias (n=63 primary studies).
Reviews
We found one “systematic review” investigating the role of fomites [Bedrosian 2020] ( Table 2). The authors searched two electronic sources - articles were last downloaded on July 10, 2020. There was no published protocol, and the authors did not assess the quality of included studies. A total of 35 relevant studies were included. Over half of the studies (25/35, 75%) were conducted in healthcare settings, and four compared environmental contamination before and after standard disinfection procedures. No study assessed viral infectivity or viability, but all tested the presence or absence of SARS-CoV-2 RNA.
Primary studies
We found 63 primary studies ( Table 1). In general, the studies did not report any hypothesis but investigated epidemiological or mechanistic evidence for fomite transmission. Forty-one studies (65.1%) were conducted in Asia, 15 (23.8%) in Europe, five (7.9%) in North America, and one each in Africa and South America (1.6% each). A total of 44 studies were conducted exclusively in hospital settings, two in hospital and quarantine facilities, three in the laboratory, and the remaining in other non-healthcare settings (public places, community, banknotes, workplace, cruise ship, quarantine rooms and hospital outdoors). Four studies were conducted exclusively in ICU and another three in ICU plus hospital wards. Five studies used before and after study design.
In 59 studies (96.7%), fomite transmission was examined in high-frequency touch surfaces ( Table 1); the remaining four studies examined circulating banknotes (1), disposable chopsticks (1) hospital staff PPE (1), and unspecified (1). The timing and frequency of sample collection and disinfection procedures were heterogeneous across studies (see Table 3). Fourteen studies (23%) performed sample collection before disinfection procedures, five studies collected samples before and after disinfection procedures, while 11 studies collected samples after disinfection. In 33 studies, the timing of sampling in relation to disinfection was not specified. In one study [Ryu 2020], disinfection procedures were not performed as required because of a lack of PPE and staff being afraid of contracting SARS-CoV-2. The number of samples per study ranged from five [Jin 2020] to 5400 [Marshall 2020].
Table 3. Studies sample collection characteristics.
| Study ID | Frequency of
sample collection |
Timing of sample collection |
|---|---|---|
| Abrahão 2020 | Not specified | Not specified |
| Akter 2020 | NA | N/A |
| Amoah 2020 | Twice | Unspecified |
| Ben-Shmuel 2020 | Not specified | Not specified |
| Bloise 2020 | Not specified | Not specified |
| Cheng 2020 | Not specified | Not specified |
| Cheng 2020a | Once | Before daily disinfection |
| Chia 2020 | Not specified | Not specified |
| Colaneri 2020 | Not specified | Not specified |
| Colaneri 2020a | Once | After disinfection |
| D'Accolti 2020 | Not specified | After disinfection |
| Declementi 2020 | Twice | After disinfection |
| Ding 2020 | Not specified | Before disinfection |
| Döhla 2020 | Not specified | Not specified |
| Escudero 2020 | Not specified | Not specified |
| Feng 2020 | Not specified | Not specified |
| Fernández-de-Mera 2020 | Not specified | Not specified |
| Ge 2020 | 1 to 3 times | Not specified |
| Guo 2020 | Not specified | Not specified |
| Harvey 2020 | Twice: Pilot phase
and full-scale phase |
N/A |
| Hu 2020 | Not specified | Not specified |
| Hu 2020a | 3 times | 4 h after case confirmation |
| Jerry 2020 | Not specified | 4 h after disinfection |
| Jiang 2020 | Not specified | Not specified |
| Jiang 2020a | Not specified | Before disinfection |
| Jin 2020 | Not specified | 2 h after disinfection |
| Kim 2020 | Not specified | Not specified |
| Lee 2020 | Not specified | After disinfection (hospital)
Before disinfection (mass facilities) |
| Lei 2020 | Twice | Before disinfection |
| Lui 2020 | N/A | N/A |
| Lv 2020 | Not specified | Not specified |
| Ma 2020 | Not specified | Not specified |
| Maestre 2020 | Not specified | Not specified |
| Marshall 2020 | End of work shift | Before disinfection |
| Moore 2020 | Not specified | Not specified |
| Nakamura 2020 | Not specified | After disinfection |
| Nelson 2020 | Not specified | Not specified |
| Ong 2020 | 5 separate time
points |
Before disinfection |
| Ong 2020a | 5 days over a
2-week period |
Before and after (33.3%:66.7%) |
| Ong 2020b | Not specified | Not specified |
| Pasquarella 2020 | Once | After disinfection |
| Peyrony 2020 | Not specified | Not specified |
| Piana 2020 | Not specified | Before disinfection |
| Razzini 2020 | Not specified | Before disinfection |
| Ryu 2020 | Not specified | Not specified |
| Santarpia 2020 | Not specified | Not specified |
| Seyedmehdi 2020 | Not specified | Not specified |
| Shin 2020 | Twice daily | After disinfection (4 days) |
| Suzuki 2020 | Not specified | Before disinfection |
| Wang 2020 | Not specified | Not specified |
| Wang 2020a | Not specified | Not specified |
| Wee 2020 | Not specified | Before disinfection |
| Wei 2020 | Not specified | After disinfection |
| Wei 2020a | Not specified | Before disinfection |
| Wong 2020 | Not specified | Before and after (50%:50%) |
| Wu 2020 | Not specified | Before disinfection |
| Ye 2020 | Three sets over a
20-day period |
Not specified |
| Yuan 2020 | Not specified | After disinfection |
| Yung 2020 | Not specified | Not specified |
| Zhang 2020 | Not specified | Not specified |
| Zhou 2020 | Not specified | Not specified |
| Zhou 2020a | Not specified | Not specified |
| Zuckerman 2020 | Not specified | Before disinfection |
Table 4. Quality of included studies.
