Key Points
Question
What is the association of the common MYOC p.(Gln368Ter) variant with different types of glaucoma in a Finnish population where the variant is highly enriched?
Findings
This genetic association study found that individuals with heterozygous MYOC p.(Gln368Ter) genotype had higher odds of primary open-angle glaucoma, exfoliation glaucoma, and glaucoma-related operations. The association could not be replicated with normal-tension glaucoma.
Meaning
This study found that the MYOC p.(Gln368Ter) variant was associated with glaucoma subtypes with increased intraocular pressure, including exfoliation glaucoma.
This genetic association study examines the association and penetrance of MYOC p.(Gln368Ter) (rs74315329) variant with different types of glaucoma in Finland.
Abstract
Importance
The c.1102C>T, p.(Gln368Ter) variant in the myocilin (MYOC) gene is a known risk allele for glaucoma. It is the most common MYOC risk variant for glaucoma among individuals of European ancestry, and its prevalence is highest in Finland. Furthermore, exfoliation syndrome has high prevalence in Scandinavia, making the Finnish population ideal to study the association of the variant with different types of glaucoma.
Objectives
To examine the association and penetrance of MYOC p.(Gln368Ter) (rs74315329) variant with different types of glaucoma in a Finnish population.
Design, Setting, and Participants
This genetic association study included individuals of Finnish ancestry in the FinnGen project. The participants were collected from Finnish biobanks, and the disease end points were defined using nationwide registries. The MYOC c.1102C>T variant was either directly genotyped or imputed with microarrays. Recruitment of samples to FinnGen was initiated in 2017, and data analysis was performed between December 2019 and May 2020.
Main Outcomes and Measures
The main outcomes were odds ratios (ORs) and penetrance with different types of glaucoma and in different age groups.
Results
A total of 218 792 individuals were included in this study (mean [SD] age 52.4 [17.5] years; 123 579 women [56.5%]), including 8591 (3.9%) with glaucoma, 3412 (1.6%) with primary open-angle glaucoma, 1515 (0.7%) with exfoliation glaucoma, 892 (0.4%) with normal-tension glaucoma, and 4766 (2.2%) with suspected glaucoma. The minor allele frequency of MYOC p.(Gln368Ter) was 0.28%. Individuals with the heterozygous variant had higher odds of primary open-angle glaucoma (OR, 3.36; 95% CI, 2.55-4.37), overall glaucoma (OR, 2.58; 95% CI, 2.12-3.13), suspected glaucoma (OR, 2.53; 95% CI, 1.93-3.26), exfoliation glaucoma (OR, 2.61; 95% CI, 1.60-4.02), and undergoing glaucoma-related operations (OR, 5.45; 95% CI, 2.95-9.28). The penetrance of heterozygous MYOC p.(Gln368Ter) was 5.2% in individuals with primary open-angle glaucoma, 9.6% in individuals with glaucoma, 5.4% in individuals with suspected glaucoma, and 1.9% in individuals with exfoliation glaucoma. There was no significant association with normal-tension glaucoma (OR, 1.69; 95% CI, 0.72-3.35).
Conclusions and Relevance
This genetic association study found that the MYOC p.(Gln368Ter) variant was associated with exfoliation glaucoma. The association with normal-tension glaucoma could not be replicated. These findings suggest that MYOC p.(Gln368Ter) was associated with open-angle glaucoma and exfoliation glaucoma in a Finnish population.
Introduction
Glaucoma is a group of diseases characterized by progressive optic neuropathy that leads to visual field loss, and it is a major cause of irreversible blindness in the world. Primary open-angle glaucoma (POAG) is the most common type of glaucoma, and its global age-standardized prevalence for individuals older than 40 years is 3.05% (95% CI, 1.69%-5.27%).1 Normal-tension glaucoma (NTG) is thought to be a subtype of POAG with intraocular pressure (IOP) 21 mm Hg or lower. Exfoliation glaucoma is caused by an accumulation of whitish, extracellular fibrillary material in the ocular tissues.2 The highest prevalence of exfoliation syndrome has been reported from Northern European countries.3 Currently, IOP is the only modifiable risk factor for POAG. Increasing age is another major risk factor.
