Key Points
Question
Among residents and staff of skilled nursing and assisted living facilities with high risk of SARS-CoV-2 exposure, what is the effect of bamlanivimab on the incidence of COVID-19?
Findings
This randomized phase 3 clinical trial included 966 participants who were residents and staff at US skilled nursing and assisted living facilities with at least 1 confirmed SARS-CoV-2 index case and who were negative at baseline for SARS-CoV-2 infection and serology, enrolled from August to November 2020. The incidence of COVID-19 infection among those treated with bamlanivimab vs placebo was 8.5% vs 15.2%, respectively, a difference that was statistically significant.
Meaning
Bamlanivimab monotherapy compared with placebo reduced the risk of COVID-19 in residents and staff of skilled nursing and assisted living facilities.
Abstract
Importance
Preventive interventions are needed to protect residents and staff of skilled nursing and assisted living facilities from COVID-19 during outbreaks in their facilities. Bamlanivimab, a neutralizing monoclonal antibody against SARS-CoV-2, may confer rapid protection from SARS-CoV-2 infection and COVID-19.
Objective
To determine the effect of bamlanivimab on the incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities.
Design, Setting, and Participants
Randomized, double-blind, single-dose, phase 3 trial that enrolled residents and staff of 74 skilled nursing and assisted living facilities in the United States with at least 1 confirmed SARS-CoV-2 index case. A total of 1175 participants enrolled in the study from August 2 to November 20, 2020. Database lock was triggered on January 13, 2021, when all participants reached study day 57.
Interventions
Participants were randomized to receive a single intravenous infusion of bamlanivimab, 4200 mg (n = 588), or placebo (n = 587).
Main Outcomes and Measures
The primary outcome was incidence of COVID-19, defined as the detection of SARS-CoV-2 by reverse transcriptase–polymerase chain reaction and mild or worse disease severity within 21 days of detection, within 8 weeks of randomization. Key secondary outcomes included incidence of moderate or worse COVID-19 severity and incidence of SARS-CoV-2 infection.
Results
The prevention population comprised a total of 966 participants (666 staff and 300 residents) who were negative at baseline for SARS-CoV-2 infection and serology (mean age, 53.0 [range, 18-104] years; 722 [74.7%] women). Bamlanivimab significantly reduced the incidence of COVID-19 in the prevention population compared with placebo (8.5% vs 15.2%; odds ratio, 0.43 [95% CI, 0.28-0.68]; P < .001; absolute risk difference, −6.6 [95% CI, −10.7 to −2.6] percentage points). Five deaths attributed to COVID-19 were reported by day 57; all occurred in the placebo group. Among 1175 participants who received study product (safety population), the rate of participants with adverse events was 20.1% in the bamlanivimab group and 18.9% in the placebo group. The most common adverse events were urinary tract infection (reported by 12 participants [2%] who received bamlanivimab and 14 [2.4%] who received placebo) and hypertension (reported by 7 participants [1.2%] who received bamlanivimab and 10 [1.7%] who received placebo).
Conclusions and Relevance
Among residents and staff in skilled nursing and assisted living facilities, treatment during August-November 2020 with bamlanivimab monotherapy reduced the incidence of COVID-19 infection. Further research is needed to assess preventive efficacy with current patterns of viral strains with combination monoclonal antibody therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT04497987
This randomized clinical trial assesses the effect of a single intravenous infusion of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities.
Introduction
SARS-CoV-2 continues to spread globally, resulting in associated morbidity, increased mortality, and overall strain on health care resources. Outbreaks of COVID-19 within nursing homes and assisted living facilities have been associated with high rates of morbidity and mortality.1,2,3 In the United States, as of February 2021, long-term care facilities accounted for 5% of reported COVID-19 cases but a disproportionate 37% of deaths.4 Residents have an increased risk of development of severe COVID-19 and associated mortality as they are generally older and have multiple comorbidities.5,6,7,8 Close living environments, presymptomatic and asymptomatic transmission of SARS-CoV-2,1,9,10 and frequent staff contact required to meet the care needs of frail residents have contributed to SARS-CoV-2 transmission.
While the emergence of efficacious vaccines is a critical step in addressing the COVID-19 pandemic, other preventive interventions, such as passive immunization, may protect vulnerable populations until widespread immunization is achieved. Additionally, vaccines may have decreased efficacy or durability in such populations.11 Neutralizing monoclonal antibodies that recognize the spike protein of SARS-CoV-2 may provide protection against COVID-19 or SARS-CoV-2 infection.12 Bamlanivimab, alone or in combination with etesevimab, received an Emergency Use Authorization for the treatment of mild to moderate COVID-19 in individuals at high risk of severe COVID-1913,14 based on data from an ongoing phase 2/3 study (NCT04427501). Treatment with bamlanivimab reduced nasopharyngeal viral load and COVID-19–related hospitalizations or emergency department visits compared with placebo.15
Preclinical studies have provided support for the premise that bamlanivimab may be a viable clinical prophylactic intervention, as demonstrated by reduced viral replication and load in the upper and lower airways in rhesus macaques in SARS-CoV-2 challenge studies.12 The phase 3 BLAZE-2 trial was designed to evaluate the efficacy of bamlanivimab in preventing COVID-19 in skilled nursing and assisted living facility residents and staff.
Methods
Trial Design and Participants
This clinical trial is an ongoing, multipart, phase 3, randomized, double-blind, placebo-controlled, single-dose study evaluating the efficacy and safety of bamlanivimab in preventing COVID-19 and SARS-CoV-2 infection.
The trial complied with the Declaration of Helsinki,16 the Council for International Organizations of Medical Sciences, the International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable laws and regulations. The study was reviewed and approved by a centralized institutional review board (Advarra). Study participants or their legal representatives provided written informed consent prior to treatment randomization.
The trial enrolled residents and staff at 74 skilled nursing and assisted living facilities in the United States (California, Colorado, Florida, Illinois, Indiana, Kentucky, Missouri, North Carolina, Ohio, Pennsylvania, and Virginia). All 74 facilities had at least 1 confirmed SARS-CoV-2 index case. The original and final protocols for the phase 3 trial, including the original and final statistical analysis plans, appear in Supplement 1.