| Study | Description of
methods and sufficient detail to replicate |
Sample
sources clear |
Analysis &
reporting appropriate |
Is bias
dealt with |
Applicability |
|---|---|---|---|---|---|
| Abrahão 2020 | Unclear | Yes | Yes | Unclear | Yes |
| Akter 2020 | Yes | Yes | Yes | Unclear | Yes |
| Amoah 2020 | Unclear | Yes | Yes | No | Yes |
| Bloise 2020 | Unclear | Yes | Unclear | No | Yes |
| Ben-Shmuel 2020 | Yes | Yes | Yes | Unclear | Yes |
| Cheng 2020 | Unclear | Yes | Yes | No | Yes |
| Cheng 2020a | Unclear | Yes | Yes | Unclear | Yes |
| Chia 2020 | No | Yes | Yes | Unclear | Yes |
| Colaneri 2020 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Colaneri 2020a | Yes | Yes | Unclear | Unclear | Yes |
| D'Accolti 2020 | Yes | Yes | No | No | Yes |
| Declementi 2020 | Unclear | Yes | Yes | Unclear | Yes |
| Ding 2020 | Yes | Yes | Yes | Unclear | Yes |
| Döhla 2020 | Unclear | Unclear | Yes | No | Yes |
| Escudero 2020 | Yes | Yes | Yes | Unclear | Yes |
| Feng 2020 | Unclear | Yes | Unclear | Unclear | Yes |
| Fernández-de-Mera 2020 | Unclear | Yes | Unclear | No | Yes |
| Ge 2020 | Unclear | Yes | Unclear | Unclear | Yes |
| Guo 2020 | Unclear | Yes | Unclear | No | Unclear |
| Harvey 2020 | Yes | Yes | Yes | Unclear | Yes |
| Hu 2020 | No | Unclear | No | No | Unclear |
| Hu 2020a | Unclear | Yes | Yes | Unclear | Yes |
| Jerry 2020 | Yes | Yes | Yes | Unclear | Yes |
| Jiang 2020 | Yes | Yes | Unclear | Unclear | Yes |
| Jiang 2020a | Unclear | Yes | Yes | No | Yes |
| Jin 2020 | Yes | Yes | Unclear | Unclear | Yes |
| Kim 2020 | Yes | Yes | Unclear | Unclear | Yes |
| Lee 2020 | Unclear | Yes | Yes | Unclear | Yes |
| Lei 2020 | Unclear | Yes | Yes | No | Yes |
| Lui 2020 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Lv 2020 | Yes | Yes | Unclear | No | Yes |
| Ma 2020 | No | Unclear | Yes | No | Yes |
| Maestre 2020 | Yes | Yes | Yes | Unclear | Yes |
| Marshall 2020 | Unclear | Yes | Yes | Unclear | Yes |
| Moore 2020 | Yes | Yes | Yes | Unclear | Yes |
| Nakamura 2020 | Yes | Yes | Yes | Unclear | Yes |
| Nelson 2020 | Unclear | Yes | Unclear | Unclear | Yes |
| Ong 2020 | Yes | Yes | Yes | No | Yes |
| Ong 2020a | Unclear | Yes | Yes | Unclear | Yes |
| Ong 2020b | Unclear | Yes | Unclear | Unclear | Yes |
| Pasquarella 2020 | Unclear | Yes | Unclear | Unclear | Yes |
| Peyrony 2020 | Yes | Yes | Yes | Unclear | Yes |
| Piana 2020 | Yes | Yes | Yes | Unclear | Yes |
| Razzini 2020 | Yes | Yes | Yes | Unclear | Yes |
| Ryu 2020 | Yes | Yes | Yes | Unclear | Yes |
| Santarpia 2020 | Yes | Yes | Unclear | Unclear | Yes |
| Seyedmehdi 2020 | No | Unclear | Unclear | No | Unclear |
| Shin 2020 | Unclear | Unclear | Yes | Unclear | Yes |
| Suzuki 2020 | Yes | Yes | Unclear | Unclear | Yes |
| Wee 2020 | Yes | Yes | Yes | Unclear | Yes |
| Wei 2020 | Yes | Yes | Unclear | Unclear | Yes |
| Wei 2020a | Unclear | Yes | Yes | Unclear | Yes |
| Wang 2020 | Yes | Yes | Yes | Unclear | Yes |
| Wang 2020a | Unclear | Yes | Yes | Unclear | Yes |
| Wong 2020 | Unclear | Yes | Yes | Unclear | Yes |
| Wu 2020 | Unclear | Yes | Unclear | Unclear | Yes |
| Ye 2020 | Unclear | Yes | Yes | Unclear | Yes |
| Yuan 2020 | Yes | Yes | Yes | Unclear | Yes |
| Yung 2020 | Unclear | Yes | Yes | No | Yes |
| Zhang 2020 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Zhou 2020 | Yes | Yes | Yes | Unclear | Yes |
| Zhou 2020a | Unclear | Yes | Yes | Unclear | Yes |
| Zuckerman 2020 | Yes | Yes | Yes | Unclear | Yes |
| 30 | 55 | 40 | 0 | 57 | |
| 63 | 63 | 63 | 63 | 63 | |
| Yes | No/
Unclear |
||||
|
Description of methods and
sufficient detail to replicate |
47.6% | 52.4% | |||
| Sample sources clear | 87.3% | 12.7% | |||
|
Analysis & reporting
appropriate |
63.5% | 36.5% | |||
| Is bias dealt with | 0.0% | 100.0% | |||
| Applicability | 90.5% | 9.5% |
Eleven studies (17.5%) set out to perform viral cultures; nine of these utilised the Vero E6 cell lines method while two did not specify the method used (see Table 1). Thirteen studies (20.6%) reported cycle thresholds (Ct) for test positivity: ≤40 (8 studies); ≤43 (1 study); <35 (1 study); <36 (1 study); <37 (1 study) and <38 (1 study).