Genetic factors play an important role in glaucoma risk. A family history with glaucoma is associated with a 3-fold excess age-adjusted risk of open-angle glaucoma, while the risk ratio for developing glaucoma has been reported at 9.2 among individuals with a first-degree relative with the disease.4,5 Myocilin (MYOC) was the first gene identified in families with a monogenic form of autosomal dominant open-angle glaucoma.6 Other rare disease-causing variants have also been reported, but MYOC variants are believed to be most common variants behind the monogenic form of glaucoma.7 MYOC risk variants have been identified in 2% to 4% of patients with adult-onset POAG and in 8% to 36% of patients with juvenile-onset open-angle glaucoma with diagnosis age 4 to 40 years.8
The most common glaucoma-associated MYOC variant among individuals of European ancestry is c.1102C>T, p.(Gln386Ter) (rs74315329) (eFigure in Supplement 1). In the Genome Aggregation Database (gnomAD), the allele frequency was reported as 0.16% in a European population, with the highest prevalence among the Finnish population, at 0.33%.9 The MYOC p.Gln368Ter variant has been associated with up to 1.6% of patients with open-angle glaucoma.10 The gain-of-function missense variants have been identified as causative monogenic variants,11 but it appears that loss-of-function variants act as variants with reduced penetrance and merely increase the risk for glaucoma. A recent study from the UK Biobank (UKB) population, report that among individuals of White British ancestry aged between 40 and 69 years at the time of recruitment, there were increased odds of p.(Gln368Ter) in individuals with POAG (odds ratio [OR], 6.76; 95% CI, 4.05-11.29), a composite glaucoma outcome (ie, POAG, self-reported, and unspecified glaucoma) (OR, 4.40; 95% CI, 3.38-5.71), ocular hypertension (OHT) (OR, 3.56; 95% CI, 2.53-4.92), and OHT and glaucoma combined (OR, 4.18; 95% CI, 3.05-5.67). The study also demonstrated that the penetrance of the MYOC p.(Gln368Ter) variant was 7.6% in patients with glaucoma, 24.3% in individuals with OHT, and 30.8% in individuals with OHT and glaucoma combined.12 In pedigree- and registry-based studies, the prevalence has been reported even higher in the individuals with glaucoma.12,13,14 MYOC variants have been thought to be associated with an increased IOP, but, interestingly, the p.(Gln368Ter) variant was recently associated with NTG (OR, 2.3; 95% CI, 0.98-5.3).15
The highest population prevalence of the MYOC p.(Gln368Ter) variant in the world has been reported in Finland, making it an ideal population to examine the penetrance and association of the variant with different types of glaucoma, including exfoliation glaucoma. In this study, we report ORs and penetrance of the MYOC c.1102C>T, p.(Gln368Ter) variant with glaucoma and its subtypes in the FinnGen project, including more than 210 000 individuals.
Methods
FinnGen16 is a public-private collaboration project that combines genotype data of currently 218 792 individuals (at data freeze 5) from Finnish biobanks, prospective epidemiological cohorts (initiated as far back as 1992), and disease-based cohorts with digital health record data from national health registries. The FinnGen study protocol adhered to the tenets of the Declaration of Helsinki17 and was approved by the Finnish Institute for Health and Welfare (THL), Digital and population data service agency, Social Insurance Institution, and Statistics Finland. Individuals gave written informed consent based on the Finnish Biobank Act. Recruitment protocols followed the biobank protocols approved by the National Supervisory Authority for Welfare and Health, Valvira. The FinnGen research project is an academic industrial collaboration aiming to identify genotype-phenotype correlations in the Finnish founder population, and individuals of Finnish ancestry were originally included in the data. Older research cohorts collected before FinnGen (prior to August 2017), were collected based on study-specific consents and later transferred to the Finnish biobanks after approval by Valvira. The ethics review board of the Hospital District of Helsinki and Uusimaa approved the FinnGen study protocol. The Biobank Access Decisions for FinnGen data include THL Biobank, Finnish Red Cross Blood Service Biobank, Helsinki Biobank, Auria Biobank, Biobank Borealis of Northern Finland, Biobank of Eastern Finland, Finnish Clinical Biobank Tampere, Central Finland Biobank, and Terveystalo Biobank. All DNA samples and data in this study were pseudonymized. The samples were linked with health record data by a unique national personal identification number assigned to all Finnish citizens and residents. The FinnGen data freeze 4 is now publicly available,16 and the data freeze 5 will be available after 1 year embargo.
Recruitment of samples to FinnGen was initiated in August 2017, and the data analysis was performed between December 2019 and May 2020. Carriers of the MYOC c.1102C>T, p.(Gln368Ter) (rs74315329) variant were identified either by direct genotyping or imputation with Illumina and Affymetrix microarrays (Illumina and Thermo Fisher Scientific). Variants with minimum allele counts of less than 5 or imputation information for at least 0.6 alleles were excluded from the analysis. The genotyping and imputation processes of FinnGen are described previously.18 We used the SAIGE software as developed by Zhou et al19 for running a phenome-wide association study. SAIGE is a mixed-model logistic regression for R version 3.4.1 (R Project for Statistical Computing) C++ package.
The disease end points were defined using nationwide registries for deaths, hospital discharges, outpatient specialist appointments, harmonizing over the International Classification of Diseases (ICD), revisions 8,20 9,21 and 10,22 NOMESCO procedure codes, Finnish-specific Social Insurance Institute drug reimbursement codes and Anatomical Therapeutic Chemical-codes. Individuals with ocular hypertension (IOP ≥22 mm Hg) or with suspected glaucomatous signs in the optic disc were classified as having suspected glaucoma. Glaucoma-related operations included trabeculectomy and iridectomy, glaucoma shunt operation, nonpenetrating glaucoma surgery, and other filtering operation. The definitions of clinical end points and corresponding control groups are provided in detail elsewhere.23 The definitions of glaucoma end points are provided in the eTable in Supplement 1.