Within 7 days of a reported confirmed SARS-CoV-2 case at a facility, residents and staff of the facility were screened for enrollment, and, if eligible, randomly assigned and dosed with 4200 mg of intravenous bamlanivimab or placebo (saline). All participants were aged 18 years or older and had no known history of COVID-19. Participants provided both nasal and nasopharyngeal swabs (baseline samples) for detection of SARS-CoV-2 by reverse transcriptase–polymerase chain reaction (RT-PCR) and blood samples for SARS-CoV-2 serology tests. Serum was tested for anti–SARS-CoV-2 antibodies using the Elecsys anti–SARS-CoV-2 electrochemiluminescence immunoassay intended for qualitative detection of antibodies to SARS-CoV-2 on a cobas e 602 (Roche Diagnostics). Additional information about the methods used to detect SARS-CoV-2 by RT-PCR and to test for anti-SARS-CoV-2 antibodies is available in the eAppendix in Supplement 2. Participants were randomized and received the trial product (bamlanivimab or placebo) before the results were available. Those who were negative at baseline for SARS-CoV-2 by RT-PCR (nasopharyngeal and nasal swabs) and serology comprised the prevention population. Participants positive at baseline for SARS-CoV-2 by RT-PCR and negative for serology comprised a treatment population for which results will be presented in more detail in a future publication. The safety population included all participants who received bamlanivimab or placebo regardless of baseline serostatus.
Given the reported disparities in the prevalence of COVID-19 across racial/ethnic subgroups,17 information on this variable was collected by study personnel based on fixed categories selected by the study participants.
Randomization
Participants enrolled in the study from August 2 to November 20, 2020. Database lock was on January 13, 2021. Eligible patients were randomized to receive 4200 mg of bamlanivimab or placebo. All participants were centrally randomized to study intervention using an interactive web response system (IWRS). Before the study was initiated, the log-in information and directions for the IWRS were provided to each site. To achieve between-group comparability, block randomization within each facility was used (block size of 4). Randomized participants within the facility were stratified by role within the facility (resident vs facility staff) and by sex.
Intervention
Participants received a single intravenous infusion of 4200 mg of bamlanivimab or placebo. The evaluation period was 8 weeks, with follow-up to 24 weeks. This article includes results from the prevention population in part 1 of this multipart study (see protocol and statistical analysis plan in Supplement 1).
Clinical and Laboratory Monitoring
Nasal swabs were obtained on study days 1, 8, 15, 22, 29, 36, 43, 50, and 57 and during postevaluation follow-up for detection of SARS-CoV-2 by RT-PCR. Serology samples were obtained at baseline, study day 29, and study day 57 during the 8-week evaluation period. Participants completed a questionnaire at screening and daily during the evaluation period on symptoms and signs associated with COVID-19 experienced during the past 24 hours. Vital signs, hospitalization events, clinical symptoms, and interventions of interest were recorded daily. Participants testing positive for SARS-CoV-2 were treated per standard of care.
Outcomes
The primary outcome was cumulative incidence within 8 weeks of randomization of COVID-19, defined as detection of SARS-CoV-2 by RT-PCR and presence of mild or worse disease severity within 21 days of detection. Mild or worse disease severity included mild, moderate, severe, and critical disease severity and death due to COVID-19 (eTable 1 in Supplement 2). Key secondary outcomes included cumulative incidence within 8 weeks of randomization of moderate or worse COVID-19 severity, defined as detection of SARS-CoV-2 by RT-PCR and moderate or worse disease severity within 21 days of detection, and cumulative incidence within 4 weeks of randomization of SARS-CoV-2 infection, defined as detection of SARS-CoV-2 by RT-PCR. Moderate or worse disease severity included moderate, severe, and critical disease severity and death due to COVID-19 (eTable 1). eTable 1 provides a list of symptoms and signs associated with mild, moderate, severe, and critical COVID-19. There were 4 other secondary end points, including mortality attributed to COVID-19. Other secondary end points not reported herein include incidence of SARS-CoV-2 infection 8 weeks after randomization, frequency of hospitalization or death due to COVID-19, and characterization of participant clinical status. Exploratory end points included characterization of SARS-CoV-2 viral end points in participants who became positive for SARS-CoV-2 infection by RT-PCR (proportion of participants who achieved SARS-CoV-2 clearance, defined as a single negative SARS-CoV-2 RT-PCR test result within 1, 2, or 3 weeks of an initial positive SARS-CoV-2 RT-PCR test result; and time to SARS-CoV-2 clearance). For a full list of secondary and exploratory outcomes, see the protocol in Supplement 1.
Sample Size Calculation
The trial was designed as event driven. Thirty-three events were calculated to be necessary to show statistical superiority of bamlanivimab, 4200 mg, over placebo in each of the primary and key secondary end points using the formula for proportional hazards models.18 The prespecified minimum detectable effect size was a relative risk of 0.33, which was chosen based on what was considered medically meaningful by the clinical experts on the author team. Assuming 90% power for the primary and key secondary end points and an 8-week placebo-group event rate of 4% for moderate-severity or worse COVID-19, a sample size of approximately 1300 participants in the prevention population was expected to yield the needed number of events for each end point.
Statistical Analysis
The National Institute of Allergy and Infectious Diseases data and safety monitoring board (DSMB) monitored the safety and efficacy of the trial monthly and conducted the review of an interim analysis of the first 300 facility residents and a subsequent analysis after all participants reached study day 57. At primary database lock, the DSMB recommended that the study should continue to obtain full outcome data for all participants while maintaining blinding of all investigators, participants, and study staff.
All randomized participants (regardless of whether they received any doses of study treatment or if they received the correct treatment) who were SARS-CoV-2 RT-PCR negative and serology negative at baseline were included in the efficacy analysis. Participants were analyzed according to the treatment group to which they were randomized (see section 6.1.1 of the statistical analysis plan in Supplement 1).
For the primary and key secondary end points, logistic regression was used to compare the treatments, with treatment, facility, and the stratification factors of sex and role in the facility (resident or staff) included as fixed effects. The inclusion of facility in the model was important, as it accounted for potentially highly heterogeneous extent of outbreak and, hence, levels of exposure to SARS-CoV-2, across facilities. Treatment effects were compared using 2-sided tests with an α = .05 unless otherwise stated. Two-sided 95% Wald confidence intervals for odds ratios and P values from a Rao test are presented. To control for multiplicity, a graphical testing sequence approach was prespecified to test the primary and key secondary end points. If the primary end point of mild or worse COVID-19 was met at the 2-sided α = .05 significance level, the end points of moderate or worse COVID-19 and SARS-CoV-2 infection were tested at the α = .04 and α = .01 levels, respectively. If either of these end points were met, the remaining end point was then tested at the α = .05 level. All other analyses were conducted at the 2-sided α = .05 significance level without adjustments for multiplicity, and the results should be interpreted as exploratory. Only confirmed SARS-CoV-2 infections and COVID-19 cases were included in the analysis. If a participant discontinued prior to a confirmed event for a given end point, he or she did not contribute any event in the logistic regression and was censored at the time of discontinuation in the time-to-event analysis. Less than 4% of participants discontinued within the end-point windows. For other approaches to handling of missing data, see sections 6.3.1 and 6.3.2 of the statistical analysis plan in Supplement 1.