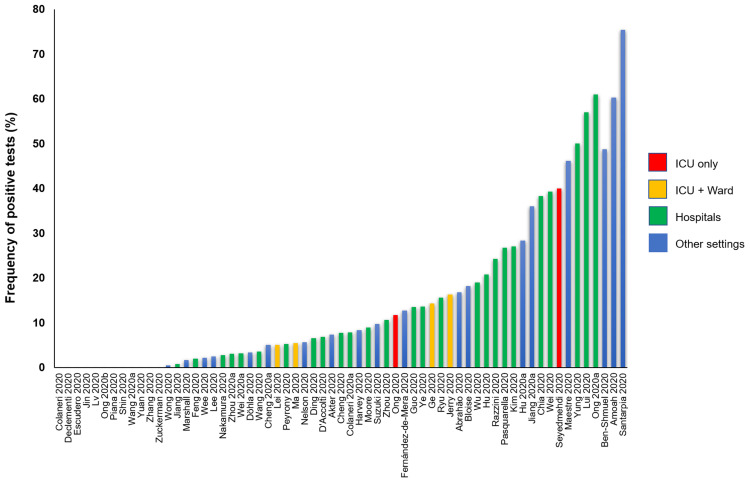
Frequency of SARS-CoV-2 positive test
All studies reported data on the frequency of positive tests ( Table 5). ( Figure 3 shows the graphical representation of these frequencies.) The frequency of positive SARS-CoV-2 tests across 51 studies (via RT-PCR) ranged from 0.5% to 75%; 12 studies (19%) reported no positive tests. The highest frequency of positive tests was found in residential isolation rooms. Of the three studies conducted in ICU [Escudero 2000, Jin 2000, Ong 2000 and Seyedmehdi 2000], two reported positive test results (11.7% and 40%). All the four studies [Lei 2000, Ma 2000, Ge 2020, Jerry 2000] conducted in both ICU and general wards reported positive tests: 5%, 5.4%, 14.3% and 16.3%, respectively. One of the three laboratory studies [Bloise 2000] reported frequency of 18.2%; a second study [Lv 2020] reported no positive test with the conventional RT-PCR tests but reported 21.3% positivity with droplet digital PCR (ddPCR) tests; the third study [Zuckerman 2020] reported no positive tests. In a cross-sectional study of SARS-CoV-2 positive subjects confined to their cabins in a cruise ship [Suzuki 2020], the frequency of positive test was 9.7% (58/601); no positive test was detected in the non-case cabins. In one study of home quarantined subjects [Maestre 2020], 46.2% (12/26) of samples were positive for SARS-CoV-2 at two months (one month after the resolution of symptoms). In another study of two hospital patients who were SARS-CoV-2 positive [Shin 2020], no positive samples were detected after 41 days following weekly disinfection. One study conducted in a high-prevalence community setting [Döhla 2020] reported no significant association in the frequencies of positive tests between human and environmental samples (p=0.76). In all four before and after studies, there was a substantial reduction in the frequency of positive tests after surface disinfection.
Figure 3. Rates of positive SARS-Cov-2 tests in studies assessing fomite transmission.
Table 5. Findings of included studies.
| Study ID | Frequency of COVID-19 positive
tests |
Concentration of
samples |
Cycle Threshold | Viral culture | Notes |
|---|---|---|---|---|---|
| Abrahão 2020 | 17/101 (16.8%) | 70-2990 genomic
units/m 2 |
Not reported | Not performed | Viral load was highest in the hospital front
door ground |
| Akter 2020 | 31/425 (7.3%) | Not reported | CT values increased
significantly with time on banknotes spiked with nasopharyngeal samples (p<0.05) |
Not performed | Banknotes sampled from the ticket vendors
and collectors at inter-city transport (bus) tested negative. |
| Amoah 2020 | Tap handle 68.8%
Toilet floor 60% Toilet seat 60% Cistern handle 60% Internal latch 53.3% |
25.9 to 132.69
gc/cm 2 |
Not reported | Not performed | Viral load was consistently lower with RNA
extraction versus direct detection across all sites, except with floor swab samples. No significant difference in the prevalence across sites (p ≥ 0.05). Significant differences in the concentration between the different contact surfaces (p ≤ 0.05) Use of the toilet facilities 2 to 3 times daily was observed to increase the risks of infection. |
| Bloise 2020 | 4/22 (18.2%) | Not reported | 33.75 to 38.80 | Not performed | qRT-PCR is unable to differentiate between
infectious and non-infectious virus present on fomites |
|
Ben-Shmuel
2020 |
Symptomatic patients:29/55
(52.7%) Asymptomatic patients:16/42 (38%) Hospital isolation units Non-ventilated patients' rooms: 9/21 (43%) Mechanically ventilated patients' rooms: 13/18 (72%) Quarantine hotel:16/42 (38%) |
Not reported | 34 to 37.9 | None of the samples
was culturable. No viable virus was recovered from plastic or metal coupons after 4–14 days of incubation |
On viral-contaminated plastic coupons, titres
of viable virus decreased by 3.5 orders of magnitude after 24 h. On metal coupons a faster reduction of 4 orders of magnitude was observed after 6 h of incubation, and similar levels of viable virus were detected at 24 h. A further decrease in viability on metal surfaces was detected at days 2 and 3. |
| Cheng 2020 | 1/13 (7.7%) | 6.5 × 10
2 copies/
mL of VTM |
Not reported | Not performed | |
| Cheng 2020a | 19/377 (5%) | 1.1 × 10
2 to 9.4 ×
10 4 copies/mL |
Not reported | Not performed | The contamination rate was highest on
patients’ mobile phones (6/77, 7.8%), followed by bed rails (4/74, 5.4%) and toilet door handles (4/76, 5.3%) |
| Chia 2020 | Floor: 65%
Bedrail: 59% Bedside locker: 47% Cardiac table: 40% Toilet seat: 18.5% ICU rooms: 0% |
Not reported | 28.45–35.66 | Not performed | High touch surface contamination occurred
in 10/15 patients (66.7%) in the first week of illness, and 3/15 (20%) beyond the first week of illness (p = 0.01). Presence of surface contamination was higher in week 1 of illness, showed some association with the Ct (P = 0.06), but was not associated with the presence of symptoms. |
| Colaneri 2020 | 0/16 (0%) | Not reported | Not reported | Not performed | |