Statistical Analysis
Outlier removal and principal component analysis has been described previously.18 In SAIGE phenome-wide association analysis, age, sex, 10 principal components, and genotyping batch were used as covariates.24 Each genotyping batch was included as a covariate for an end point if there were at least 10 cases and 10 controls in that batch to avoid convergence issues. Descriptive statistics are presented as means and SDs for continuous variables and numbers and percentages for categorical variables. We extracted the genotypes using bcftools version 1.9 and identified homozygote and heterozygote carriers of the MYOC c.1102C>T variant. To examine FinnGen disease end points among carriers of homozygous and heterozygous variants, we compared the number of disease end point cases in individuals with homozygous and heterozygous variants with the number of cases in the FinnGen samples using Fisher exact test. This comparison was also conducted in different age groups: younger than 40 years, younger than 50 years, 50 to 59 years, 60 to 65 years, and older than 65 years. We used the basic packages in analyses in R statistical software. All tests were 2-tailed.
Results
In total, 218 792 individuals (mean [SD] age, 52.4 [17.5] years; 123 579 women [56.5%]) from FinnGen were included in this study (Table 1). Of these, 8591 (3.9%) had glaucoma, 3412 (1.6%) had POAG, 1515 (0.7%) had exfoliation glaucoma, 892 (0.4%) had NTG, and 4766 (2.2%) had suspected glaucoma (Table 2). A total of 459 individuals (0.2%) had undergone glaucoma-related surgical operations.
Table 1. Characteristics of Study Participants.
| Characteristic | Participants, age, No. (%) (N = 218 792) | ||||
|---|---|---|---|---|---|
| <40 y (n = 56 083) | <50 y (n = 89 784) | 50-59 y (n = 46 581) | 60-65 y (n = 24 713) | >65 y (n = 57 714) | |
| Age, mean (SD), y | 28.7 (8.0) | 34.9 (10.4) | 55.1 (2.9) | 62.5 (1.4) | 73.0 (5.9) |
| Sex | |||||
| Men | 21 043 (37.5) | 35 124 (39.1) | 21 324 (45.8) | 11 536 (46.7) | 27 229 (47.2) |
| Women | 35 040 (62.5) | 54 660 (60.9) | 25 257 (54.2) | 13 177 (53.3) | 30 485 (52.8) |
Table 2. Disease Prevalence, Penetrance, and Association of the Heterozygous Myocilin (MYOC) c.1102C>T, p.(Gln368Ter) Genotype With Different Types of Glaucoma in the FinnGen Biobank Cohort.
| Phenotype | No. (%)a | Odds ratio (95% CI) | P valueb | ||
|---|---|---|---|---|---|
| Total | rs74315329 (TC) | rs74315329 (CC) | |||
| Individuals | 218 792 | 1235 (0.6) | 217 553 (99.6) | NA | NA |
| Glaucoma | 8591 (3.9) | 118 (9.6) | 8473 (3.9) | 2.58 (2.12-3.13) | <.001 |
| POAG | 3412 (1.6) | 61 (5.2) | 3351 (1.5) | 3.36 (2.55-4.37) | <.001 |
| Suspected glaucoma | 4766 (2.2) | 64 (5.4) | 4702 (2.2) | 2.53 (1.93-3.26) | <.001 |
| Exfoliation glaucoma | 1515 (0.7) | 21 (1.9) | 1494 (0.7) | 2.61 (1.6-4.02) | <.001 |
| Glaucoma-related operations | 459 (0.2) | 14 (1.1) | 445 (0.2) | 5.45 (2.95-9.28) | <.001 |
| NTG | 892 (0.4) | 8 (0.7) | 884 (0.4) | 1.69 (0.72-3.35) | .16 |
Abbreviations: NA, not applicable; NTG, normal-tension glaucoma; POAG, primary open-angle glaucoma.
Penetrance in cohort.
Fisher exact test, 2-tailed, in individuals with heterozygous variant.
Of all individuals, 1235 (0.6%) carried the heterozygous CT genotype of the MYOC p.(Gln368Ter) variant. The minor allele frequency (MAF) of MYOC p.(Gln368Ter) in this cohort population was 0.28%. Only 4 individuals with TT homozygotes were observed, and they had no history of eye diseases. Therefore, the genotype analyses were performed using individuals with heterozygous MYOC p.(Gln368Ter) variants, and we refer to the MYOC p.(Gln368Ter) variant as its heterozygous CT genotype.