Exploratory subgroup analyses of primary and key secondary end points within the resident (prespecified) and high-risk (post hoc) populations were also conducted. High-risk participants included all residents of the skilled nursing or assisted living facilities and staff who satisfied at least 1 of the following at the time of screening: aged 65 years or older with a body mass index of 35 or higher, chronic kidney disease, type 1 or type 2 diabetes, or immunosuppressive disease or receiving immunosuppressive treatment; or aged 55 years or older with cardiovascular disease, hypertension, chronic obstructive pulmonary disease, or other chronic respiratory disease. Details regarding these methods are provided in section 6 of the statistical analysis plan in Supplement 1.
In the process of closing out the study, it was discovered that for a subset of participants who required legally authorized representatives for consent, more symptom data were collected than permitted by the protocol. A sensitivity analysis that excluded those symptom data was carried out.
The statistical analyses were performed using SAS version 9.4 or higher (SAS Institute Inc), FACTS version 6.0 or higher (FACT Software Group), and/or R version 3.6 or higher (R Foundation).
Results
Participants
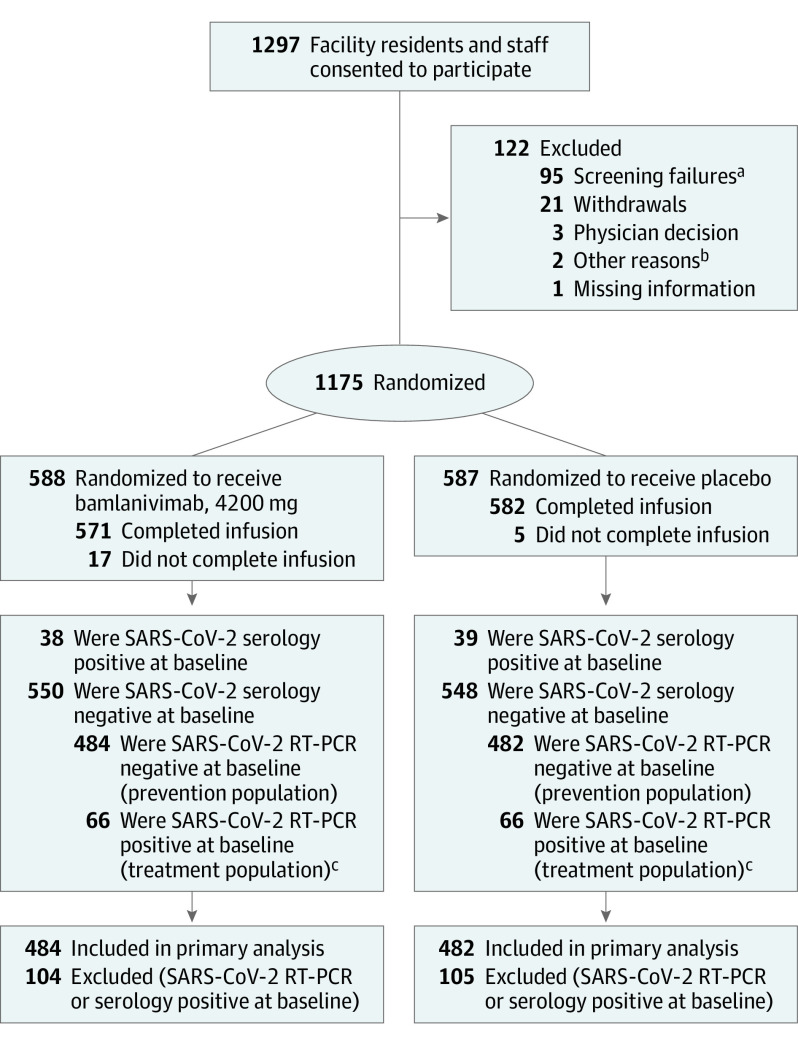
A total of 1297 participants enrolled in the study. Of the 1175 randomized participants who received study drug, 588 received 4200 mg of bamlanivimab and 587 received placebo (Figure 1); 966 participants comprised the prevention population (negative at baseline for SARS-CoV-2 by RT-PCR and serology), of which 300 were residents. A total of 132 participants comprised the treatment population (positive at baseline for SARS-CoV-2 infection and negative for SARS-CoV-2 serology at baseline) with either presymptomatic or asymptomatic infection. Data from the treatment population will be reported in a future publication.
Figure 1. Flow of Participants Through the BLAZE-2 Randomized Clinical Trial.
RT-PCR indicates reverse transcriptase–polymerase chain reaction.
aA majority of the 95 screening failures were due to a lack of venous access sufficient to allow intravenous infusions and blood sampling per protocol (45 participants) or due to a serious concomitant systemic disease, condition, or disorder that in the opinion of investigators should preclude participation (34 participants), as described in sections 5.1 and 5.2 of the trial protocol (Supplement 1).
bOne participant provided signed consent but did not return for infusion, and for 1 participant, infusion nurses were unable to secure intravenous access despite multiple attempts.
cThe treatment population will be reported on in a future publication.
Baseline demographics and risk factors were balanced between the bamlanivimab group and the placebo group (Table 1; eTables 2-4 in Supplement 2). Among participants in the prevention population, the median age was 53.0 (range, 18-104) years, 29.2% (282 of 966) were aged 65 years or older, and 74.7% (722 of 966) were female. Among the resident participants in the prevention population, the median age was 76.0 (range, 31-104) years, 78.3% (235 of 300) were aged 65 years or older at the time of randomization, and 59.7% (179 of 300) were female.