| Colaneri 2020a | 2/26 (7.8%) | Not reported | Not reported | None of the inoculated
samples induced a cytopathic effect on day 7 of culture. |
|
| D'Accolti 2020 | Inside patients’ rooms: 3/22 (13.6%)
Outside patients’ rooms: 0% |
Not reported | 29.54 to >35 | Not performed | All samples tested positive for IC control,
confirming the appropriate efficiency of the whole analysis process. |
|
Declementi
2020 |
0/24 (0%) | Not reported | Not reported | Not performed | |
| Ding 2020 | 7/107 (6.5%) | 407 to 723 RNA
copies |
36.1 to 37.9 | Not performed | Positive samples were from inside door handle
of the isolation rooms and toilet seat cover |
| Döhla 2020 | 4/152 (3.4 %) | Not reported | Not reported | No infectious virus could
be isolated under cell culture conditions |
No correlation between PCR-positive
environmental samples and PCR-positive human samples, p = 0.76 |
| Escudero 2020 | 0/237 (0%) | Not reported | Not reported | Not performed | |
| Feng 2020 | Private surfaces: 4/132 (3%)
Public surfaces: 0/70 (0%) |
Not reported | Not reported | Could not perform viral
culture due to the low virus quantity in the positive samples. |
|
|
Fernández-de-
Mera 2020 |
7/55 (12.7%) | Not reported | 36.05 to 41.06 | Not performed | |
| Ge 2020 | 16/112 (14.3%) | Not reported | Not reported | Not performed | 15/16 of positive samples were from ICU. |
| Guo 2020 |
Intensive Care Unit:
Contaminated area: 27/70 (43.5%) Semi-contaminated area: 3/33 (8.3%) Clean area: 0/12 (0%) General Ward: Contaminated area: 9/105 (8.6%) Semi-contaminated area: 0/24 (0%) Clean area: 0/46 (0%) |
ICU Contaminated
area: 1.5 × 10 5 to 7.1 × 10 3 copies/ sample NA, not applicable; ND, not determined for other sites |
Not reported | Not performed | The rate of positivity was higher for surfaces
frequently touched by medical staff or patients. The highest rates were for computer mice (ICU 6/8, 75%; GW 1/5, 20%), followed by trash cans (ICU 3/5, 60%; GW 0/8), sickbed handrails (ICU 6/14, 42.9%; GW 0/12), and doorknobs (GW 1/12, 8.3%). |
| Harvey 2020 | 29/348 (8.3%) | Majority of our
positive samples not quantifiable. 2.54 to 102.53 gc/cm 2 |
Not reported | Not performed | The estimated risk of infection from touching
a contaminated surface was low (less than 5 in 10,000). The percent of positive samples per week was inversely associated with daily maximum temperature (p=0.03) and absolute humidity (p=0.02). Temperature was inversely correlated with COVID-19 case numbers (p=0.01). |
| Hu 2020 | 5/24 (20.8%) | Viral RNA ranged
from 1.52 × 10 3 to 4.49 × 10 3 copies/ swab |
Not reported | Not performed | |
| Hu 2020a | 1st batch: 11/23 (47.8%)
2nd batch: 2/23 (8.7%) |
Not reported | 26 to 39 | Not performed | 70% of samples taken from the bedroom
tested positive for SARS-CoV-2, followed by 50% of samples taken from the bathroom and that of 33% from the corridor. The inner walls of toilet bowl and sewer inlet were the most contaminated sites with the highest viral loads. |
| Jerry 2020 | COVID-19 patient room: 11/26
(42.3%) Post-disinfection: 1/25 (4%) Nurses station: 1/29 (3.4%) |
Not reported | Not reported | Not performed | |
| Jiang 2020 | 1/130 (0.8%) | Not reported | Not reported | Not performed | |
| Jiang 2020a | 8/22 (36%) | Not reported | 28.75 to 37.59 | Not performed | All control swab samples were negative for
SARS-CoV-2 RNA. |
| Jin 2020 | 0/5 (0%) | Not reported | Not reported | Not performed | |
| Kim 2020 | All surfaces: 89/320 (27%)
Rooms without routine disinfection: 52/108 (48%) Rooms with routine disinfection: 0% |
Not reported | Ct values varied
across rooms: ≤ 35; > 35 and ≤ 40 |
Not performed | |
| Lee 2020 | Hospitals: 0/68 (0%)
Mass facilities: 2/12 (16.7%) |
Not reported | 27.4 to 34.8 | Not performed | Note: Hospitals were disinfected. |
| Lei 2020 | 9/182 (5%) | Not reported | Patient's facemask
(Ct = 38.6) Floor of a patient's room (Ct = 42.4 and 41.2) Patient's mobile phones (Ct = 44.1 and 41.0) |
Not performed | |
| Lui 2020 | 8/14 (57%) | 3.4 × 10
3 copies/
mL |
Not reported | Not performed | The concentration of SARS-CoV-2 RNA
detected from chopsticks was significantly lower than those of nasopharyngeal swabs and sputum samples, p<0.001 |
| Lv 2020 | qRT-PCR: 0%; ddPCR: 13/61 (21.3%) | From 0.84
copies/cm 2 to 37.4 copies/cm 2 |
Not reported | Not performed | |
| Ma 2020 | All surfaces: 13/242 (5.4%)
Object handles: 0/26 (0%) |
Not reported | 36.38 ± 1.92 | Not performed | |
| 13/242 (5.4%) | 33.5 to 39.54 | Not performed | |||
| Maestre 2020 | 12/26 (46.2%) | 20 copies/cm
2 in
master bedroom used by both occupants |
Not reported | Not performed | The highest SARS-CoV-2 RNA signal was
observed on the top of the TV surface. The surfaces in the bathroom did not yield any SARS-CoV-2 signal, except for the toilet handle. |
| Marshall 2020 | Locations with positive employees:
1.7% Locations without positive employees: 0.13% |
Not reported | 35 to 38 | Not performed | Locations with positive environmental surfaces
had 10 times greater odds (P≤0.05) of having positive employees compared to locations with no positive surfaces. |