The penetrance and association of the heterozygous MYOC p.(Gln368Ter) genotype with different types of glaucoma in FinnGen data freeze 5 is summarized in Table 2. Heterozygous carriers of MYOC p.(Gln368Ter) had increased odds of glaucoma (OR, 2.58; 95% CI, 2.12-3.13; P < .001), POAG (OR, 3.36; 95% CI, 2.55-4.37; P < .001), suspected glaucoma (OR, 2.53; 95% CI, 1.93-3.26; P < .001), and exfoliation glaucoma (OR, 2.61; 95% CI, 1.6-4.02; P < .001). Individuals with the heterozygous MYOC p.(Gln368Ter) variant were also more likely to undergo glaucoma-related operations (OR, 5.45; 95% CI, 2.95-9.28; P < .001). The association of heterozygous MYOC p.(Gln368Ter) genotype with NTG was not significant (OR, 1.69; 95% CI, 0.72-3.35; P = .16). The penetrance of MYOC p.(Gln368Ter) in individuals with the heterozygous variant was 9.6% (n = 118 of 1117) in individuals with glaucoma, 5.2% (n = 61 of 1117) in individuals with POAG, 5.4% (n = 64 of 1117) in individuals with suspected glaucoma, and 1.9% (n = 21 of 1117) in individuals with exfoliation glaucoma. The penetrance was 1.1% (n = 14 of 1221) in individuals who had undergone glaucoma-related operations.
The age-stratified associations and frequencies of MYOC p.(Gln368Ter) with different glaucoma types in individuals with heterozygous MYOC p.(Gln368Ter) are summarized in Table 3. Frequency here reports the number of newly diagnosed individuals in each age group. The frequency of glaucoma in individuals with MYOC p.(Gln368Ter) was highest among individuals aged 60 to 65 years, with 21 of 156 carriers (13.5%) having glaucoma. For POAG, the frequency was highest in individuals older than 65 years (35 of 442 individuals [7.9%]). The frequency of exfoliation glaucoma in MYOC p.(Gln368Ter) carriers was highest in the population older than 65 years (14 of 421 individuals [3.3%]), with an OR of 2.63 (95% CI, 1.36-4.3; P = .002).
Table 3. Age-Stratified Disease Frequency and Odds Ratios Among Carriers of the Heterozygous MYOC c.1102C>T, p.(Gln368Ter) Variant in Different Glaucoma Types in FinnGen Cohort.
| Age, y | Carriers, No. (%) | OR | P valuea |
|---|---|---|---|
| Glaucoma | |||
| <40 | 10 (4.7) | 3.34 (1.57-6.31) | .001 |
| <50 | 26 (6.7) | 3.17 (2.04-4.75) | <.001 |
| 50-59 | 17 (7.5) | 1.87 (1.07-3.08) | .02 |
| 60-65 | 21 (13.5) | 3.04 (1.82-4.86) | <.001 |
| >65 | 54 (11.7) | 2.60 (1.91-3.46) | <.001 |
| POAG | |||
| <40 | 1 (0.5) | 2.33 (0.06-13.49) | .35 |
| <50 | 9 (2.4) | 4.81 (2.16-9.35) | <.001 |
| 50-59 | 8 (3.7) | 2.59 (1.10-5.24) | .02 |
| 60-65 | 9 (6.3) | 3.21 (1.43-6.33) | .003 |
| >65 | 35 (7.9) | 3.55 (2.43-5.04) | <.001 |
| Suspected glaucoma | |||
| <40 | 10 (4.7) | 5.88 (2.75-11.7) | <.001 |
| <50 | 19 (5.0) | 3.77 (2.24-6.01) | <.001 |
| 50-59 | 8 (3.7) | 1.44 (0.61-2.89) | .28 |
| 60-65 | 9 (6.3) | 2.36 (1.05-4.64) | .02 |
| >65 | 28 (6.4) | 2.63 (1.72-3.87) | <.001 |
| Exfoliation glaucoma | |||
| <40 | 0 | NA | NA |
| <50 | 2 (0.6) | 5.90 (0.70-22.41) | .05 |
| 50-59 | 4 (1.9) | 5.96 (1.58-15.86) | .005 |
| 60-65 | 1 (0.7) | 0.92 (0.02-5.28) | >.99 |
| >65 | 14 (3.3) | 2.52 (1.36-4.3) | .002 |
Abbreviations: NA, not applicable; OR, odds ratio; POAG, primary open-angle glaucoma.
Fisher exact test, 2-tailed.
In phenome-wide association analysis, compared with noncarriers, carriers of the MYOC p.(Gln368Ter) risk allele had higher odds for glaucoma (OR, 4.62; 95% CI, 4.27-4.96; P < .001), POAG (OR, 9.92; 95% CI, 9.36-10.49; P < .001), suspected glaucoma (OR, 4.19; 95% CI, 3.74-4.64; P < .001), exfoliation glaucoma (OR, 5.83; 95% CI, 5.00-6.66; P < .001), glaucoma-related operations (OR, 94.90; 95% CI, 93.18-96.62; P < .001), and NTG (OR, 2.06; 95% CI, 1.13-2.98; P = .13) (Table 4). The phenome-wide association Manhattan plot of MYOC p.(Gln368Ter) is presented in the Figure.