Table 1. Baseline Participant Characteristicsa.
| Characteristics | Residents | Staff | ||
|---|---|---|---|---|
| Bamlanivimab (n = 161) | Placebo (n = 139) | Bamlanivimab (n = 323) | Placebo (n = 343) | |
| Age | ||||
| Median (range), y | 76.0 (31-104) | 75.0 (41-96) | 43.0 (18-82) | 42.0 (18-74) |
| No. (%) ≥65 y | 126 (78.3) | 109 (78.4) | 19 (5.9) | 28 (8.2) |
| Sex, No. (%) | ||||
| Female | 95 (59.0) | 84 (60.4) | 260 (80.5) | 283 (82.5) |
| Male | 66 (41.0) | 55 (39.6) | 63 (19.5) | 60 (17.5) |
| Race, No./total (%)b | ||||
| White | 145/160 (90.6) | 126/138 (91.3) | 284/322 (88.2) | 303/340 (89.1) |
| Black or African American | 13/160 (8.1) | 11/138 (8.0) | 25/322 (7.8) | 30/340 (8.8) |
| American Indian or Alaska Native | 0 | 0 | 4/322 (1.2) | 1/340 (0.3) |
| Asian | 1/160 (0.6) | 0 | 5/322 (1.6) | 5/340 (1.5) |
| Native Hawaiian or other Pacific Islander | 1/160 (0.6) | 0 | 1/322 (0.3) | 1/340 (0.3) |
| Multiple | 0 | 1/138 (0.7) | 3/322 (0.9) | 0 |
| Hispanic or Latino ethnicity, No./total (%)b | 3/160 (1.9) | 7/139 (5.0) | 17/323 (5.3) | 21/343 (6.1) |
| Body mass index, median (range)c | 28.2 (15.4-64.7) | 29.1 (14.1-77.4) | 29.9 (16.4-62.0) | 30.3 (16.5-65.7) |
| At high risk of severe COVID-19, No. (%)d | 161 (100) | 139 (100) | 132 (40.9) | 143 (41.7) |
Participants were negative at baseline for SARS-CoV-2 by reverse transcriptase–polymerase chain reaction and serology.
Race and ethnicity were self-reported by participants.
Body mass index is calculated as weight in kilograms divided by the square of height in meters.
All residents were high risk by definition. Staff were high risk if they met 1 or more of the following at the time of screening: (1) aged ≥65 years with body mass index ≥35, chronic kidney disease, type 1 or type 2 diabetes, immunosuppressive disease, or receiving immunosuppressive treatment or (2) aged ≥55 years with cardiovascular disease, hypertension, chronic obstructive pulmonary disease, or other chronic respiratory disease.
Participants in the prevention population who were at high risk of severe COVID-19 comprised the high-risk prevention population (described in eTable 1 in Supplement 2). All 300 resident participants and 41.3% (275 of 666) of the staff participants were included in the high-risk prevention population.
Primary Outcome
For the overall prevention population, 114 participants (11.9%) experienced mild or worse COVID-19 by day 57; participants who received bamlanivimab had significantly reduced incidence of mild or worse COVID-19 compared with participants who received placebo (8.5% vs 15.2%; odds ratio, 0.43; 95% CI, 0.28-0.68; P < .001), with an absolute risk difference of −6.6 (95% CI, −10.7 to −2.6) percentage points (eFigure 1 in Supplement 2). In the resident prevention population, incidence of mild or worse COVID-19 was significantly lower in the bamlanivimab group compared with the placebo group (8.8% vs 22.5%; odds ratio, 0.20; 95% CI, 0.08-0.49; P < .001), for an absolute risk difference of −13.7 (95% CI, −21.9 to −5.4) percentage points (Figure 2). Among the staff in the prevention population, incidence of mild or worse COVID-19 was not significantly different in the bamlanivimab group compared with the placebo group (8.4% vs 12.2%; odds ratio, 0.58; 95% CI, 0.33-1.02; P = .06), with an absolute risk difference of −3.8 (95% CI, −8.4 to 0.8) percentage points (P = .12 for interaction) (Figure 2). In the high-risk prevention population, a post hoc analysis showed that the incidence of mild or worse COVID-19 was also lower in the bamlanivimab group compared with the placebo group (eFigure 1).
Figure 2. Time From Infusion to Development of Mild or Worse COVID-19 With Bamlanivimab vs Placebo Among Resident and Staff Participants.
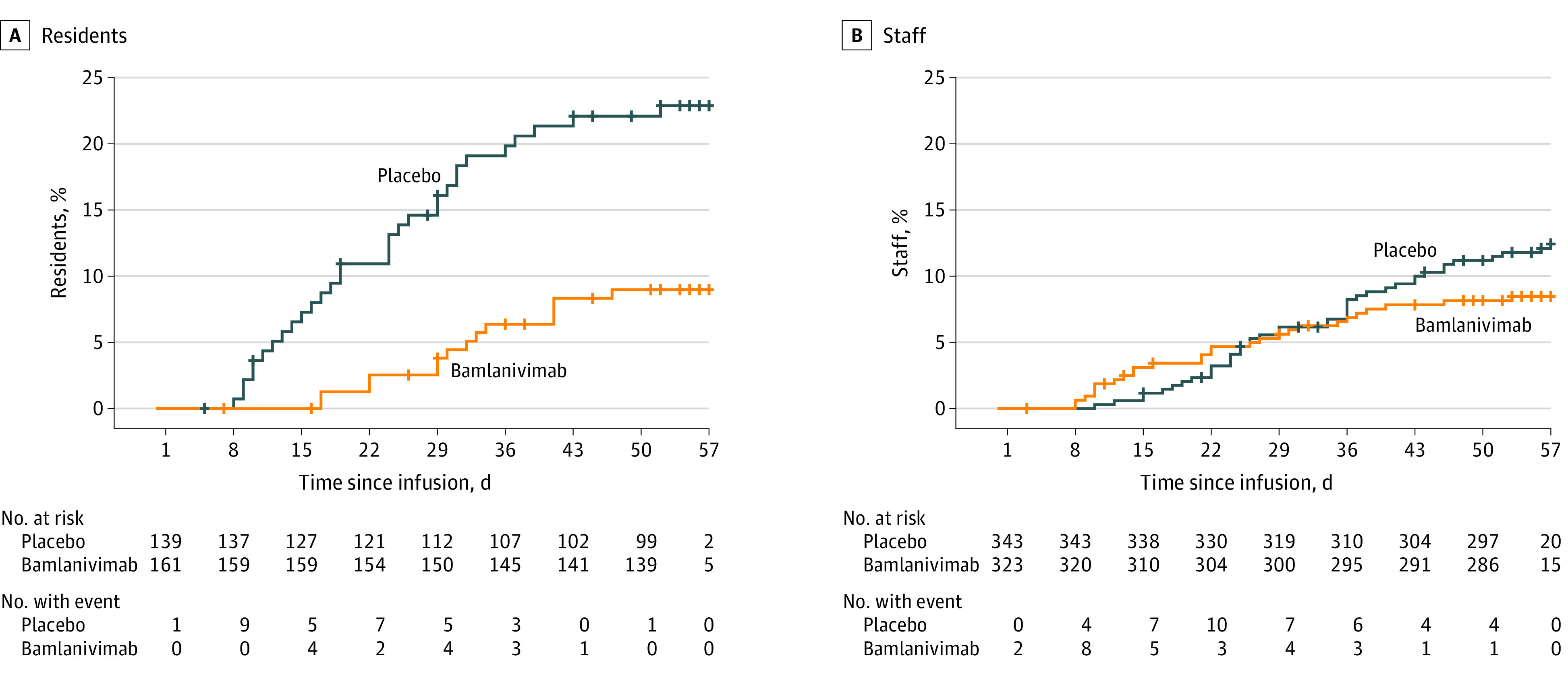
Participants were negative at baseline for SARS-CoV-2 by reverse transcriptase–polymerase chain reaction and serology. More than 95% of participants were followed up beyond day 57. Similar data for other subgroups are shown in eFigure 1 in Supplement 2.