| Moore 2020 | 30/336 (8.9%) | 2·2 × 10
5 to 59
genomic copies/ swab |
28·8 to 39·1 | No CPE or a decrease
in Ct values across the course of three serial passages were observed suggesting the samples did not contain infectious virus |
|
|
Nakamura
2020 |
4/141 (2.8%) | 2.96 × 10
3 copies/
swab to 4.78 × 10 3 copies/swab |
Not reported | Not performed | |
| Nelson 2020 | All surfaces: 5/89 (5.6%)
BP cuffs: 5/9 (44%) |
Not reported | 37.38 to 39.18 | Not performed | |
| Ong 2020 | ICU ward common areas: 6/60 (10%)
Staff pantry: 2/15 (13.3%) |
Not reported | Not reported | All samples
in common areas and staff pantry were negative on viral cell culture. |
Viral cell culture was not attempted on patient
room samples due to resource limitations. |
| Ong 2020a | Environmental sites: 17/28 (61%)
PPE: 1/10 (10%) Post-disinfection: 0% |
Not reported | 30.64 to 38.96 | Not performed | |
| Ong 2020b | 0/90 (0%) | Not reported | 20.8 to 32.23 | Not performed | |
|
Pasquarella
2020 |
4/15 (26.7%) | Not reported | 31 to 35 | Not performed | |
| Peyrony 2020 | 10/192 (5.2%) | Not reported | 35.71 to 39.69 | Because of weak
amounts of viral RNA in positive samples, there was no attempt to isolate viruses in cell culture |
|
| Piana 2020 | 0/96 (0%) | Not reported | Not reported | Not performed | |
| Razzini 2020 | 9/34 (24.3%) | Not reported | 21.5 to 24 | Not performed | |
| Ryu 2020 | Hospital A: 10/57 (17.5%)
Hospital B: 3/22 (13.6%) |
Not reported | Not reported | Not performed | Hospital A (more severe patients in well-
equipped isolation rooms) Hospital B (less severe patients in common hospital rooms) |
| Santarpia 2020 | All personal items: 70.6%
Toilets: 81.0% Room surfaces: 75.0% Cellular phones: 77.8% Bedside rails and tables: 75% Window ledges: 72.7% |
Mean
concentration ranged from 0.17 to 0.82 copies/µL across surfaces tested |
Not reported | Due to the low
concentrations recovered in these samples cultivation of virus was not confirmed |
|
|
Seyedmehdi
2020 |
4/10 (40%) | 3227 ± 3674
copies/mL |
Not reported | Not performed | |
| Shin 2020 | 0/12 (0%) | Not reported | 27.97 to 39.78 | Not performed | |
| Suzuki 2020 | 58/601 (9.7%) | Not reported | 26.21–38.99 | No virus was cultured | SARS-CoV-2 RNA was detected from about
two-thirds of all case-cabins swabbed, while it was not detected from any non-case cabins. |
| Wang 2020 | ICU: 2/28 (7.1%)
General ward: 0/34 (0%) |
Not reported | 37.56 and 39.00 | Not performed | |
| Wang 2020a | 0/45 (0%) | No positive
samples |
No positive
samples |
No positive samples | |
| Wee 2020 | 10/445 (2.2%) | Not reported | 32.69 | Not performed | Of the 4 index cases who required
supplemental oxygen in the general ward, 75.0% (3/4) had positive environmental surveillance samples for SARS-CoV-2, compared with 8.2% (2/24) among those not on supplemental oxygen (P = 0.01) |
| Wei 2020 | 44/112 (39.3%) | Not reported | Not reported | Not performed | |
| Wei 2020a | 3/93 (3.2%) | Not reported | 17.5 to 32.9 | Not performed | |
| Wong 2020 | Before disinfection: 2/428 (0.5%)
Post-disinfection: 0% |
Not reported | Not reported | Not performed | |
| Wu 2020 | 38/200 (19%) | Not reported | Not reported | Not performed | |
| Ye 2020 | 85/626 (13.6%) | Not reported | Not reported | Not performed | The most contaminated objects were self-
service printers (20%), desktops/keyboards (16.8%), and doorknobs (16%). Hand sanitizer dispensers (20.3%) and gloves (15.4%) were the most frequently contaminated PPE. |
| Yuan 2020 | 0/38 (0%) | Not reported | Not reported | Not performed | |
| Yung 2020 | Environmental sites: 3/3 (100%)
PPE: 0/3 (0%) |
Not reported | 28.7, 33.3, and 29.7 | Not performed | |
| Zhang 2020 | 0/13 (0%) | Not reported | Not reported | Not performed | |
| Zhou 2020 | 23/218 (10.6%) | 10
1 to 10
4 genome
copies per swab |
>30. | No virus was cultured | Viral RNA was detected on 114/218 (52.3%) of
all surfaces and 91/218 (41.7%) of "suspected" surfaces |
| Zhou 2020a | 10/318 (3.1%) | 3–8 viruses/cm 2 | Not reported | Not performed | |
|
Zuckerman
2020 |
0/6 (0%) | Not reported | Not reported | Not performed |
Viral load and concentration
A total of 17 studies reported data on viral concentration ( Table 5); the units of measure used to report this data varied across the studies and included genomic copies/swab (4 studies), genomic copies/cm 2 (4 studies), genomic copies/mL (4 studies), and 1 study each for mean concentration, viruses/cm 2, genomic units/m 2, genomic copies/sample and RNA copies. We found it impossible to make any comparisons across the studies because of the heterogeneity in units of measurement.
Cycle thresholds
A total of 28 studies (44.4%) reported data in Ct with values ranging from 20.4 to 44.1 ( Table 5). One study of ICU patients [Razzini 2020] reporting positive rates of 24.3% (9/34) had the lowest range of Ct (21.5-24), while another study of ICU and isolation ward patients [Lei 2020] reporting positive rates of 5% (9/182) had the highest range of Ct (38.6-44); in both studies, the Ct for positivity was ≤40.