Table 4. Phenome-Wide Carrier Analysis Results for MYOC c.1102C>T, p.(Gln368Ter) in FinnGen for Different Glaucoma Types.
| Glaucoma type | No. | βa | OR (95% CI) | P value |
|---|---|---|---|---|
| Any | 8591 | 1.5 (0.18) | 4.62 (4.27-4.96) | 2.3E-18 |
| POAG | 3412 | 2.3 (0.29) | 9.92 (9.36-10.49) | 2.2E-15 |
| Suspected glaucoma | 4766 | 1.4 (0.23) | 4.19 (3.74-4.64) | 3.3E-10 |
| Exfoliation glaucoma | 1515 | 1.8 (0.43) | 5.83 (5.00-6.66) | 3.4E-5 |
| Glaucoma-related operations | 459 | 4.6 (0.88) | 94.90 (93.18-96.62) | 2.0E-7 |
| NTG | 892 | 0.72 (0.47) | 2.06 (1.13-2.98) | 1.3E-1 |
Abbreviations: NTG, normal-tension glaucoma; OR, odds ratio; POAG, primary open-angle glaucoma.
Estimated with SAIGE for the alternative allele.
Figure. The Phenome-Wide Association Manhattan Plot of the Myocilin (MYOC) c.1102C>T, p.(Gln368Ter) Variant Across All End Points in FinnGen Data Freeze 5.
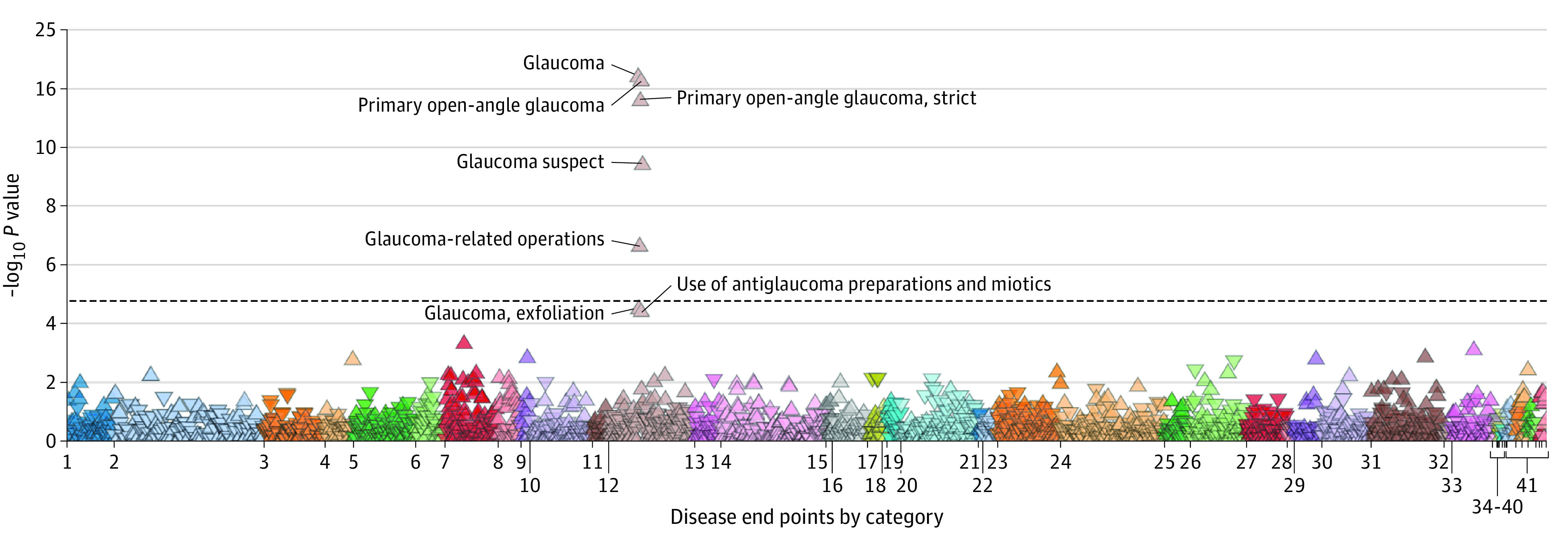
1 Indicates certain infectious and parasitic diseases; 2, neoplasms from hospital discharges; 3, neoplasms, from cancer register; 4, diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism; 5, endocrine, nutritional and metabolic diseases; 6, diabetes end points; 7, mental and behavioral disorders; 8, psychiatric end points; 9, alcohol-related diseases; 10, diseases of the nervous system; 11, neurological end points; 12, diseases of the eye and adnexa; 13, diseases of the ear and mastoid process; 14, diseases of the circulatory system; 15, cardiometabolic end points; 16, diseases of the respiratory system; 17, asthma and related end points; 18, chronic obstructive pulmonary disorder and related end points; 19, interstitial lung disease end points; 20, diseases of the digestive system; 21, dental end points; 22, gastrointestinal end points; 23, diseases of the skin and subcutaneous tissue; 24, diseases of the musculoskeletal system and connective tissue; 25, rheumatoid end points; 26, diseases of the genitourinary system; 27, pregnancy, childbirth and the puerperium; 28, certain conditions originating in the perinatal period; 29, congenital anomalies, deformations, and chromosomal anomalies; 30, symptoms, signs, and anomalous clinical and laboratory findings, not elsewhere classified; 31, injury, poisoning and certain other consequences of external causes; 32, external causes of morbidity and mortality; 33, factors influencing health status and contact with health services; 34, drug purchase end points; 35, operation end points; 36, diseases marked as autoimmune origin; 37, demonstration end points; 38, other, not yet classified end points (same as miscellaneous); 39, common end point; 40, miscellaneous, not yet classified end points; 41, comorbidity end points.