Key Secondary Outcomes
Two key secondary end points were assessed within the multiplicity-controlled serial gatekeeping testing scheme: the incidence of moderate or worse severity COVID-19 by day 57 and the incidence of SARS-CoV-2 infection by day 29. In the overall prevention population, administration of bamlanivimab significantly reduced the incidence of moderate or worse COVID-19 compared with placebo (8.3% vs 14.1%; odds ratio, 0.46; 95% CI, 0.29-0.73; P < .001), with an absolute risk difference of −5.8 (95% CI, −9.8 to −1.8) percentage points (eFigure 2A in Supplement 2). In the resident prevention population, incidence of moderate or worse COVID-19 was also lower among bamlanivimab recipients compared with placebo recipients (8.8% vs 21.7%; odds ratio, 0.20; 95% CI, 0.08-0.49), with an absolute risk difference of −12.9 (95% CI, −21.1 to −4.8) percentage points (eFigure 2A). Among the staff in the prevention population, 8.1% of staff participants in the bamlanivimab group and 11.1% of staff participants in the placebo group experienced moderate or worse COVID-19 by day 57 (odds ratio, 0.61; 95% CI, 0.34-1.09), with an absolute risk difference of −3.0 (95% CI, −7.4 to 1.5) percentage points (P = .11 for interaction) (eFigure 2A). In the high-risk prevention population, a post hoc analysis showed that fewer cases of moderate or worse COVID-19 occurred in participants who received bamlanivimab compared with those who received placebo (eFigure 2A).
Assessment of infection with SARS-CoV-2 by RT-PCR within 4 weeks of randomization revealed that 198 participants (20.6%) in the prevention population tested positive for SARS-CoV-2. Bamlanivimab was associated with significantly lower incidence of SARS-CoV-2 infection compared with placebo (17.9% vs 23.3%; odds ratio, 0.66; 95% CI, 0.46-0.94; P = .02), with an absolute risk difference of −5.4 (95% CI, −10.5 to −0.3) percentage points (eFigure 2B in Supplement 2). Bamlanivimab was also associated with lower incidence of SARS-CoV-2 infection by week 4 in the resident and high-risk prevention populations. In the resident prevention population, 15.1% (24 of 159) of resident participants who received bamlanivimab and 31.9% (44 of 138) of residents who received placebo tested positive for SARS-CoV-2 by RT-PCR (odds ratio, 0.24; 95% CI, 0.12-0.51]), with an absolute risk difference of −16.8 (95% CI, −26.4 to −7.2) percentage points (Figure 3A). Among the staff in the prevention population, 19.3% of staff participants in the bamlanivimab group and 19.8% in the placebo group tested positive for SARS-CoV-2 by RT-PCR by week 4 (odds ratio, 0.96; 95% CI, 0.62-1.49), with an absolute risk difference of −0.5 (95% CI, −6.5 to 5.5) percentage points (P = .005 for interaction) (Figure 3B). In the high-risk prevention population, a post hoc analysis showed that fewer participants in the bamlanivimab group tested positive for SARS-CoV-2 by RT-PCR compared with the placebo group (eFigure 2B).
Figure 3. Time From Infusion to Detection of SARS-CoV-2 by RT-PCR With Bamlanivimab vs Placebo and Viral Load in Participants Who Tested Positive for SARS-CoV-2 During the Study.
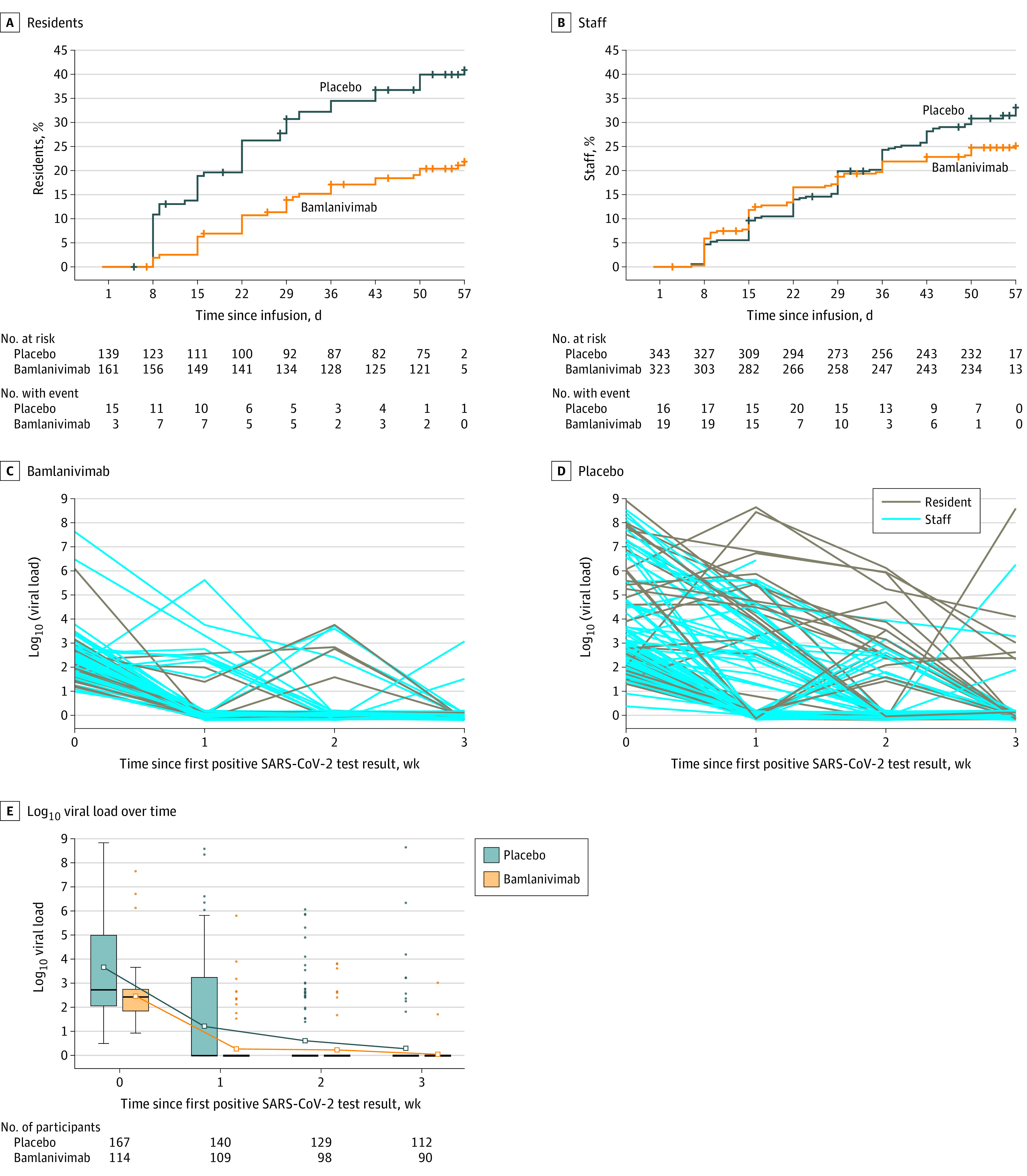
A and B, Kaplan-Meier curves for time from infusion to detection of SARS-CoV-2 by reverse transcriptase–polymerase chain reaction (RT-PCR) with bamlanivimab vs placebo among resident and staff participants. Participants were negative at baseline for SARS-CoV-2 by RT-PCR and serology. More than 95% of participants were followed up beyond day 57. Similar data for other subgroups are shown in eFigure 2B in Supplement 2. C and D, Spaghetti plots for log10 viral load over time for resident and staff participants. Participants were negative at baseline for SARS-CoV-2 by RT-PCR and serology. Participants with at least 1 positive postbaseline SARS-CoV-2 RT-PCR test result: bamlanivimab group, n = 23 residents and n = 62 staff; placebo group, n = 44 residents and n = 68 staff. Time 0 represents the time of a participant’s first positive RT-PCR test result. Each line represents a participant. E, Box plot for log10 viral load over time for participants with at least 1 positive postbaseline SARS-CoV-2 RT-PCR test result. Time 0 represents the time of a participant’s first positive RT-PCR test result. Box tops and bottoms indicate interquartile ranges; solid horizontal bars, medians; hollow squares, means; whiskers, lowest and highest observations within 1.5 interquartile ranges of the first and third quartiles; and dots, outliers. In the later weeks, the 25th, 50th, and 75th percentiles are coincident, so the boxes appear as solid horizontal bars.
