Key Points
Question
What is the definition of adherence to and persistence with intravitreal therapy in neovascular age-related macular degeneration?
Findings
This expert consensus survey used a modified Delphi technique to establish a set of definitions for the terms adherence, nonadherence, persistence, nonpersistence, planned discontinuation, and transfer of care. A classification system based on the World Health Organization dimensions of adherence was developed for the reasons for nonadherence and nonpersistence.
Meaning
These definitions provide a framework when assessing patient engagement to intravitreal therapy, which may be useful in future studies identifying rates or risk factors for patient nonadherence or nonpersistence.
This systematic review and expert consensus survey study describes the development and validation of terminology for patient nonadherence and nonpersistence to anti–vascular endothelial growth factor therapy for neovascular age-related macular degeneration.
Abstract
Importance
Poor adherence or persistence to treatment can be a barrier to optimizing clinical practice (real-world) outcomes to intravitreal injection therapy in patients with neovascular age-related macular degeneration (nAMD). Currently, there is a lack of consensus on the definition and classification of adherence specific to this context.
Objective
To describe the development and validation of terminology on patient nonadherence and nonpersistence to anti–vascular endothelial growth factor therapy.
Design, Setting, and Participants
Following a systematic review of currently used terminology in the literature, a subcommittee panel of retinal experts developed a set of definitions and classification for validation. Definitions were restricted to use in patients with nAMD requiring intravitreal anti–vascular endothelial growth factor therapy. Validation by the full nAMD Barometer Leadership Coalition was established using a modified Delphi approach, with predetermined mean scores of 7.5 or more signifying consensus. Subsequent endorsement of the definitions was provided from a second set of retinal experts, with more than 50% members agreeing or strongly agreeing with all definitions.
Main Outcomes and Measures
Development of consensus definitions for the terms adherence and persistence and a classification system for the factors associated with treatment nonadherence or nonpersistence in patients with nAMD.
Results
Nonadherence was defined as missing 2 or more treatment or monitoring visits over a period of 12 months, with a visit considered missed if it exceeded more than 2 weeks from the recommended date. Nonpersistence was defined by nonattendance or an appointment not scheduled within the last 6 months. The additional terms planned discontinuation and transfer of care were also established. Reasons for treatment nonadherence and nonpersistence were classified into 6 dimensions: (1) patient associated, (2) condition associated, (3) therapy associated, (4) health system and health care team associated, (5) social/economic, and (6) other, with subcategories specific to treatment for nAMD.
Conclusions and Relevance
This classification system provides a framework for assessing treatment nonadherence and nonpersistence over time and across different health settings in the treatment of nAMD with current intravitreal anti–vascular endothelial growth factor treatments. This may have additional importance, given the potential association of the coronavirus pandemic on adherence to treatment in patients with nAMD.
Introduction
Since its introduction, intravitreal anti–vascular endothelial growth factor (anti-VEGF) injection therapy has transformed the treatment of neovascular age-related macular degeneration (nAMD).1 However, outcomes observed in clinical practice (the real world) generally do not reach those seen in clinical trials,2 potentially because of lack of adherence or nonpersistence to the recommended trial regimens. Even within strict clinical trial settings, deviations from recommended protocols have often been associated with poorer visual health outcomes, with a recent secondary analysis3 of the Comparison of Age-Related Macular Degeneration Treatment Trial (CATT) reporting worse visual acuity at 2 years in patients with missed or delayed visits.
The World Health Organization defines adherence to long-term therapy as “the extent to which a person’s behaviour, corresponds with agreed recommendations from a health care provider.”4(p3) In contrast, persistence is defined as “the duration of time from initiation to discontinuation of therapy.”5(p44) Previous discussions in ophthalmology have largely focused on how this behavior is associated with outcomes in glaucoma therapy. The concept of what constitutes adherence and persistence in nAMD, however, has not been clearly established. A recent systematic review6 of factors affecting treatment nonadherence and nonpersistence to anti-VEGF therapy in nAMD identified considerable variations in both terminology and descriptions of adherence and persistence, concluding that uniform definitions, specific to this field, are required.
The development of consensus definitions is important because it enables consistent reporting and comparison of the true prevalence of nonadherence and nonpersistence. The effectiveness of proposed interventions can also be analyzed. In this study, we describe the development and validation of definitions for terms associated with adherence and persistence to anti-VEGF therapies in nAMD.
