Abstract
Introduction and importance
Burkitt's lymphoma is one of the fastest growing human cancers and it needs a rapid diagnosis.
Case presentation
A young woman presented to our institution with acute abdominal pain, tenderness and constipation. Ultrasound reported a right ovarian mass; at laparoscopy, we discovered ascites, peritoneal carcinomatosis and a voluminous pelvic mass.
Clinical discussion
Diagnosis was confirmed as non-Hodgkin sporadic Burkitt's lymphoma: the careful workup was the key to initiate multiagent chemotherapy.
Conclusion
Primary ovarian Burkitt's lymphoma, in a young woman in a non-endemic zone, is a rarity that represents a strong diagnostic challenge, but rapid identification can lead the patient to appropriate therapies and improvement of prognosis.
Abbreviations: BL, Burkitt's lymphoma; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; NHL, Non-Hodgkin's lymphoma
Keywords: Carcinomatosis, Laparoscopy, Lymphoma, Non-Hodgkin, Ovarian Burkitt
Highlights
-
•
Burkitt's lymphoma is one of the fastest growing human cancers and it needs a rapid diagnosis
-
•
A young woman presented acute abdominal pain, tenderness and constipation: after diagnostic laparoscopy, we discovered ascites, peritoneal carcinomatosis and a voluminous pelvic mass.
-
•
Diagnosis was confirmed as non-Hodgkin sporadic Burkitt's lymphoma: the careful workup was the key to initiate multiagent chemotherapy.
-
•
Primary ovarian Burkitt's lymphoma, in a young woman in a non-endemic zone represents a strong diagnostic challenge because of its rarity, but rapid identification can lead the patient to appropriate therapies and improvement of prognosis.
1. Introduction
Burkitt's lymphoma (BL) is a rare and very aggressive B-cell lymphoma that represents less than 1% of all non-Hodgkin lymphomas [2]. Nevertheless, it is the most frequent in children and adolescents (up to 34% of young age lymphomas) [3]. Its main feature is the deregulation and translocation of the c-MYC gene on chromosome 8, rearranging with immunoglobulin heavy or light chain genes [4].
BL shows three clinical subtypes:
-
-
endemic form (the most frequent): it affects mainly the facial bones, typically the maxilla. In African children it has an incidence of 3 to 6 cases/100.000/year [5];
-
-
immunodeficiency-related form (HIV-infected patients, patients with autoimmune diseases, patients with primary immunodeficiency disorders): BL represents 25–30% of NHL in HIV patients (that in these cases often have CD4 counts greater than 200 cells/μL), with the atypical presentation of an extra-nodal disease (liver, lung or other organs) [[6], [7], [8]];
-
-
sporadic form: it affects mainly the terminal ileum, the cecum and the intra-abdominal lymph nodes. It is more common among Caucasians, and more among males [5]
This paper reports the case of a 24-year-old female, emphasizing the clinical features and the findings in a sporadic form of suspected primary ovarian BL.
2. Case presentation
A 24-year-old female patient presented to the Emergency Department with abdominal tenderness, diffuse pain, constipation lasting for approximately one month and a single episode of vomit. A few days before she had performed a transvaginal ultrasound and a gynecological examination, showing a voluminous mass of suspected right ovarian origin.
The physical examination revealed abdominal distention and ascites without clinical signs of peritonitis of intestinal obstruction.
After the screening for SARS-Cov2-RNA, that resulted negative, this woman was admitted to our Unit.
The laboratory findings were as follows: serum lactate dehydrogenase (LDH) 601 U/L (normal <250 U/L), white blood cells 11.060/mL, C-reactive protein 46.5 mg/L (normal <10), serum CA-125363 U/mL (normal <35 U/mL); CA 19–9, CA 15–3, carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) were within normal limits.
During her stay, the patient underwent a rectosigmoidoscopy, that excluded the presence of malignant colonic lesions. In addition, a thoracoabdominal CT scan was performed, revealing the presence of ascites, omental cake appearance of the greater omentum, and a voluminous meso-hypogastric and pelvic mass encasing ileum and cecum with inhomogeneity of uterus and left ovary (Fig. 1).
Fig. 1.