Other Secondary Outcomes
An additional prespecified secondary end point of this study was mortality due to COVID-19. All 5 COVID-19–related deaths in the study occurred in resident participants randomized to receive placebo. By day 57, in the prevention population, 4 COVID-19-related deaths occurred among participants in the placebo group (n = 482) vs 0 in the bamlanivimab group (n = 484) (eTable 5 in Supplement 2). In the treatment population (see Methods section), 1 COVID-19–related death occurred among resident participants in the placebo group (n = 66) vs 0 in the bamlanivimab group (n = 66). Deaths occurred in residents ranging in age from 65 to 89 years.
Exploratory End Points
A total of 282 participants (29.3%) in the prevention population tested positive for SARS-CoV-2 by RT-PCR within 8 weeks of randomization. Participants who received bamlanivimab had lower viral loads (converted from cycle threshold values; see Section 6 of the statistical analysis plan in Supplement 1) compared with placebo recipients at the time of their first positive test result (2.44 vs 3.64 log10 viral load) and the decrease in mean viral load at first positive RT-PCR status to mean viral load at week 1 was greater in the bamlanivimab group compared with the placebo group (−0.8 [95% CI, −1.22 to −0.39] log10 viral load; P < .001) (Figure 3, C-E). The proportion of participants who achieved viral clearance after testing positive for SARS-CoV-2 was greater in the bamlanivimab group compared with the placebo group at 1 week (14.9% vs 8.3%), 2 weeks (86.0% vs 61.3%), and 3 weeks (93.0% vs 78.0%) (eFigure 3 in Supplement 2).
Post Hoc Analyses
In a post hoc analysis of the participants in the prevention population who became infected with SARS-CoV-2 (tested positive by RT-PCR prior to day 57), fewer participants were SARS-CoV-2 serology positive in the bamlanivimab group compared with the placebo group by study day 57 (2.6% vs 15.0%) (eFigure 4 in Supplement 2).
Sensitivity Analysis
In the sensitivity analysis, some symptom data were excluded for 128 residents. For the primary end point, the number of events was reduced by 4, with 3 fewer events in the bamlanivimab group and 1 fewer event in the placebo group (eTables 7-8 in Supplement 2). The results remained statistically significant, with a similar effect size.
Adverse Events
Of the 1175 participants included in the overall safety population, serious adverse events were reported in 3.7% of bamlanivimab recipients and 3.2% of placebo recipients (Table 2). The number of deaths not attributed to COVID-19 were 5 (0.9%) and 6 (1.0%) in the bamlanivimab and placebo groups, respectively. Adverse events were reported in 20.1% of the bamlanivimab group and 18.9% of the placebo group. Percentages of mild, moderate, and severe adverse events were balanced between both groups. Rates of adverse events occurring in 1% or more of the safety population were generally balanced across both treatment groups (Table 2; eTable 6 in Supplement 2).
Table 2. Adverse Events (Safety Population).
| Adverse eventsa | No. (%) | |
|---|---|---|
| Bamlanivimab (n = 588) | Placebo (n = 587) | |
| Participants with ≥1 treatment-emergent adverse eventb | 118 (20.1) | 111 (18.9) |
| Severity of treatment-emergent adverse eventb,c | ||
| Severe | 19 (3.2) | 17 (2.9) |
| Moderate | 29 (4.9) | 31 (5.3) |
| Mild | 66 (11.2) | 61 (10.4) |
| Most common treatment-emergent adverse events (occurring in ≥1% of bamlanivimab or placebo recipients)d | ||
| Urinary tract infection | 12 (2.0) | 14 (2.4) |
| Hypertension | 7 (1.2) | 10 (1.7) |
| Fall | 2 (0.3) | 6 (1.0) |
| Dizziness | 4 (0.7) | 6 (1.0) |
| Arthralgia | 6 (1.0) | 4 (0.7) |
| Serious adverse eventse | 22 (3.7) | 19 (3.2) |
| Deaths resulting from adverse eventf | 5 (0.9) | 6 (1.0) |
| Discontinuation from study participation due to adverse event (including death) | 5 (0.9) | 6 (1.0) |
Includes full randomized population that received at least 1 infusion. Study-specific clinical events related to COVID-19 were reported separately and not as adverse events (per protocol).