Methods
Subcommittee and Validation Group
The nAMD Barometer Leadership Coalition is an international group of experts (with 14 members; M.O., T.Y.W., P.M., B.E., S.J.T., T.A., V.D., F.J.R., R.G., J.B., R.P.F., and A.L. and 2 nonauthors) in the field of nAMD, vision care, and healthy aging. The nAMD Barometer program is a multiphase initiative established to develop robust evidence and provide recommendations on improving treatment in nAMD. As part of phase 1 of this program, a subcommittee (with 8 members; T.A., J.B., V.D., R.G., A.L., P.M., M.O., and T.Y.W.) was formed to lead the development and consensus validation of terms associated with adherence and persistence in nAMD.
External endorsement of definitions was carried out by the wider members of the Vision Academy group. The Vision Academy is an international collaboration of more than 80 expert physicians who provide guidance on management of various retinal diseases (a full list of members is at https://www.visionacademy.org/meet-our-members). Financial support for the nAMD Barometer program and the Vision Academy initiative is provided by Bayer Consumer Care AG, Basel, Switzerland.
Systematic Literature Review
A systematic review with no date restrictions was conducted to identify original studies that included a definition of treatment adherence or persistence to anti-VEGF therapy for nAMD. Databases, including MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), were searched on June 1, 2019. No eligibility restrictions were placed on the type of anti-VEGF or treatment regimen used. Studies were excluded if interventions other than anti-VEGF injections or retinal conditions other than nAMD were evaluated. Terms such as compliance, nonattendance, discontinuation, dropout, cessation, and loss to follow-up were considered synonymous. Current definitions and usage of the terms nonadherence and nonpersistence were extracted from the literature. The reasons for nonadherence and/or nonpersistence were derived from articles included in a recently published systematic review6 conducted by the same nAMD Barometer group.
Definition Development, Validation, and Endorsement
The term adherence, the preferred term in recent health literature, was chosen for validation because it reflects a more proactive health care interaction compared with the more passive term compliance.4 The negative connotations of blame associated with compliance have also led to its decreasing use. Similarly, the term persistence was chosen instead of discontinuation to mirror this shared health-engaging behavior.
A modified Delphi approach was used to establish consensus definitions (Figure; further details are provided in the eAppendix in the Supplement). Using the results of the systematic review as a starting point, proposed definitions for adherence and persistence were drafted. These initial definitions were discussed and refined among subcommittee members via virtual meetings and email correspondence to determine the most appropriate definitions to put forward for validation.
Figure. Modified Delphi Consensus Process and Development of Validated Definitions.
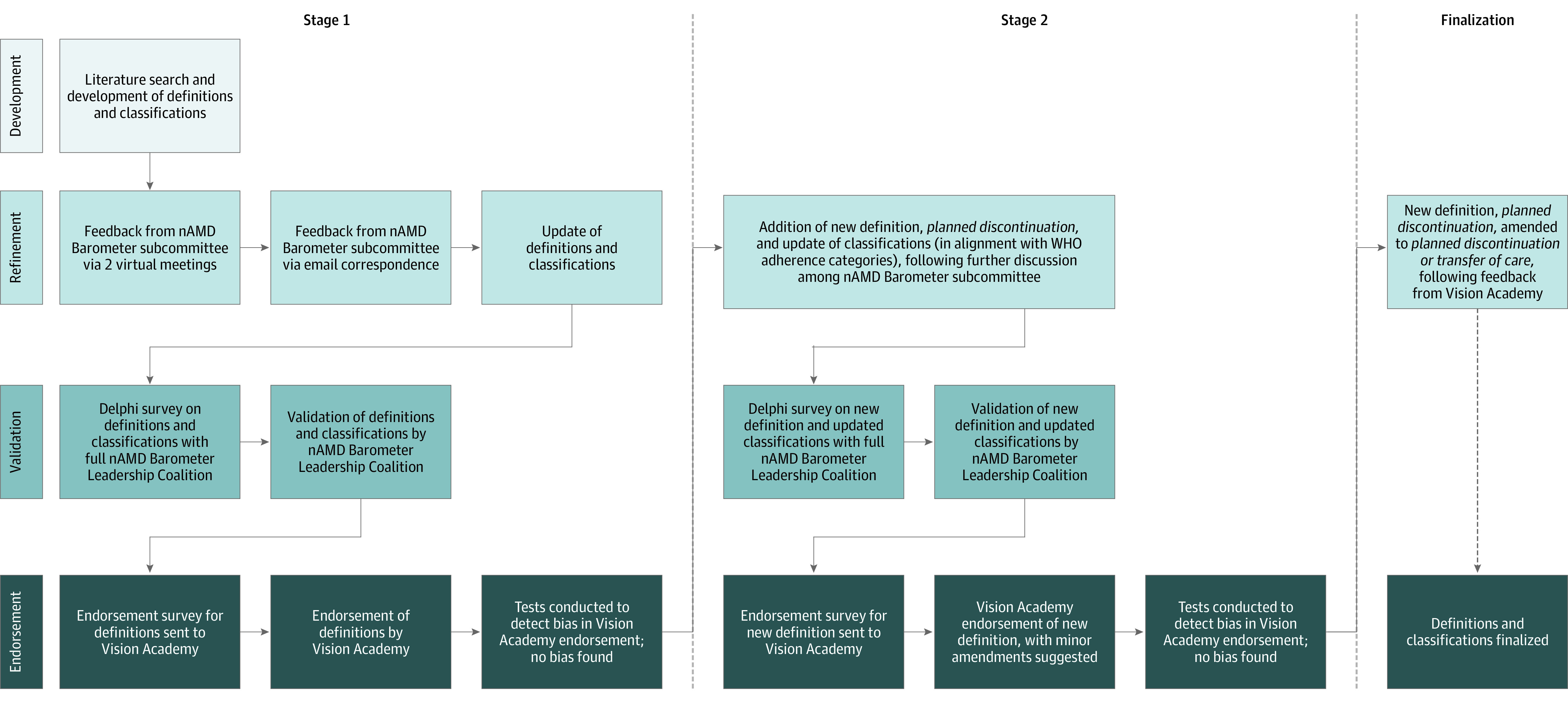
nAMD indicates neovascular age-related macular degeneration; WHO, World Health Organization.