Viral culture
Of the 11 studies that planned to perform viral culture, only two (18.2%) reported Ct values that could act as prompts to undertake viral isolation ( Table 6). Only two studies provided information on the timing of sample collection for viral culture but were missing key details with respect to collection related to the timing of the onset of symptoms of the patients with respect to the collection and timing. One study of subjects in a cruise ship [Suzuki 2020] reported collecting samples for viral culture from 1–17 days after the cabin was vacated on a cruise ship and at least 17 days after the quarantining to cabins was ordered and 8 days after the first cabin cleaning, while another study of patients in residential isolation [Santarpia 2020] reported collecting the samples on “days 5–9” or “day 10” of occupancy at a medical centre or quarantine unit, all of whom were evacuated from the same cruise ship reported previously and would have been at least 2 weeks from the last day of quarantine [Suzuki 2020]. The incubation period ranged from 4–7 days and there were subtle differences in the culture media used across the studies ( Table 6). None of the studies reported success with viral culture despite positive RT-PCR detection tests. There were methodological issues with the techniques employed for viral culture across the studies (see Table 6).
Table 6. Findings of included studies: viral culture.
| Study ID | Threshold
for viral culture |
Timing of viral
culture |
Method used for viral culture | Cycle
Threshold |
Results of viral culture |
|---|---|---|---|---|---|
|
Ben-
Shmuel 2020 |
Not specified | Not specified | Applied 200 μL from 10-fold serial sample dilutions upon VERO E6 cell cultures
in 24-well plates. After 1 h, wells were overlaid with 1 mL of MEM medium supplemented with 2% foetal calf serum (FCS), MEM non-essential amino acids, 2 mM L-glutamine, 100 units/mL penicillin, 0.1% streptomycin, 12.5 units/mL nystatin and 0.15% sodium bicarbonate. Cells were incubated for 5 days (37°C, 5% CO2), and CPEs were observed after fixation with crystal violet solution. |
34 to 37.9 | None of the samples
was culturable. No viable virus was recovered from plastic or metal coupons after 4–14 days of incubation |
|
Colaneri
2020a |
All 26
samples were inoculated onto susceptible Vero E6 cells |
Not specified | A 200-μL sample was inoculated onto a Vero E6 confluent 24-well microplate
for virus isolation. After 1 hour of incubation at 33°C in 5% CO2 in air, the inoculum was discarded and 1 mL of medium for respiratory viruses was added (Eagle's modified minimum essential medium supplemented with 1% penicillin, streptomycin and glutamine, and 5 mg/mL trypsin) to each well. Cells were incubated at 33°C in 5% CO2 in air and observed by light microscopy every day for cytopathic effect. After a 7-day incubation, 200 μL of supernatant was used for molecular assays. |
Not reported | None of the inoculated
samples induced a cytopathic effect on day 7 of culture. |
|
Döhla
2020 |
Not specified | Not specified | Seeded Vero E6 cells in 24 well plates or T25 flasks at a density of 70–80 %. Cells
were incubated with 200µl (24 well) – 1000 µl (T25 flask) of the sample material supplemented with 1x penicillin/streptomycin/amphotericin B and incubated for 1 h at 37°C in 5 % CO2. For water samples, 10% (v/v) of inoculation volume was replaced by 10xPBS to obtain a final concentration of 1xPBS. After 1 h of incubation, the inoculum was removed, Dulbecco’s Modified Eagle’s medium (Gibco) with 3 % foetal bovine serum (Gibco) and 1x penicillin/streptomycin/ amphotericin B was added. Cells were incubated over several days at 37°C, 5% CO2 and observed for development of a cytopathic effect that typically occurs for growth of SARS-CoV-2 on Vero E6 cells. |
Not reported | No infectious virus could
be isolated under cell culture conditions from any sample |
| Feng 2020 | Not specified | Not specified | Not reported | Not reported | Could not perform viral
culture due to the low virus quantity in the positive samples. |
|
Moore
2020 |
<34 | Not specified | Vero E6 cells (Vero C1008; ATCC CRL-1586) in culture medium [MEM
supplemented with GlutaMAX-I, 10% (v/v) fetal bovine serum (FBS), 1X (v/v) non-essential amino acids and 25 mM HEPES] were incubated at 37oC. Cells (1 x 106 cells/25 cm2 flask) were washed with 1X PBS and inoculated with ≤1 mL environmental sample and incubated at 37°C for 1 h. Cells were washed with 1X PBS and maintained in 5 mL culture medium (4% FBS) with added antibiotic– antimycotic (4X), incubated at 37°C for 7 days and monitored for cytopathic effects (CPE). Cell monolayers that did not display CPE were subcultured up to three times, providing continuous cultures of ~30 days. |
28·8 to 39·1 | No CPE or a decrease
in Ct values across the course of three serial passages were observed suggesting the samples did not contain infectious virus |
| Ong 2020 | Positive
swabs from PCR |
Not specified | Monolayers of Vero C1008 cells (ATCC-1586) in T25 flasks were inoculated
with 1 mL inoculum (500 µL of the swab sample and 500 µL of Eagle’s MEM) and cultured at 37°C, 5% CO2 with blind passage every 7 days. Also, 140 µL cell culture was used for RNA extraction and real-time PCR twice per week to monitor changes in target SARS-CoV-2 genes as an indication of successful viral replication. In the absence of CPEs and real-time PCR indication of viral replication, blind passages continued for a total of 4 passages before any sample was determined to be negative of viable SARS-CoV-2 virus particles. |
Not reported | All samples in common
areas and staff pantry were negative on viral cell culture. |
|
Peyrony
2020 |
Not specified | Not specified | Not specified | 35.71 to
39.69 |
Because of weak amounts
of viral RNA in positive samples, there was no attempt to isolate viruses in cell culture |
|
Santarpia
2020 |
Subset of
samples that were positive for viral RNA by RT-PCR |
Days 5–9 of patient
occupancy for one site and day 10 occupancy for the second site. No information is provided on the date of onset of patient symptoms |
Vero E6 cells. Several indicators were utilized to determine viral replication
including cytopathic effect (CPE), immunofluorescent staining, time course PCR of cell culture supernatant, and electron microscopy. |
Not reported | Cultivation of virus on cell
culture was not confirmed including the air sample. |
|
Suzuki
2020 |
Some
samples from which viral RNA was present |
No details provided
from time of symptom onset but ranged from 1–17 days after the cabin was vacated and at least 17 days after the quarantining to cabins was ordered and 8 days after the first cabin cleaning |