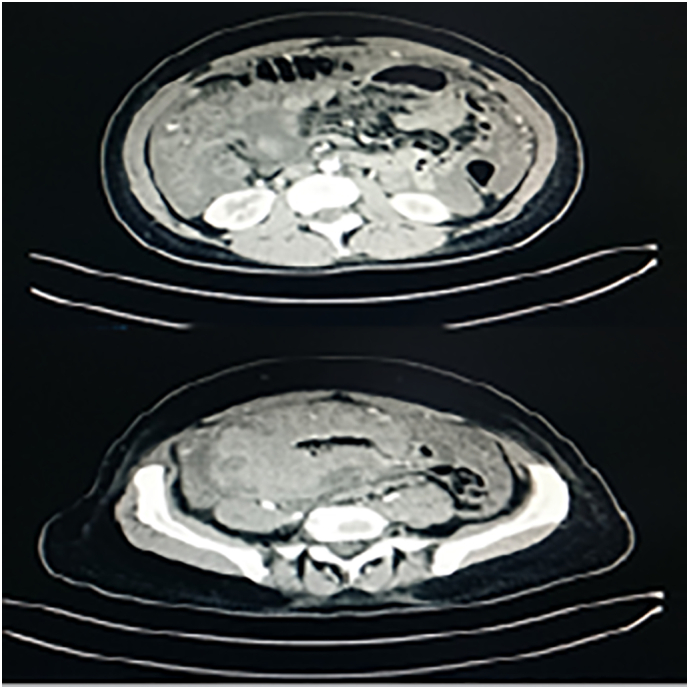
CT scan revealed a voluminous mass that include bowel and features as peritonitis carcinomatosis.
The PET scan revealed increased fluorodeoxyglucose (FDG) uptake in the abdominopelvic regions, consistent with peritoneal carcinomatosis (Fig. 2).
Fig. 2.
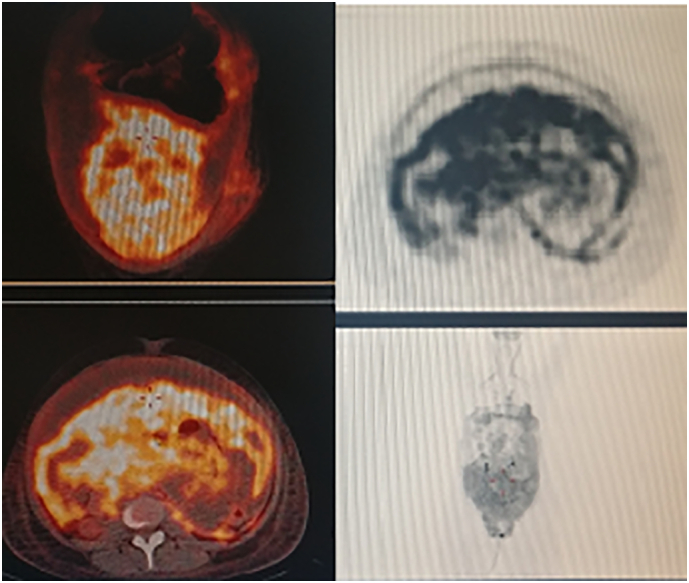
PET-FDG confirmed CT-scan findings.
The multidisciplinary discussion had proposed a virtual colonoscopy, but the patient underwent a diagnostic laparoscopy before, due to an exacerbation of symptoms in the following 24–48 h.
The exploration revealed a massive peritoneal carcinomatosis and a considerable amount of ascites (approximately 3 L).
The post-operative course was uneventful and the patient was discharged 4 days later; she was referred to the Hematology Department for systemic treatment.
The cytological exam of ascitic fluid and multiple biopsies of peritoneal nodules revealed the presence of malignant cells.
Histochemical studies revealed positivity for CD20 and c-myc, poor focal expression of Bcl6, no expression of CD3, CD5, CD10, CD30, Bcl2, cyclin-D1 and EBER; Ki-67 index was determined to be 95%.
The pathological diagnosis reported a high-grade non-Hodgkin's B-cell lymphoma. The high proliferation index, together with the morphological and immunohistochemical features were consistent with the diagnosis of Burkitt's lymphoma.
3. Discussion
Burkitt's lymphoma is an uncommon kind of malignant B-cell NHL (5%); it is highly aggressive and probably the fastest growing human tumor, and one of the first tumors showing a chromosomal translocation (chromosome 14) that activates an oncogene (c-MYC) [[9], [10], [11]].
The epidemiological and laboratory findings, initially described between 1958 and 1962 in sub-Saharan African children, suggested that the combination of very early infections of P. falciparum malaria and Epstein-Barr virus (EBV) in childhood causes B cell hyperplasia and triggers lymphomagenesis [[11], [12], [13]].
In endemic areas, BL usually affects the maxilla and presents with jaw tumors in over half of those patients, but it was soon understood that the initial presentations were variable and that distal ileum, caecum, ovaries, kidney or the breast could also be involved. In untreated cases patients die with wide-spread metastases to the liver, kidneys and other organs [14,15].
HIV-related Burkitt's lymphoma is associated with EBV in approximately 40% of cases: the incidence is very high, with an aggressive clinical course in such immunosuppressed patients [11,16].