The Delphi method, a structured tool for establishing group consensus, was then used to validate the initial set of definitions.7 This approach involved providing experts with a circulating series of questionnaires. After each round, the questionnaire was modified according to anonymized group feedback before it was re-sent, with the goal of working toward mutual agreement in the subsequent round. Specifically, for this study, a draft of the definitions and factors affecting treatment adherence or persistence developed by the subcommittee was circulated to the full Leadership Coalition for consensus validation. Each member of the Leadership Coalition was asked to assign a score from 1 to 10 to indicate their level of agreement with the proposed definitions, where 1 indicated strongly disagreeing and 10 strongly agreeing. If respondents disagreed, they were required to provide anonymized feedback on reasons for disagreement and suggested changes to the proposed definition. The mean score from all respondents was calculated, and a predetermined cutoff value of 7.5 or more was established for consensus. If the mean score was 7.5 or more, consensus was reached and the term validated. If the mean score was less than 7.5, then consensus was not reached, and the definition was amended according to the feedback and sent back for a further round of evaluation. This process was repeated until consensus was reached on all terms. The modified Delphi consensus and validation process was completed over a period from November 2019 to May 2020.
The validated set of definitions was then sent to the wider Vision Academy group members for endorsement via an online survey. Respondents were asked to rate their agreement with the proposed definitions with the options strongly agree, agree, neither agree nor disagree, disagree, and strongly disagree. A target of more than 50% of members responding was required for the survey to be valid. Participants were also asked for their country of practice as well as the reimbursement status of treatment (ie, mostly reimbursed or mostly out of pocket) to ascertain if this may have influenced the response. Biases were assessed using χ2. Endorsement was established if 50% of respondents or more either agreed or strongly agreed. Means and SDs were calculated using Excel version 16.48 (Microsoft).
Results
Current Definitions in the Literature
The systematic review identified 21 studies8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 that reported definitions of compliance, adherence, persistence, discontinuation, and/or loss to follow-up. Additional insights into reasons for nonadherence and nonpersistence were also included from 9 studies.29,30,31,32,33,34,35,36,37 Definitions were extracted from the existing literature (Table 1).
Table 1. Current Definitions Extracted From the Systematic Review.
| Term | Definitions in the existing literature8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30 |
|---|---|
| Nonadherence |
|
| Synonyms: noncompliance, absenteeism, and nonattendance | |
| Nonpersistence |
|
| Synonyms: discontinuation, dropout, cessation, and loss to follow-up |
Proposed Definitions for Adherence and Persistence
The new validated definitions are described in Table 2. A single definition for each term was developed to ensure consistency and simplicity of use in everyday practice. To facilitate this, the validated definitions used attendance at any scheduled clinic visit (both monitoring and injection visits) as a measure of adherence or persistence. This enabled the terminology to be used across different injection regimens (ie, both as needed and treat and extend), as well as different practice settings.