Samples were mixed with Dulbecco’s modified Eagle medium supplemented with
typical concentrations of penicillin G, streptomycin, gentamicin, amphotericin B and 5% fetal bovine serum. They were inoculated on confluent VeroE6/TMPRSS2 cells. Culture medium at 0- or 48-hours post-infection (hpi) were collected and diluted10-fold in water, then boiled for 5 minutes. CPE observation after 4 days. |
26.21-38.99 | No virus was cultured |
|
Wang
2020a |
Not specified | Not specified | Samples were obtained and inoculated on Vero-E6 cells for virus culture. The
cytopathic effect (CPE) was observed after 96 h. |
No positive
samples |
No positive samples |
| Zhou 2020 | Ct value <30 | Not specified | Vero E6 and Caco2 cells were used to culture virus. The cells were cultured
in DMEM supplemented with heat inactivated fetal bovine serum (10%) and Penicillin/Streptomycin (10, 000 IU/mL &10, 000 µg/mL). For propagation, 200 µL of samples were added to 24 well plates. After 5–7 days, cell supernatants were collected, and RT-qPCR to detect SARS-CoV-2 performed. Samples with at least one log increase in copy numbers for the E gene (reduced Ct values relative to the original samples) after propagation in cells were considered positive. |
>30. | No virus was cultured |
Discussion
We found 63 primary studies investigating the role of fomites in SARS-CoV-2 transmission. The results of the majority of these studies show that SARS-CoV-2 RNA can be frequently detected on surfaces in both healthcare and non-healthcare settings. However, there were no positive culture results for studies that attempted to culture for viable virus. There is a wide variation in study setting and designs across studies, and the overall quality of published studies is low to moderate. The heterogeneity in study design and methodology makes it difficult to compare results across studies. The results of the systematic review (n=35) [Bedrosian 2020] showed that surface contamination was greatest in laboratories and least in households; however, none of the included studies addressed viral infectivity. The review authors did not assess the reporting quality of the primary studies and the search periods are now outdated.
The inability to culture the virus despite positive PCR detection tests suggests that SARS-CoV-2 RNA is more stable (and likely found in greater concentrations) on fomites than infective SARS-CoV-2 virus 11 . Factors known to affect the ability of fomites to serve as transmitters of respiratory viruses include the rate of decay of the virus on the surface and on the hands, the virus transfer rate (surface to hand, and hand to face), the frequency of touch between the hands and face, the dose-response curve of the virus, temperature and humidity, amongst others 12 .
The substantial reduction in positive detection rates before and after studies (and in some ICU settings) suggests that good hygiene procedures can minimise the risk of surface contamination. The inconsistency in describing a priori Ct values across the studies, coupled with the wide range in actual Ct values, suggests that the reported positive SARS-CoV-2 RNA detection rates are markers of previous viral presence from non-viable virus.
In a systematic review assessing the role of fomites in virus transmission in the Middle East Respiratory Syndrome (MERS) 13 , the authors reported possible evidence of fomite contamination but the evidence for fomite transmission was anecdotal. Our review findings are consistent with these observations. In an observational study of four hospitalised patients with MERS 14 , there was positive viral culture from fomites including bed sheets, bed rails, intravenous fluid hangers, and radiograph devices. In contrast to that study, published research on SARS-CoV-2 shows no evidence of positive viral culture to date. Our review findings support several national and international guidelines recommending good hygiene practices to reduce the spread of SARS-CoV-2 15– 17 .
We identified one non-peer-reviewed (pre-print) systematic review that assessed SARS-CoV-2 contamination in fomites 18 . The authors concluded that the quality of measurements was poor, and the reliability of the data is uncertain. Our findings are consistent with these. Compared to that review, we searched more databases, included more than twice the number of included studies, and accounted for the reporting quality of included studies.
Although there has been much research into fomite transmission of SARS-CoV-2, much uncertainty remains, and it is difficult to draw meaningful conclusions. Firstly, the variation in Ct across the studies suggests that there is no standardized threshold for detection of SARS-CoV-2 RNA. Some studies have shown that lower Ct correlates with higher genomic load 19 .
The studies included in this review used Ct of <35 to <43; these threshold values indicate that some of the positive tests reported in the studies may be misleading. Future research aimed at establishing internationally accepted Ct values should be considered a priority. The discrepancies in units of measurements for viral load and/or concentration also creates confusion. Therefore, standardized checklists for reporting of studies investigating SARS-CoV-2 transmission should be developed, including mandatory publishing of protocols, including the timing of the collection of any environmental specimens with respect to patient symptom onset. Looking for viable virus long after a patient has developed a significant innate and adaptive immunologic response will consistently yield negative results.
That all 11 culture studies failed to isolate the virus with significant fundamental methodological flaws indicates that the threshold for transmissibility from contaminated surfaces is unknown and more rigorous and carefully orchestrated studies are required before any conclusions may be drawn. One factor likely relates to the timing of sample collection after the onset of infection. Two studies reported the timeframe for sample collection but without precision while nine did not report any timelines. The mean incubation period of SARS-CoV-2 is 5–6 days 2 ; therefore, sample collection within the first few days of infection onset is likely to yield greater viral RNA load and result in better infectivity and culture results. Future studies should endeavour to collect surface samples of likely contaminated surfaces and medical equipment within useful timeframes and should also report this variable with their results.