To determine if there was a true association, we analyzed the number of individuals with exfoliation glaucoma who also had POAG as a comorbidity or earlier diagnosis. We found 567 individuals who had both diagnoses in digital health records (6 of 21 carriers of the variant with exfoliation glaucoma), and we created a new exfoliation glaucoma strict end point phenotype containing only individuals with exfoliation glaucoma without any record of POAG. The OR for carriers of the MYOC p.(Gln368Ter) variant for this exfoliation glaucoma strict end point was 2.98 (95% CI, 1.65-4.96; P < .001).
Discussion
In this genetic association study, we found that the MYOC p.(Gln368Ter) variant was associated with different types of glaucoma with high IOP in a Finnish population, where the prevalence of this variant is highest in the world. The MYOC p.(Gln368Ter) variant was significantly associated with different types of glaucoma, including POAG, suspected glaucoma, and exfoliation glaucoma. We did not find an association with NTG.
To our knowledge, this is the first large population cohort association analysis of the MYOC p.(Gln368Ter) variant with exfoliation glaucoma, also finding a robust association. In exfoliation syndrome, extracellular fibrillary material can be discovered on the surface of the lens, the pupillary border, and elsewhere in the anterior segment of the eye. Exfoliation glaucoma is the most common identifiable cause of secondary glaucoma, and it is relatively common in Nordic countries, with a prevalence of nearly 20% in individuals aged 70 years or older in Finland, and almost 30% of these individuals develop glaucoma in their lifetime.3,25 In our study cohort, a relatively large number of patients (1515 individuals [0.7% of the study population]) had exfoliation glaucoma, and the OR of carriers of the heterozygous variant to develop exfoliation glaucoma was 2.61 (95% CI, 1.6-4.02). The OR for carriers of the MYOC p.(Gln368Ter) variant for the exfoliation glaucoma strict end point, containing only individuals without any record of POAG, was still high, at 2.98 (95% CI, 1.65-4.96; P < .001). In many individuals, POAG may be a preliminary diagnosis before later detection of exfoliation material. Exfoliation material accumulates within the trabecular meshwork and the Schlemm canal, increasing IOP, and while many of its components have been identified, including fibrous proteins, inhibitors of proteinases, complement factors, lysyl oxidase-like 1 (LOXL1), and apolipoprotein E (ApoE), myocilin has not been detected in this glycosylated proteinaceous complex.2 Our findings suggest that the MYOC p.(Gln368Ter) variant was also associated with exfoliation glaucoma, and we speculate that the variant may have contributed to increase in IOP along with the exfoliation material. Unfortunately, the FinnGen sample does not have recorded IOP levels to investigate this in more detail.
We also studied the association of the MYOC p.(Gln368Ter) variant with NTG. In a 2019 study, Alward and colleagues15 observed that MYOC p.(Gln368Ter) was associated with NTG (OR, 2.3; 95% CI, 0.98-5.3; P = .04) in their study population of 1333 individuals with NTG. We had a relatively large NTG population of 892 individuals, and we did not find a significant association. The coding and diagnosis of NTG in clinical practice could vary significantly, affecting the association results. Also, as with all glaucoma types, not all individuals with NTG receive diagnoses, which affects the results. Still, our results are in line with the hypothesis that myocilin contributes to glaucoma primarily through increasing IOP.