Table 2. Validated Definitions for Adherence and Persistence to Anti–Vascular Endothelial Growth Factor Therapy in Neovascular Age-Related Macular Degeneration.
| Definition | Mean (SD) Delphi score | |
|---|---|---|
| Stage 1 | Stage 2 | |
| Adherence | ||
| Full adherence | ||
| Attendance at every scheduled clinic visit (treatment or monitoring) and undergoing every treatment or monitoring procedure advised by the treating physician over 12 mo | 9.69 (0.61) | 9.91 (0.31) |
| Adherence | ||
| Missing ≤1 treatment or monitoring visit scheduled as advised by the treating physician over 12 moa,b | 8.75 (2.20) | 9.82 (0.40) |
| Nonadherence | ||
| Missing ≥2 treatment or monitoring visits scheduled as advised by the treating physician over 12 mob | 8.33 (2.17) | 9.67 (0.67) |
| Persistence | ||
| Persistence | ||
| Maintaining treatment or monitoring as advised by the treating physician and attending the most recent appointment within the last 6 moc | 9.31 (0.82) | 9.82 (0.40) |
| Nonpersistence | ||
| Not attending any treatment or monitoring visit for any reason within the last 6 mo or not scheduling follow-up appointments for any reason for 6 mod | 9.50 (0.67) | 9.27 (1.27) |
| Planned discontinuation and transfer of care | ||
| Planned discontinuation | NA | 9.27 (1.27) |
| Lack of treatment response (treatment futility) or no disease activity requiring ongoing treatment, as judged by the treating physician | ||
| Transfer of care | ||
| The ongoing management of the patient’s neovascular age-related macular degeneration, transferred to another physician | ||
Abbreviation: NA, not applicable.
A visit is considered missed if the recommended appointment date is exceeded by more than 2 weeks for any reason. The number of missed visits is determined based on the total potential visits missed during the nonadherent period, using the last recommended visit interval.
The period of 12 months begins from the time of the first injection. For subsequent years of treatment, adherence is calculated every 12 months.
A patient is not required to be adherent to be persistent.
The first day of the 6-month period after the most recent appointment attended should serve as the date of onset of nonpersistence. A minimum of 6 months since the first injection is required to assess persistence.
Adherence and Nonadherence
The term adherence was broken down into 2 categories: fully adherent and adherent (Table 2). The term fully adherent refers to ideal practice, with complete observance of all scheduled visits. However, in clinical practice, this is often unrealistic, and most patients would be classified as nonadherent using this all-or-nothing approach. Therefore, a less stringent assessment of adherence was also established to provide a stepdown level of gauging adherence, which, although imperfect, was more achievable in clinical practice. A definition of no more than 1 missed appointment over a 12-month period was chosen because this reflects the commonly used definition of more than an 80% cutoff for classification of what constitutes good adherence to general medications.38,39 When a patient is nonadherent and misses appointments, the number of missed visits is determined by the total potential visits during the nonadherent period using the last known visit interval. For example, if a patient is recommended to have injections every 4 weeks but does not return for 4 months, then the number of missed visits is 3 if the patient attends all follow-up visits for the remaining 12-month period. If there is a further period of missed appointments within this 12-month period, the results are cumulative. Adherence is determined every 12 months, so changes in adherence patterns over time can be assessed per year (eg, for a patient with 3 years of treatment, adherence is given per year [ie, for years 1, 2, and 3]).
The timing of the visit was also considered important for calculating adherence, with a margin of 2 weeks’ delay allowed, after which the physician-recommended visit is considered missed. The 2-week cutoff was based on logistics of scheduling appointments in clinical practice. Visits outside of this recommended time frame were also recorded as missed, regardless of whether an appointment was actually booked. This accounted for variations in health care models, with some systems requiring patients to call up and initiate the next appointment vs others in which the clinic automatically makes the bookings. Delays attributable to systemic factors, such as lack of clinic capacity, as a reason for nonadherence were also captured in this way.
For patients with bilateral nAMD, adherence was also calculated per patient rather than per eye, using the eye with the shortest visit interval for determination. For example, a patient receiving injections once every 12 weeks in 1 eye and once every 4 weeks in the fellow eye who does not attend any visits for 12 weeks is considered nonadherent. This allows for factors such as bilaterality in disease or nonsimultaneous injections to be easily identified as barriers to treatment.