The sporadic form of BL occurs anywhere in the world and is associated to EBV only in 5 to 15% of cases. Adult sporadic BL is commonly a disease of men in their twenties and thirties, with an incidence of 0.6 per million [17]: distal ileum, caecum and mesentery are most frequently affected, and the presenting symptoms include abdominal swelling (due to large mesenteric retroperitoneal or pelvic mass), abdominal pain and symptoms of bowel obstruction [14].
Usually, patients with BL do not have the typical early non-Hodgkin's lymphoma symptoms (fever, night sweats and weight loss), because these transformed cells compromise host immune system and develop mechanisms to escape its surveillance [18]. In case of rapidly growing extranodal intra-abdominal tumor, patients can present with symptoms of bowel obstruction, intussusception or appendicitis [19].
Our patient, a young woman, presented with abdominal pain, tenderness and constipation, which are a rare presentation, in a sporadic BL not associated with immunocompromised status (HIV negative) or EBV infection, which was also unexpected. Symptoms were attributable to mass effect and peritoneal carcinomatosis, but the absence of clear signs of intestinal obstruction are more in favor of primary ovarian origin than ileocolic one.
The right diagnosis in these cases are often post-operative. It is very difficult, especially in adults, to make a distinction between BL and diffuse large B-cell lymphoma: doubtful cases should undergo genetic testing and additional analysis because the two diseases are treated differently [[20], [21], [22]].
Early diagnosis of BL is crucial to prevent life-threatening complications related to high tumor burden and fast increase in size; aggressive chemotherapy is required: in addition to malaria prophylaxis, intensive chemotherapy with CODOX-M/IVAC (cyclophosphamide, vincristine, doxorubicin and high dose methotrexate, alternating with ifosfamide, etoposide, and cytarabine) is the definitive treatment [18,[23], [24], [25]] that can improve the outcome and the 5-year survival rate for disseminated BL in children and adolescents [9,10].
4. Conclusion
Burkitt's lymphoma represents a medical emergency requiring immediate diagnostic and therapeutic interventions, as it is one of the most aggressive tumors. Usually, clinical presentation is a diagnostic dilemma as it mimics other syndromes; primary ovarian BL, in a young woman in our latitudes, is also a rarity which further complicated the diagnosis. Pathological and immunohistochemical testing remain the investigations of choice for Burkitt's lymphoma, because it is of utmost importance to initiate the right treatment as soon as possible.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
In our institute, the approval of the ethics committee for the retrospective analysis of a clinical case report is not required.
Sources of funding
None funding were used.
Author contribution
William Sergi: Design of work and manuscript writing; Tiziana Rita Lucia Marchese: Co-author; Ivan Botrugno: Review of surgical technique literature and author of introduction; Arturo Baglivo: Data collection; Marcello Spampinato: Supervisor.
Guarantor
Spampinato Marcello.
Registration of research studies
The submitted case report is not a research study.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declares no conflict of interests.
Footnotes
The work was written in line with the SCARE criteria [1].
Consent to the processing of data for scientific purposes is requested and signed at the time of admission and kept in the medical record; the authors confirm that the patient's parents have signed consent to the publication of the data.
References
- 1.Agha R.A., Franchi T., Sohrabi C., Mathew G., for the SCARE Group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 2.Cadavid L., Sastoque J.M., Gutiérrez C. Primary osseous Burkitt lymphoma with nodal and intracardiac metastases in a child. Radiol. Case Rep. 2017;12:185–190. doi: 10.1016/j.radcr.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molyneux E.M., Rochford R., Griffin B. Burkitt’s lymphoma. Lancet. 2012;379:1234–1244. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 4.Magrath I. Epidemiology: clues to the pathogenesis of Burkitt lymphoma. Br. J. Haematol. 2012;156:744. doi: 10.1111/j.1365-2141.2011.09013.x. [DOI] [PubMed] [Google Scholar]