A treatment-emergent adverse event was defined as an event that first occurred or worsened in severity after baseline.
Patients with multiple occurrences of these categories were counted once for each category. Patients may be counted in more than 1 category.
The preferred terms were defined according to the Medical Dictionary for Regulatory Activities, version 23.0.
The serious adverse events in the placebo group were pneumonia, bacteremia, sepsis, septic shock, upper respiratory tract infection, urinary tract infection (bacterial), cardiac failure (congestive), cardiorespiratory arrest, cerebrovascular accident, dementia, syncope, femur fracture, corneal injury, open-globe injury, abdominal distension, ascites, mouth hemorrhage, increased blood creatinine, hyperkalemia, chronic kidney disease, ureterolithiasis, chronic obstructive pulmonary disease, epistaxis, thyrotoxic crisis, and hypovolemic shock. The serious adverse events in the bamlanivimab group were urinary tract infection (n = 4), atrial fibrillation (n = 2), pneumonia, AIDS, gastroenteritis, groin abscess, cardiac failure (congestive), cardiorespiratory arrest, acute myocardial infarction, coronary artery disease, cerebrovascular accident, headache, hypoesthesia, paresthesia, transient ischemic attack, vascular dementia, road traffic incident, spinal compression fracture, anemia, iron-deficiency anemia, thrombocytopenia, small intestinal obstruction, increased ammonia level, hypoglycemia, bile duct stone, lumbar spinal stenosis, and psychotic disorder.
Five deaths reported in the bamlanivimab group were due to cardiorespiratory arrest, AIDS, acute myocardial infarction, pneumonia, and vascular dementia. Six deaths reported in the placebo group were due to cardiorespiratory arrest, cardiac failure (congestive), chronic obstructive pulmonary disease, dementia, hypovolemic shock, and upper respiratory tract infection.
Hypersensitivity reactions within 24 hours of dose administration were reported in 3 participants (0.5%) in the bamlanivimab group and none in the placebo group. The percentage of participants who received a complete infusion of randomized treatment was 97.1% for bamlanivimab and 99.1% for placebo.
Discussion
This phase 3 clinical trial conducted in skilled nursing and assisted living facilities demonstrated that bamlanivimab was effective in reducing the incidence of mild or worse COVID-19 in residents and participants at high risk of severe COVID-19.
Bamlanivimab was also associated with lower rates of infection in residents and high-risk individuals. Participants in the prevention population who received bamlanivimab and acquired SARS-CoV-2 had lower baseline viral loads and shorter time to viral clearance compared with participants who received placebo. Since higher viral loads may correlate with higher degrees of infectiousness,19 decreasing viral load through prophylaxis could potentially slow further spread of disease, although this has yet to be tested.
All COVID-19–related deaths reported during this trial occurred among participants in the placebo group. This potential protective effect of bamlanivimab in reducing mortality could be due to fewer participants acquiring SARS-CoV-2 infections, as well as lower viral loads among those infected, since persistently high viral load has previously been identified as a risk factor for worse outcomes.15
In contrast to the resident and high-risk prevention populations, there was no significant difference in incidence of COVID-19 or SARS-CoV-2 infection in low-risk staff participants who received bamlanivimab compared with placebo. Interpretation of this observation yields various hypotheses given that staff (vs residents) could have differential exposures to infected persons (in the facility and in the community); however, these findings are consistent with the hypothesis that administration of neutralizing monoclonal antibodies has maximal effect in older people and in those at high risk of severe disease whose immune response to SARS-CoV-2 infection may be suboptimal.11 Additionally, the endogenous immune response in the majority of individuals infected with SARS-CoV-2 can effectively fight infection,20 supporting the findings from this study that show no benefit of bamlanivimab over placebo among the entire staff population.
Among those in the prevention population who tested positive for SARS-CoV-2 infection by RT-PCR by day 57, seroconversion was less frequent among participants who received bamlanivimab compared with placebo, possibly due to faster viral clearance and decreased antigenic exposure. It has been reported that some people with asymptomatic infection and limited inflammatory response may not demonstrate seroconversion as evidence of COVID-19 infection.21
The Emergency Use Authorization for bamlanivimab as monotherapy was revoked by the US Food and Drug Administration (FDA). It noted that “based on…the sustained increase of SARS-CoV-2 viral variants that are resistant to bamlanivimab alone resulting in the increased risk for treatment failure, the FDA has determined that the known and potential benefits of bamlanivimab, when administered alone, no longer outweigh the known and potential risks for its authorized use.”22 However, authorization of bamlanivimab in combination with etesevimab remains in place. The BLAZE-2 study started before such variants were spreading in the United States. As such, the results presented herein primarily represent validation of the use of neutralizing monoclonal antibodies as protective passive immunotherapy against COVID-19, with potential clinical relevance for antibody therapies in continued distribution.
Limitations
This study has several limitations. First, the study was conducted prior to widespread vaccine rollout, and any participant vaccinated against SARS-CoV-2 prior to screening was excluded. Studies are necessary to determine whether neutralizing monoclonal antibodies affect vaccine performance in this population. Second, while both nasopharyngeal and nasal swabs were obtained for detection of SARS-CoV-2 infection prior to dose administration, nasal swabs alone were obtained for subsequent SARS-CoV-2 detection during the evaluation and follow-up period. Use of nasal swabs for detection of SARS-CoV-2 may have lower sensitivity than nasopharyngeal swabs.23
Third, a variety of SARS-CoV-2 variants have recently been identified outside of this study,24,25,26 some of which have reduced susceptibility to vaccine-induced protection and to neutralization by convalescent serum and several monoclonal antibodies.27 Such variants represent a challenge in the selection of agents for prevention and treatment of SARS-CoV-2 and have already triggered use of combinations of monoclonal antibodies15,20 and discovery of novel therapeutic antibodies.
Fourth, there was very little racial diversity in the participant population. This was a reflection of the populations in facilities who agreed to participate in the trial.