Persistence and Nonpersistence
The term nonpersistence was defined as nonattendance of any treatment or monitoring visit within the last 6 months. In determining the 6-month nonattendance cutoff, the subcommittee agreed that a 4-month period was too short, because some treat-and-extend regimens allow extension up to 4 months (16 weeks). In contrast, 12 months was considered too long, because there are very few circumstances in which patients would not have either a monitoring visit or injection for a full year and still be considered to be engaging in therapy for nAMD.
Accordingly, a minimum assessment period of 6 months since the first injection is required to gauge levels of persistence, because this is the least amount of elapsed time that meets the definition of nonpersistence. For example, a patient who received an injection and was scheduled to return in 4 weeks but did not return for either monitoring or further injections for 7 months would be considered nonpersistent. However, if at the time of assessment, only 4 months had passed since their last visit, although the patient would be considered nonadherent (having missed 3 potential visits), persistence cannot be determined. The tolerance threshold of missed appointments for patients who are adherent was still stricter than that allowed in the definition for persistence. In this way, a patient could be classified as persistent while not necessarily being adherent, but not vice versa.
Planned Discontinuation or Transfer of Care
An additional 2 terms, planned discontinuation and transfer of care, were also developed to account for those patients for whom treatment cessation is intentional and not because of nonpersistence. Patients are recorded as persistent if they attend visits with other physicians or clinics, as long as it was for the purpose of monitoring or treating their nAMD and it was possible to obtain ongoing treatment details. If a patient is known to have followed up with another physician but treatment details are not known, then the patient journey would be designated as a transfer of care.
Proposed Classification of Factors Affecting Nonadherence and Nonpersistence
The reasons for treatment nonadherence and nonpersistence were classified according to the World Health Organization dimensions of adherence4: (1) patient associated, (2) condition associated, (3) therapy associated, (4) health system and health care team associated, and (5) social/economic, and (6) other. Within these dimensions, subcategories specific to intravitreal therapy to nAMD were created (Table 3). The subcategories were determined based on common factors identified from the previously published systematic review.6 There was no limit on number of factors per patient, since reasons may be multifaceted or interconnected.
Table 3. Validated Classification of Reasons for Treatment Nonadherence or Nonpersistence to Therapy in Neovascular Age-Related Macular Degeneration.
| Classification | Subcategories | Mean (SD) Delphi score |
|---|---|---|
| Patient associated |
|
9.27 (1.56) |
| Condition associated |
|
9.27 (1.42) |
| Therapy associated |
|
8.64 (2.34) |
| Health system and health care team associated |
|
8.73 (2.33) |
| Social/economic |
|
8.82 (2.34) |
| Other |
|
9.64 (0.67) |
Discussion
There is currently no universal agreement of what adherence and persistence to intravitreal injection therapy is in nAMD. In this study, we have provided a set of definitions that assess the extent and cause of treatment nonadherence or nonpersistence specific to this context.
The definitions proposed here are designed to be sufficiently flexible to cover all currently used injection regimens for nAMD. Although there has been a transition to favoring the treat-and-extend protocol in recent years, using timing of scheduled visits rather than the number of injections enables these definitions to be used by practitioners across different health systems. However, it was decided to restrict these definitions and classification to therapy for nAMD with anti-VEGF only, because intravitreal injection is usually more time critical in this condition compared with other indications, such as diabetic macular edema. The reasons behind nonadherence or nonpersistence are also more likely to differ in this population of older patients, compared with those with macular edema from other retinal diseases.40
This new classification system also addresses some of the shortcomings in previous definitions, one of which was the grouping of patient death or planned discontinuation because of treatment futility with other reasons for nonpersistence.6 Clearly, these represent different scenarios than patients who are nonpersistent because of factors such as lack of transportation, for example. In addition, the distinction between the terminology of adherence and persistence is also clarified here, because patients can be nonadherent yet still persistent.
The classification system for reasons for nonadherence or nonpersistence was also modeled on the World Health Organization dimensions of adherence but had subcategories specific to patients receiving intravitreal injections. This helps to align this system with other discussions of adherence in the health literature yet keeps it relevant to the management of nAMD. However, it is worth noting that although factors have been classified into distinct dimensions, causes can be bidirectional or interdependent. For example, a patient’s perceived treatment burden may be associated with system issues, such as the distance to specialist treatment, which is also associated with social barriers, such as access to transportation. Therefore, for an individual patient, there can be multiple attributable reasons.