We found that the MYOC p.(Gln368Ter) genotype was a risk factor for POAG (OR 3.36; 95% CI, 2.6-4.4) with an association similar to that of a positive family history (OR, 3.3; 95% CI, 2.0-5.6) as reported by Hollands et al.26 When taking account only individuals with previous glaucoma-related operations, indicating more severe disease, the OR was significantly higher, at 5.45 (95% CI, 2.95-9.28). SAIGE phenome-wide association analysis19 is a widely used method in genome-wide association studies, revealing the association of a single allele, rather than genotype, with different disease end points, and it uses age, sex, principal components, and genotyping batch as covariates. The OR for POAG in allelic SAIGE phenome-wide association analysis was 9.92 (95% CI, 9.36-10.49), which is higher than that reported in a UKB study by Han et al12 (6.76), these numbers being more comparable than those from our genotype analysis.26 POAG was defined more broadly in the UKB compared with our study. The UKB included secondary open-angle glaucoma types and NTG within the POAG group, and the numbers are not totally comparable. Nevertheless, population differences could partly explain the difference in ORs. The SAIGE phenome-wide association study results19 are not directly comparable with heterozygote ORs owing to the aforementioned differences. The SAIGE phenome-wide association analysis may overestimate ORs when the number of cases is small, as the number of individuals with the variant with glaucoma-related operations was in this study, and the genotype analysis may provide better estimates on the association in these situations. Hence, we think it is informative to present both analyses, since genome-wide association results are commonly presented.
The strength of the study was the very high-quality imputation and direct genotyping of the MYOC c.1102C>T, p.(Gln368Ter) variant with an imputation INFO score of 99.8% (range, 0.88-1). The high MAF of MYOC p.(Gln368Ter) in Finland increases power to estimate ORs. The MAF in our biobank-based sample was 0.28%, which is very close to the that reported in gnomAD version 2.1.1 (0.33%) by Karczewski et al,9 although the FinnGen cohort cannot be used to estimate an exact prevalence. In general, validation studies have observed good specificity and sensitivity of the health events recorded in the Finnish registries.27,28 Furthermore, Finland has a rather isolated population with recent bottlenecks, which offers advantages in studying rare variants in complex diseases.29
Limitations
This study had several limitations. The MYOC p.(Gln368Ter) variant only increases the risk for glaucoma, thus having a reduced penetrance. A 2020 study by Craig et al30 was able to develop a polygenic risk score for glaucoma and reported a 6-fold increase in the penetrance of glaucoma from the lowest to the highest tertile polygenic risk score group in carriers of p.(Gln368Ter) at age 60 years. Additionally, many Finnish biobanks have collected informed consents from patients during hospital visits, and thus FinnGen is not a healthy population-based sample, as the UKB is, for example. This increases the number of individuals, including those with ophthalmic diseases, and challenges the evaluation of true penetrance or ORs, as it may overestimate the disease prevalence. In the case of penetrance, our findings describe only the situation in this cohort and possibly not the real penetrance of the variant in the general population. The phenotype data in the FinnGen project were obtained from several national health care registries using, among others, ICD-8, -9, and -10 diagnosis codes and procedure codes, and some minor local variation in the use of codes cannot be excluded. It is also estimated 50% of individuals with glaucoma remain undiagnosed.31,32 Because the study was based on reported codes in national health registries instead of diagnoses based on novel ophthalmic examinations, some individuals with glaucoma could be unreported or undiagnosed. Furthermore, if the MYOC p.(Gln368Ter) variant increases the IOP without glaucoma, it could potentially lead to misdiagnosis of glaucoma in individuals who carry the variant and overestimate the penetrance. Still, in our cohort, we estimated prevalence of glaucoma at 3.9%, POAG at 1.6%, and exfoliation glaucoma at 0.7%; a previous study estimated the prevalence of glaucoma in Europe at 2.93% and of POAG at 2.51%.1 These numbers are relatively similar despite the addressed selection bias in recruitment. Additionally, individuals with glaucoma or another comorbidity may be less active in participating in biobank studies compared with healthy individuals.
Conclusions
The findings of our genetic association study add to the literature on the association of the MYOC p.(Gln368Ter) with glaucoma subtypes due to increased IOP. We did not find an association with NTG. We were also able to demonstrate a robust association with exfoliation glaucoma.