Conclusions
Among residents and staff in skilled nursing and assisted living facilities, treatment during August-November 2020 with bamlanivimab monotherapy reduced the incidence of COVID-19 infection. Further research is needed to assess preventive efficacy with current patterns of viral strains with available combination monoclonal antibody therapy.
Trial Protocol and Statistical Analysis Plan
eAppendix. Supplemental Methods
eFigure 1. Time From Infusion to Development of Mild or Worse Severity COVID-19 With Bamlanivimab Versus Placebo
eFigure 2A. Time From Infusion to Development of Moderate or Worse Severity COVID-19 With Bamlanivimab Versus Placebo
eFigure 2B. Time From Infusion to Detection of SARS-CoV-2 by RT-PCR With Bamlanivimab Versus Placebo
eFigure 3. Time From First Positive SARS-CoV-2 Test to Clearance
eFigure 4. Time From First Positive RT-PCR for SARS-CoV-2 to Seropositivity
eTable 1. Definitions for COVID-19 Severity
eTable 2. Demographics of Participants in the Safety Population at Baseline
eTable 3. Demographics of High-Risk Participants in the Prevention Population at Baseline
eTable 4. Demographics of Participants With Positive SARS-CoV-2 Status by RT-PCR and Negative Serology at Baseline (Treatment Population)
eTable 5. Deaths Among Participants SARS-CoV-2 Serology Negative at Baseline
eTable 6. Adverse Events in the Prevention Population, Resident Prevention Population, and Staff Prevention Population
eTable 7. Sensitivity Analysis – All Prevention Population
eTable 8. Sensitivity Analysis – Resident Prevention Population
eReferences
Nonauthor Collaborators. BLAZE-2 Investigators
Data Sharing Statement
References
- 1.Arons MM, Hatfield KM, Reddy SC, et al. ; Public Health–Seattle and King County and CDC COVID-19 Investigation Team . Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081-2090. doi: 10.1056/NEJMoa2008457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham NSN, Junghans C, Downes R, et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020;81(3):411-419. doi: 10.1016/j.jinf.2020.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMichael TM, Currie DW, Clark S, et al. ; Public Health–Seattle and King County, EvergreenHealth, and CDC COVID-19 Investigation Team . Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005-2011. doi: 10.1056/NEJMoa2005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaiser Family Foundation . State COVID-19 data and policy actions. Published May 18, 2021. Accessed February 7, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/state-covid-19-data-and-policy-actions/#long-term-care-cases-deaths
- 5.Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. doi: 10.1001/jamainternmed.2020.7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisman DN, Bogoch I, Lapointe-Shaw L, McCready J, Tuite AR. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. JAMA Netw Open. 2020;3(7):e2015957. doi: 10.1001/jamanetworkopen.2020.15957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis. 2021;72(9):e206-e214. doi: 10.1093/cid/ciaa1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 10.White EM, Santostefano CM, Feifer RA, et al. Asymptomatic and presymptomatic severe acute respiratory syndrome coronavirus 2 infection rates in a multistate sample of skilled nursing facilities. JAMA Intern Med. 2020;180(12):1709-1711. doi: 10.1001/jamainternmed.2020.5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996-1012. doi: 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones BE, Brown-Augsburger PL, Corbett KS, et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021;13(593):eabf1906. doi: 10.1126/scitranslmed.abf1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. Published November 9, 2020. Accessed February 7, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
- 14.US Food and Drug Administration . Coronavirus (COVID-19) update: FDA authorizes monoclonal antibodies for treatment of COVID-19. Published February 9, 2021. Accessed March 18, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19-0
- 15.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi: 10.1001/jama.2021.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Karaca-Mandic P, Georgiou A, Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med. 2021;181(1):131-134. doi: 10.1001/jamainternmed.2020.3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499-503. doi: 10.2307/2531021 [DOI] [PubMed] [Google Scholar]
- 19.Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral cultures for COVID-19 infectious potential assessment—a systematic review. Clin Infect Dis. Published online December 3, 2020. doi: 10.1093/cid/ciaa1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators . REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi: 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staines HM, Kirwan DE, Clark DJ, et al. IgG seroconversion and pathophysiology in severe acute respiratory syndrome coronavirus 2 infection. Emerg Infect Dis. 2021;27(1):85. doi: 10.3201/eid2701.203074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration . Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Updated April 16, 2021. Accessed April 21, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
- 23.Péré H, Podglajen I, Wack M, et al. Nasal swab sampling for SARS-CoV-2: a convenient alternative in times of nasopharyngeal swab shortage. J Clin Microbiol. 2020;58(6):e00721-e00720. doi: 10.1128/JCM.00721-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. Preprint posted December 22, 2020. doi: 10.1101/2020.12.21.20248640 [DOI]
- 25.Voloch CM, da Silva Francisco R Jr, de Almeida LGP, et al. ; Covid19-UFRJ Workgroup; LNCC Workgroup . Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J Virol. Published online March 1, 2021. doi: 10.1128/JVI.00119-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rambaut A, Loman N, Pybus O, Barclay W, Barrett J, Carabelli A, et al. Preliminary genomic characterisation of an emergent SARSCoV-2 lineage in the UK defined by a novel set of spike mutations. Virological.org. Posted December 2020. Accessed February 9, 2021. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563
- 27.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130-135. doi: 10.1038/s41586-021-03398-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Supplemental Methods
eFigure 1. Time From Infusion to Development of Mild or Worse Severity COVID-19 With Bamlanivimab Versus Placebo
eFigure 2A. Time From Infusion to Development of Moderate or Worse Severity COVID-19 With Bamlanivimab Versus Placebo
eFigure 2B. Time From Infusion to Detection of SARS-CoV-2 by RT-PCR With Bamlanivimab Versus Placebo
eFigure 3. Time From First Positive SARS-CoV-2 Test to Clearance
eFigure 4. Time From First Positive RT-PCR for SARS-CoV-2 to Seropositivity
eTable 1. Definitions for COVID-19 Severity
eTable 2. Demographics of Participants in the Safety Population at Baseline
eTable 3. Demographics of High-Risk Participants in the Prevention Population at Baseline
eTable 4. Demographics of Participants With Positive SARS-CoV-2 Status by RT-PCR and Negative Serology at Baseline (Treatment Population)
eTable 5. Deaths Among Participants SARS-CoV-2 Serology Negative at Baseline
eTable 6. Adverse Events in the Prevention Population, Resident Prevention Population, and Staff Prevention Population
eTable 7. Sensitivity Analysis – All Prevention Population
eTable 8. Sensitivity Analysis – Resident Prevention Population
eReferences
Nonauthor Collaborators. BLAZE-2 Investigators
Data Sharing Statement