Limitations
There are several potential limitations to the proposed classification system. First, these definitions were established from consensus opinions and have yet to be tested on patient data sets. This is similar to the development of other classification systems currently in use, such as the definitions of atrophy associated with AMD, which was established using expert consensus at the Classification of Atrophy Meeting. However, the clinical relevance of our proposed system will be examined in the next phase of the nAMD Barometer initiative, in which these definitions will be used in observational studies of patients, clinicians, and caregivers and their perceptions of barriers to treatment.
A further limitation to this classification system is that these were established in the setting of an industry-sponsored group, which could introduce subconscious bias to the recommendations. The use of objective evidence, such as the systematic literature review and the World Health Organization dimensions of adherence, as the starting basis for developing the definitions is intended to minimize any potential bias.
An additional consideration is that the maximum number of missed visits allowed to still be considered as adherent was 1 per 12-month period, which may not truly reflect all currently used treatment regimens. The rationale for only 1 visit was based on a treat-and-extend regimen in the first year. The absolute minimum number of injections in the first 12 months, assuming a loading dose of 3 injections followed by a 2-weekly extension at every visit, would be approximately 6 injections, and the 80% calculation refers to this scenario. However, although the treat-and-extend approach is increasingly preferred, not every physician or health system uses this regimen. Furthermore, the minimum number of injections per 12-month period will depend on the patient’s disease activity and point in their treatment trajectory. For example, a patient may be on a 16-weekly interval in their third year of treatment, for which an adherence rate of 80% would be 2.4 visits of 3 expected visits per year. Nevertheless, to provide ease of use and better reflect the critical aspect of the first year of treatment, we felt that using a constant and whole number (ie, missing no more than 1 visit per 12 months), rather than percentage of visits, was a reasonable compromise. Finally, usage of this proposed classification system may be limited when used in studies where the intended treatment schedule is not recorded. For example, in some electronic or insurance databases, only the actual date of visit may be recorded. Therefore, missed visits or visits outside the 2-week margin may not necessarily be detected.
Conclusions
Understanding the prevalence and reasons behind nonadherence and nonpersistence is important, in that they remain significant barriers to optimizing outcomes for patients with nAMD. The validated definitions and classification system proposed in this article provide an opportunity to raise awareness among health care professionals and patients. It also sets out a uniform language for use in future research for easier comparison. The current COVID-19 pandemic in particular has presented unprecedented challenges for patient management. It is likely that a considerable proportion of patients with nAMD will have had their treatment interrupted during this crisis.41 Consistent terminology will be important as we begin to assess the effect of the pandemic on patient outcomes.
Consensus definitions also establish benchmarks to measure the effectiveness of interventions designed to improve adherence and persistence to anti-VEGF injections. As part of the nAMD Barometer project, initiatives currently underway include quantifying nonadherence and nonpersistence using this proposed framework in 2 separate studies. The first is a qualitative observational study using interviews with patients and caregivers (both adherent and nonadherent) to determine barriers and assess its association with visual health outcomes. The second is an audience-specific survey of physicians, clinic staff, and patients, intended to quantify perspectives, cultural differences, and health care system differences and correlate these risk factors with their effect on adherence. Future studies could examine whether a tool for triaging and identifying individuals who are at risk can help modify outcomes. Ultimately, the goal will be to develop meaningful interventions, tailored according to the patient, physician, and health system.