eTable. List of Glaucoma End Point Definitions
eFigure. Allele Frequencies of the Myocilin (MYOC) c.1102C>T, p.(Gln368Ter) Variant in Different Populations in the gnomAD Database
Nonauthor Collaborators
References
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Challa P, Johnson WM. Composition of exfoliation material. J Glaucoma. 2018;27(suppl 1):S29-S31. doi: 10.1097/IJG.0000000000000917 [DOI] [PubMed] [Google Scholar]
- 3.Vesti E, Kivelä T. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res. 2000;19(3):345-368. doi: 10.1016/S1350-9462(99)00019-1 [DOI] [PubMed] [Google Scholar]
- 4.Burr JM, Mowatt G, Hernández R, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess. 2007;11(41):iii-iv, ix-x, 1-190. doi: 10.3310/hta11410 [DOI] [PubMed] [Google Scholar]
- 5.Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1640-1645. doi: 10.1001/archopht.116.12.1640 [DOI] [PubMed] [Google Scholar]
- 6.Stone EM, Fingert JH, Alward WL, et al. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275(5300):668-670. doi: 10.1126/science.275.5300.668 [DOI] [PubMed] [Google Scholar]
- 7.Williams SE, Carmichael TR, Wainstein T, Hobbs A, Ramsay M. MYOC mutations in Black South African patients with primary open-angle glaucoma: genetic testing and cascade screening. Ophthalmic Genet. 2015;36(1):31-38. doi: 10.3109/13816810.2014.972520 [DOI] [PubMed] [Google Scholar]
- 8.Souzeau E, Burdon KP, Dubowsky A, et al. Higher prevalence of myocilin mutations in advanced glaucoma in comparison with less advanced disease in an Australasian disease registry. Ophthalmology. 2013;120(6):1135-1143. doi: 10.1016/j.ophtha.2012.11.029 [DOI] [PubMed] [Google Scholar]
- 9.Karczewski KJ, Francioli LC, Tiao G, et al. ; Genome Aggregation Database Consortium . The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434-443. doi: 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fingert JH, Héon E, Liebmann JM, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8(5):899-905. doi: 10.1093/hmg/8.5.899 [DOI] [PubMed] [Google Scholar]
- 11.Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21(4):395-428. doi: 10.1016/S1350-9462(02)00010-1 [DOI] [PubMed] [Google Scholar]
- 12.Han X, Souzeau E, Ong JS, et al. Myocilin gene Gln368Ter variant penetrance and association with glaucoma in population-based and registry-based studies. JAMA Ophthalmol. 2019;137(1):28-35. doi: 10.1001/jamaophthalmol.2018.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig JE, Baird PN, Healey DL, et al. Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology. 2001;108(9):1607-1620. doi: 10.1016/S0161-6420(01)00654-6 [DOI] [PubMed] [Google Scholar]
- 14.Allingham RR, Wiggs JL, De La Paz MA, et al. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1998;39(12):2288-2295. [PubMed] [Google Scholar]
- 15.Alward WLM, van der Heide C, Khanna CL, et al. ; NEIGHBORHOOD Consortium . Myocilin mutations in patients with normal-tension glaucoma. JAMA Ophthalmol. 2019;137(5):559-563. doi: 10.1001/jamaophthalmol.2019.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FinnGen. Accessed April 27, 2021. https://www.finngen.fi/en
- 17.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 18.Tanigawa Y, Wainberg M, Karjalainen J, et al. ; FinnGen . Rare protein-altering variants in ANGPTL7 lower intraocular pressure and protect against glaucoma. PLoS Genet. 2020;16(5):e1008682. doi: 10.1371/journal.pgen.1008682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou W, Nielsen JB, Fritsche LG, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50:1335-1341. doi: 10.1038/s41588-018-0184-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . International Classification of Diseases, Eigth Revision (ICD-8). World Health Organization; 1965. [Google Scholar]
- 21.World Health Organization . International Classification of Diseases, Ninth Revision (ICD-9). World Health Organization; 1977. [Google Scholar]
- 22.World Health Organization . International Statistical Classification of Diseases, Tenth Revision (ICD-10). World Health Organization; 1992. [Google Scholar]
- 23.FinnGen . Clinical endpoints. Accessed April 27, 2021. https://www.finngen.fi/en/researchers/clinical-endpoints
- 24.Zhou W, Nielsen JB, Fritsche LG, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50(9):1335-1341. doi: 10.1038/s41588-018-0184-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirvelä H, Tuulonen A, Laatikainen L. Intraocular pressure and prevalence of glaucoma in elderly people in Finland: a population-based study. Int Ophthalmol. 1994-1995;18(5):299-307. doi: 10.1007/BF00917834 [DOI] [PubMed] [Google Scholar]
- 26.Hollands H, Johnson D, Hollands S, Simel DL, Jinapriya D, Sharma S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma: the rational clinical examination systematic review. JAMA. 2013;309(19):2035-2042. doi: 10.1001/jama.2013.5099 [DOI] [PubMed] [Google Scholar]
- 27.Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40(6):505-515. doi: 10.1177/1403494812456637 [DOI] [PubMed] [Google Scholar]
- 28.Haukka J. Finnish health and social welfare registers in epidemiological research. Nor Epidemiol. 2009;14(1). doi: 10.5324/nje.v14i1.284 [DOI] [Google Scholar]
- 29.Lim ET, Würtz P, Havulinna AS, et al. ; Sequencing Initiative Suomi (SISu) Project . Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet. 2014;10(7):e1004494. doi: 10.1371/journal.pgen.1004494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig JE, Han X, Qassim A, et al. ; NEIGHBORHOOD consortium; UK Biobank Eye and Vision Consortium . Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet. 2020;52(2):160-166. doi: 10.1038/s41588-019-0556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996;103(10):1661-1669. doi: 10.1016/S0161-6420(96)30449-1 [DOI] [PubMed] [Google Scholar]
- 32.Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology. 1998;105(4):733-739. doi: 10.1016/S0161-6420(98)94031-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. List of Glaucoma End Point Definitions
eFigure. Allele Frequencies of the Myocilin (MYOC) c.1102C>T, p.(Gln368Ter) Variant in Different Populations in the gnomAD Database
Nonauthor Collaborators