As reported in the results, findings from one study [Lv 2020] showed that detection rates were different when qRT-PCR was compared with ddPCR. Interestingly, the authors of another included study [Bloise 2020] concluded that qRT-PCR is unable to differentiate between infectious and non-infectious viruses. Therefore, the use of RT-PCR as the gold standard for detection of SARS-CoV-2 requires further research. The positive findings from the before and after studies show that good hygiene procedures should continue to be a cornerstone for the management of SARS-CoV-2 and other communicable diseases.
Strengths and limitations
To our knowledge, this is the most comprehensive review to date that evaluates the role of fomites in SARS-CoV-2 transmission. We extensively searched the literature for published studies and included studies that are yet to undergo peer review. We also accounted for the quality of the studies and have presented summary data for some subgroups where possible. However, we recognize several limitations. We may not have identified all published studies investigating the role of fomites; indeed, several other studies may have been published after the last search date for this review. Heterogeneity due to variations in study designs and lack of uniformity in measurement metrics prevent us from statistically combining data across studies and limits the validity and applicability of the review results.
Conclusion
The evidence from published research suggests that SARS-CoV-2 RNA can be readily detected on surfaces and fomites. There is no evidence of viral infectivity or transmissibility via fomites to date but no studies to date have been found to be methodologically robust and of high enough quality to even adequately address the question. Good hygienic practices appear to reduce the incidence of surface contamination. Published studies are heterogeneous in design, methodology and viral reporting metrics and there are flaws in the reporting quality. Standardized guidelines for the design and reporting of research on fomite transmission should now be a priority.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Figshare: Extended data: SARS-CoV-2 and the Role of Fomite Transmission: A Systematic Review, https://doi.org/10.6084/m9.figshare.14247113.v1 9 .
This project contains the following extended data:
-
-
Appendix 1: Protocol
-
-
Appendix 2: Search Strategy
-
-
Appendix 3: List of excluded studies
-
-
Appendix 4: References to included studies
Reporting guidelines
Figshare: PRISMA checklist for ‘SARS-CoV-2 and the Role of Fomite Transmission: A Systematic Review’, https://doi.org/10.6084/m9.figshare.14247113.v1 9 .
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgements
This work was commissioned and paid for by the World Health Organization (WHO). Copyright on the original work on which this article is based belongs to WHO. The authors have been given permission to publish this article. The author(s) alone is/are responsible for the views expressed in the publication. They do not necessarily represent views, decisions, or policies of the World Health Organization.
Funding Statement
The review was funded by the World Health Organization: Living rapid review on the modes of transmission of SARs-CoV-2 reference WHO registration No2020/1077093. CH and ES also receive funding support from the NIHR SPCR Evidence Synthesis Working Group project 390.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. World Health Organization: WHO Coronavirus Disease (COVID-19) Dashboard. [Accessed 18/01/2021]. Reference Source [Google Scholar]
- 2. World Health Organization: Transmission of SARS-CoV-2: implications for infection prevention precautions. [Accessed 16/01/2021]. Reference Source [Google Scholar]
- 3. World Health Organization: Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. [Accessed 16/01/2021]. Reference Source [Google Scholar]
- 4. Kramer A, Schwebke I, Kampf G: How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. 10.1186/1471-2334-6-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karia R, Gupta I, Khandait H, et al. : COVID-19 and its Modes of Transmission. SN Compr Clin Med. 2020;1–4. 10.1007/s42399-020-00498-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mondelli MU, Colaneri M, Seminari EM, et al. : Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect Dis. 2020;S1473-3099(20)30678-2. 10.1016/S1473-3099(20)30678-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahman HS, Aziz MS, Hussein RH, et al. : The transmission modes and sources of COVID-19: A systematic review. Int J Surg.Open. 2020;26:125–36. 10.1016/j.ijso.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldman E: Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis. 2020;20(8):892–893. 10.1016/S1473-3099(20)30561-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Onakpoya I, Heneghan C, Spencer EA, et al. : Extended data: SARS-CoV-2 and the Role of Fomite Transmission: A Systematic Review. figshare. Journal contribution.2021. 10.6084/m9.figshare.14247113.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whiting PF, Rutjes AWS, Westwood ME, et al. : QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 11. Paton S, Spencer A, Garratt I, et al. : Persistence of SARS-CoV-2 virus and viral RNA on hydrophobic and hydrophilic surfaces and investigating contamination concentration. bioRxiv. 2021. 10.1101/2021.03.11.435056 [DOI] [Google Scholar]
- 12. Boone BA, Gerba CP: . Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73(6):1687–96. 10.1128/AEM.02051-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawson P, Malik MR, Parvez F, et al. : What Have We Learned About Middle East Respiratory Syndrome Coronavirus Emergence in Humans? A Systematic Literature Review. Vector Borne Zoonotic Dis. 2019;19(3):174–192. 10.1089/vbz.2017.2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bin SY, Heo JY, Song MS, et al. : Environmental Contamination and Viral Shedding in MERS Patients During MERS-CoV Outbreak in South Korea. Clin Infect Dis. 2016;62(6):755–60. 10.1093/cid/civ1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization: Coronavirus disease (COVID-19) advice for the public. [Accessed 18th January, 2021]. Reference Source [Google Scholar]
- 16. Public Health England: Guidance COVID-19: cleaning in non-healthcare settings outside the home.Updated 16 October 2020. [Accessed 18th January, 2021]. Reference Source [Google Scholar]
- 17. Centers for Disease Control and Prevention: COVID-19. How to Protect Yourself & Others. [Accessed 18th January, 2021]. Reference Source [Google Scholar]
- 18. Cherrie JW, Cherrie MPC, Davis A, et al. : Contamination of air and surfaces in workplaces with SARS-CoV-2 virus: a systematic review. Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zacharioudakis IM, Prasad PJ, Zervou FN, et al. : Association of SARS-CoV-2 Genomic Load with COVID-19 Patient Outcomes. Ann Am Thorac Soc. 2020. 10.1513/AnnalsATS.202008-931RL [DOI] [PMC free article] [PubMed] [Google Scholar]