eAppendix. Results From Modified Delphi Consensus Process
References
- 1.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3(3):CD005139. doi: 10.1002/14651858.CD005139.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina. 2016;36(8):1418-1431. doi: 10.1097/IAE.0000000000001142 [DOI] [PubMed] [Google Scholar]
- 3.Ramakrishnan MS, Yu Y, VanderBeek BL. Association of visit adherence and visual acuity in patients with neovascular age-related macular degeneration: secondary analysis of the Comparison of Age-Related Macular Degeneration Treatment trial. JAMA Ophthalmol. 2020;138(3):237-242. doi: 10.1001/jamaophthalmol.2019.4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabaté E. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003. [PubMed] [Google Scholar]
- 5.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44-47. doi: 10.1111/j.1524-4733.2007.00213.x [DOI] [PubMed] [Google Scholar]
- 6.Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128(2):234-247. doi: 10.1016/j.ophtha.2020.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalkey NC. The Delphi Method: An Experimental Study of Group Opinion. Rand Corporation; 1969. [Google Scholar]
- 8.Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23(2):127-140. doi: 10.1080/13548506.2016.1274040 [DOI] [PubMed] [Google Scholar]
- 9.Cohen SY, Mimoun G, Oubraham H, et al. ; LUMIERE Study Group . Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33(3):474-481. doi: 10.1097/IAE.0b013e31827b6324 [DOI] [PubMed] [Google Scholar]
- 10.Heimes B, Gunnemann F, Ziegler M, et al. Compliance von patienten mit altersabhängiger makuladegeneration unter anti-VEGF-therapie: analyse und verbesserungsvorschläge. Ophthalmologe. 2016;113(11):925-932. doi: 10.1007/s00347-016-0275-z [DOI] [PubMed] [Google Scholar]
- 11.Krivosic V, Philippakis E, Couturier A, et al. [A “fast track” to improve management of neovascular age related macular degeneration]. J Fr Ophtalmol. 2017;40(8):642-647. doi: 10.1016/j.jfo.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 12.Polat O, İnan S, Özcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turk J Ophthalmol. 2017;47(4):205-210. doi: 10.4274/tjo.28003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varano M, Eter N, Winyard S, Wittrup-Jensen KU, Navarro R, Heraghty J. Current barriers to treatment for wet age-related macular degeneration (wAMD): findings from the wAMD patient and caregiver survey. Clin Ophthalmol. 2015;9:2243-2250. doi: 10.2147/OPTH.S92548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massamba N, Dirani A, Knoeri J, Pasquier B, Ingram A, Soubrane G. Evaluating the impact of summer vacation on the visual acuity of AMD patients treated with ranibizumab. Eye (Lond). 2015;29(11):1453-1457. doi: 10.1038/eye.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Droege KM, Muether PS, Hermann MM, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1281-1284. doi: 10.1007/s00417-012-2177-3 [DOI] [PubMed] [Google Scholar]
- 16.Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2017;12:13-20. doi: 10.2147/OPTH.S151611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlken C, Wilke T, Bauer-Steinhusen U, Agostini HT, Hasanbasic Z, Müller S. Treatment of neovascular age-related macular degeneration patients with vascular endothelial growth factor inhibitors in everyday practice: identification of health care constraints in Germany-the PONS Study. Retina. 2018;38(6):1134-1144. doi: 10.1097/IAE.0000000000001681 [DOI] [PubMed] [Google Scholar]
- 18.Sachs HG, Wilke RG. [Anti VEGF therapy under real-life conditions: adherance determines long term outcome in neovascular AMD]. Klin Monbl Augenheilkd. 2016;233(8):958-964. [DOI] [PubMed] [Google Scholar]
- 19.Chang A, Stokes J, Priestman L, Holmes C, Said P. Persistence to aflibercept therapyin wet amd patients engaged with thesmartsight support program. Clin Exp Ophthalmol. 2018;46:92-93.28627769 [Google Scholar]
- 20.Curtis LH, Hammill BG, Qualls LG, et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 Medicare beneficiaries. Am J Ophthalmol. 2012;153(6):1116-24.e1. doi: 10.1016/j.ajo.2011.11.032 [DOI] [PubMed] [Google Scholar]
- 21.Lad EM, Hammill BG, Qualls LG, Wang F, Cousins SW, Curtis LH. Anti-VEGF treatment patterns for neovascular age-related macular degeneration among Medicare beneficiaries. Am J Ophthalmol. 2014;158(3):537-43.e2. doi: 10.1016/j.ajo.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 22.McGrath LA, Lee LR. Characteristics of patients who drop out from ranibizumab therapy. Asia Pac J Ophthalmol (Phila). 2013;2(5):295-299. doi: 10.1097/APO.0b013e31829dc65a [DOI] [PubMed] [Google Scholar]
- 23.Nunes RP, Nóbrega MJ, De Novelli FJ, et al. Causes of interruption of bevacizumab therapy in age-related macular degeneration. Arq Bras Oftalmol. 2010;73(2):146-149. doi: 10.1590/S0004-27492010000200009 [DOI] [PubMed] [Google Scholar]