- 5.Levine P.H., Kamaraju L.S., Connelly R.R. The American Burkitt’s lymphoma registry: eight years’ experience. Cancer. 1982;49:1016–1022. doi: 10.1002/1097-0142(19820301)49:5<1016::aid-cncr2820490527>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow S.H., Campo E., Harris N.L. Vol. 2. WHO Press; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; p. 439. [Google Scholar]
- 7.Bonnet C., Janssens A., Wu K.L. BHS guidelines for the treatment of Burkitt’s lymphoma. Belg. J. Hematol. 2015;6:61–69. [Google Scholar]
- 8.Bush L.M., Urrutia J.G., Rodriguez E.A., Perez M.T. AIDS-associated cardiac lymphoma – a review: apropos a case report. J. Int. Assoc. Provid AIDS Care. 2015;14(6):482–490. doi: 10.1177/2325957414520981. [DOI] [PubMed] [Google Scholar]
- 9.De Leval L., Hasserjan R.P. Diffuse large B-cell lymphomas and Burkitt lymphoma. Hematol. Oncol. Clin. North Am. 2009;23:791–827. doi: 10.1016/j.hoc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Klein E., Klein G. Burkitt Lymphoma. Semin. Cancer Biol. 2009;19:345–346. doi: 10.1016/j.semcancer.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Brady G., MacArthur G.J., Farrel P.J. Epstein-Barr virus and Burkitt lymphoma. J. Clin. Pathol. 2007;60(12):1397–1402. doi: 10.1136/jcp.2007.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkitt D. A sarcoma involving the jaws in African children. Br. J. Surg. 1958;46:218–223. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 13.Mulima N., Molyneux E., Jaffe H., Kamiza S., Borgstein E., Mkandawire N., Liomba G., Batumba M., Lagos D., Gratrix F., Boshoff C., Casabonne D., Carpenter L.M., Newton R. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case–control study. PLoS One. 2008;3e:2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoxha F.T., Hashani S.I., Krasniqi A.S. Intussusceptions as acute abdomen caused by Burkitt lymphoma: a case report. Cases J. 2009;2:9,322. doi: 10.1186/1757-1626-2-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bethel C.A., Bhathacharyya N., Hutchimson C., Poyman F., Cooney D.R. Alimentary tract malignancies in children. J. Pediatr. Surg. 1997;32:1004–1008. doi: 10.1016/s0022-3468(97)90387-0. (Discussion 1008–1009) [DOI] [PubMed] [Google Scholar]
- 16.Borukamm G.W. Epstein Barr virus and the pathogenesis of Burkitt’s lymphoma: more questions than answers. Int. J. Cancer. 2009;124:1245–1255. doi: 10.1002/ijc.24223. [DOI] [PubMed] [Google Scholar]
- 17.Yalmarthi S., Smith R.C. Adult intussusception: case reports and review of literature. Postgrad. Med. J. 2005;81:174–177. doi: 10.1136/pgmj.2004.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.God J.M., Hague A. Immune evasion by B-cell lymphoma. J Clin Cell Immunol. 2011;2 doi: 10.4172/2155-9899.1000e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S.M., Huang F.C., Wu C.H., Ko S.F., Lee S.Y., Hsica C.C. Ileocaecal Burkitt’s lymphoma presenting as ileocolic intussusception with appendiceal invagination and acute appendicitis. J. Formos. Med. Assoc. 2010;109:476–479. doi: 10.1016/S0929-6646(10)60080-0. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura N., Nakamine H., Tamaru J., Nakamura S., Yoshino T., Ohshima K. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod. Pathol. 2002 Jul;15(7):771–776. doi: 10.1097/01.MP.0000019577.73786.64. [DOI] [PubMed] [Google Scholar]
- 21.Bellan C, Stefano L, Giulia de F, Rogena EA, Lorenzo L. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol. Oncol. 2009 Dec;27(4):182–5. Doi: 10.1002/hon.914. [DOI] [PubMed]
- 22.Shahini L., Gašparov S., Petruševska G., Manxhuka Kerliu S., Veselaj F., Kurshumliu F. Clinical significance of VEGF-A and microvessel density in diffuse large B-cell lymphoma and low-grade follicular lymphoma. Acta Clin. Croat. 2017 Dec;56(4):588–593. doi: 10.20471/acc.2017.56.04.02. [DOI] [PubMed] [Google Scholar]
- 23.Aldoss I.T., Wesenburger D.D., Fu K., Chan W.C., Vose J.M., Bierman P.J., Bociek R.G., Armitage J.O. Adult Burkitt lymphoma: advances in diagnosis and treatment. Oncology (Williston Park) 2008;22:1508–1517. [PubMed] [Google Scholar]
- 24.Lacasce A., Howard O., Li S., Fisher D., Weng A., Neuberg D., Shipp M. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficiency with decreased toxicity. Leuk. Lymphoma. 2004;45:761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 25.Di Nicola M., Carlo-Stella C., Mariotti J., Devizzi L., Massimino M., Cabras A., Magni M., Matteucci P., Guidetti A., Gandola L., Gianni A.M. High response rate and manageable toxicity with an intensive short-term chemotherapy programme for Burkitt’s lymphoma in adults. Br. J. Haematol. 2004;126:815–820. doi: 10.1111/j.1365-2141.2004.05141.x. [DOI] [PubMed] [Google Scholar]