- 24.Oishi A, Mandai M, Nishida A, Hata M, Matsuki T, Kurimoto Y. Remission and dropout rate of anti-VEGF therapy for age-related macular degeneration. Eur J Ophthalmol. 2011;21(6):777-782. doi: 10.5301/EJO.2011.7430 [DOI] [PubMed] [Google Scholar]
- 25.Vaze A, Fraser-Bell S, Gillies M. Consequences of long-term discontinuation of vascular endothelial growth factor inhibitor therapy in the patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2014;92(8):e697-e698. doi: 10.1111/aos.12417 [DOI] [PubMed] [Google Scholar]
- 26.Westborg I, Rosso A. Risk factors for discontinuation of treatment for neovascular age-related macular degeneration. Ophthalmic Epidemiol. 2018;25(2):176-182. doi: 10.1080/09286586.2017.1397701 [DOI] [PubMed] [Google Scholar]
- 27.Krüger Falk M, Kemp H, Sørensen TL. Four-year treatment results of neovascular age-related macular degeneration with ranibizumab and causes for discontinuation of treatment. Am J Ophthalmol. 2013;155(1):89-95.e3. doi: 10.1016/j.ajo.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen A, Bloch SB, Fuchs J, et al. A 4-year longitudinal study of 555 patients treated with ranibizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(12):2630-2636. doi: 10.1016/j.ophtha.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 29.Ozturk M, Harris ML, Nguyen V, Barthelmes D, Gillies MC, Mehta H. Real-world visual outcomes in patients with neovascular age-related macular degeneration receiving aflibercept at fixed intervals as per UK licence. Clin Exp Ophthalmol. 2018;46(4):407-411. doi: 10.1111/ceo.13085 [DOI] [PubMed] [Google Scholar]
- 30.Vaze A, Fraser-Bell S, Gillies M. Reasons for discontinuation of intravitreal vascular endothelial growth factor inhibitors in neovascular age-related macular degeneration. Retina. 2014;34(9):1774-1778. doi: 10.1097/IAE.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 31.Holz FG, Tadayoni R, Beatty S, et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99(2):220-226. doi: 10.1136/bjophthalmol-2014-305327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eldem BM, Muftuoglu G, Topbaş S, et al. ; SALUTE Study Group . A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a Turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta Ophthalmol. 2015;93(6):e458-e464. doi: 10.1111/aos.12540 [DOI] [PubMed] [Google Scholar]
- 33.Jelin E, Wisløff T, Moe MC, Heiberg T. Development and testing of a patient-derived questionnaire for treatment of neovascular age-related macular degeneration: dimensions of importance in treatment of neovascular age-related macular degeneration. Acta Ophthalmol. 2018;96(8):804-811. doi: 10.1111/aos.13847 [DOI] [PubMed] [Google Scholar]
- 34.Pagliarini S, Beatty S, Lipkova B, et al. A 2-year, phase IV, multicentre, observational study of ranibizumab 0.5 mg in patients with neovascular age-related macular degeneration in routine clinical practice: the EPICOHORT study. J Ophthalmol. 2014;2014:857148. doi: 10.1155/2014/857148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subhi Y, Sørensen TL. Neovascular age-related macular degeneration in the very old (≥90 years): epidemiology, adherence to treatment, and comparison of efficacy. J Ophthalmol. 2017;2017:7194927. doi: 10.1155/2017/7194927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wintergerst MWM, Bouws J, Loss J, et al. Gründe für therapieverzögerung und -abbruch bei altersabhängiger makuladegeneration. Ophthalmologe. 2018;115(12):1035-1041. doi: 10.1007/s00347-017-0610-z [DOI] [PubMed] [Google Scholar]
- 37.Boulanger-Scemama E, Querques G, About F, et al. Ranibizumab for exudative age-related macular degeneration: a five year study of adherence to follow-up in a real-life setting. J Fr Ophtalmol. 2015;38(7):620-627. doi: 10.1016/j.jfo.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 38.Burnier M. Is there a threshold for medication adherence? lessons learnt from electronic monitoring of drug adherence. Front Pharmacol. 2019;9:1540. doi: 10.3389/fphar.2018.01540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glombiewski JA, Nestoriuc Y, Rief W, Glaesmer H, Braehler E. Medication adherence in the general population. PLoS One. 2012;7(12):e50537. doi: 10.1371/journal.pone.0050537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2017;12:13-20. doi: 10.2147/OPTH.S151611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Gage SB, Drouin J, Desplas D, et al. Intravitreal anti–vascular endothelial growth factor use in france during the coronavirus disease 2019 pandemic. JAMA Ophthalmol. Published online December 17, 2020. doi: 10.1001/jamaophthalmol.2020.5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Results From Modified Delphi Consensus Process


