Key Points
Question
What is the menopause-related quality of life in carriers of the BRCA1/2 pathogenic variant after salpingectomy with delayed oophorectomy compared with the standard salpingo-oophorectomy?
Findings
In this nonrandomized controlled trial with 577 women who were BRCA1/2 pathogenic variant carriers, the change from baseline score on the Greene Climacteric Scale was 6.7 points higher after standard salpingo-oophorectomy without hormone replacement therapy compared with salpingectomy, and 3.6 points higher with hormone replacement therapy. Both of these differences were statistically significant.
Meaning
In this study, menopause-related quality of life reported by women appeared to be better after salpingectomy than after salpingo-oophorectomy, regardless of hormone replacement therapy.
Abstract
Importance
Most women with a BRCA1/2 pathogenic variant undergo premature menopause with potential short- and long-term morbidity due to the current method of ovarian carcinoma prevention: risk-reducing salpingo-oophorectomy (RRSO). Because the fallopian tubes play a key role in ovarian cancer pathogenesis, salpingectomy with delayed oophorectomy may be a novel risk-reducing strategy with benefits of delaying menopause.
Objective
To compare menopause-related quality of life after risk-reducing salpingectomy (RRS) with delayed oophorectomy with RRSO in carriers of the BRCA1/2 pathogenic variant.
Design, Setting, and Participants
A multicenter nonrandomized controlled preference trial (TUBA study), with patient recruitment between January 16, 2015, and November 7, 2019, and follow-up at 3 and 12 months after surgery was conducted in all Dutch university hospitals and a few large general hospitals. In the Netherlands, RRSO is predominantly performed in these hospitals. Patients at the clinical genetics or gynecology department between the ages of 25 and 40 years (BRCA1) or 25 to 45 years (BRCA2) who were premenopausal, had completed childbearing, and were undergoing no current treatment for cancer were eligible.
Interventions
Risk-reducing salpingo-oophorectomy at currently recommended age or RRS after completed childbearing with delayed oophorectomy. After RRSO was performed, hormone replacement therapy was recommended for women without contraindications.
Main Outcomes and Measures
Menopause-related quality of life as assessed by the Greene Climacteric Scale, with a higher scale sum (range, 0-63) representing more climacteric symptoms. Secondary outcomes were health-related quality of life, sexual functioning and distress, cancer worry, decisional regret, and surgical outcomes.
Results
A total of 577 women (mean [SD] age, 37.2 [3.5] years) were enrolled: 297 (51.5%) were pathogenic BRCA1 variant carriers and 280 (48.5%) were BRCA2 pathogenic variant carriers. At the time of analysis, 394 patients had undergone RRS and 154 had undergone RRSO. Without hormone replacement therapy, the adjusted mean increase from the baseline score on the Greene Climacteric Scale was 6.7 (95% CI, 5.0-8.4; P < .001) points higher during 1 year after RRSO than after RRS. After RRSO with hormone replacement therapy, the difference was 3.6 points (95% CI, 2.3-4.8; P < .001) compared with RRS.
Conclusions and Relevance
Results of this nonrandomized controlled trial suggest that patients have better menopause-related quality of life after RRS than after RRSO, regardless of hormone replacement therapy. An international follow-up study is currently evaluating the oncologic safety of this therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02321228
This nonrandomized controlled trial evaluates menopausal symptoms affecting quality of life in women who were BRCA1 or BRCA2 pathogenic variant carriers after risk-reducing salpingo-oophorectomy vs risk-reducing salpingectomy.
Introduction
Cancer of the ovaries, fallopian tubes, and peritoneum is the most lethal type of gynecologic cancer. Women with a pathogenic variant (PV) in a BRCA1/2 gene have a lifetime risk of ovarian cancer of about 44% (BRCA1) or 17% (BRCA2).1 No effective screening is currently available.2 Therefore, these women are advised to undergo risk-reducing salpingo-oophorectomy (RRSO) at the age of 35 to 40 years (BRCA1-PV) or 40 to 45 years (BRCA2-PV), before incidences rise.3
Risk-reducing salpingo-oophorectomy reduces ovarian cancer risk by 96% but has several disadvantages.4 It induces premature menopause with potential short-term effects (hot flushes, sleep disturbances, and impaired sexual functioning)5 and long-term effects (risk of cardiovascular disease, osteoporosis, and cognitive impairment).6 The fallopian tube instead of the ovary is indicated as the tissue of origin of high-grade serous cancer, which is the most common type of ovarian cancer.7,8 This paradigm shift supports risk-reducing salpingectomy (RRS) with delayed oophorectomy (RRO) as a novel prevention strategy leading to postponed menopause.8 However, empirical data exist neither on quality of life (QoL) nor on the actual cancer risk after RRS. Investigating the effect on ovarian cancer (oncologic safety) would require at least 3000 participants with 10 to 15 years of follow-up. Before initiating such a trial, the association between RRS and QoL should be elucidated.
Two previous feasibility studies noted that 34% to 44% of BRCA1/2-PV carriers were interested in undergoing this novel strategy within the protection of a clinical trial.9,10 However, randomization was an important barrier for participation.9
The role of the fallopian tube in ovarian cancer pathogenesis, the interest of BRCA1/2-PV carriers in postponing premature menopause, and their reluctance to participate in a randomized trial formed the basis of this prospective multicenter preference trial to compare menopause-related QoL, sexual function, cancer worry, decisional conflict, decisional regret, and surgical outcomes after RRS with delayed RRO and RRSO.
Methods
A nationwide prospective, multicenter, nonrandomized controlled preference trial comparing women choosing RRS with delayed RRO vs RRSO was initiated in 13 Dutch hospitals. Treatment allocation was based on patients’ preferences.9 The primary comparison was between patients after RRS and RRSO without hormone replacement therapy (HRT) or hormonal therapy for menstrual cycle regulation, but comparisons were also made with women who use HRT after RRSO. The medical ethics committee of Arnhem-Nijmegen approved the trial protocol,11 and a multidisciplinary data safety monitoring board was installed. To ensure safety concerning ovarian cancer incidence, a safety rule was established to notify the study team whether the observed ovarian cancer incidence exceeded the expected incidence according to age, type of BRCA1/2-PV, and duration of follow-up. Participants provided written informed consent. No financial compensation was provided. The trial protocol has been published12 and the approved version is available in Supplement 1. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline for nonrandomized controlled trials.
In the Netherlands, RRSO without hysterectomy is advised in women between the ages of 35 and 40 years (BRCA1-PV) and 40 and 45 years (BRCA2-PV) with an uptake of 97% to 98%.13 Risk-reducing salpingo-oophorectomy is predominantly performed in university hospitals with a clinical genetics department, which all participated.
Between January 16, 2015, and November 7, 2019, women with a documented BRCA1/2-PV visiting the gynecology or clinical genetics department of a participating center were informed about the trial. To meet the inclusion criteria, patients had to be aged 25 to 40 years (BRCA1-PV) or 25 to 45 years (BRCA2-PV), premenopausal, and capable of reading and speaking Dutch, and to have completed childbearing. Patients were excluded when they had, in advance, anticipated an oophorectomy within 2 years after RRS; were legally incapable of providing informed consent; had prior bilateral salpingectomy or ovarian, fallopian tube, or peritoneal cancer; or had a malignant disease at enrollment. Eligible women were provided with a patient information letter, received face-to-face counseling, and had at least 1 week to consider participating before giving written informed consent. From November 2017, participants received an additional patient decision aid.14
Eligible women chose between the standard and novel strategies. The standard strategy consisted of RRSO within the current guideline age range with postoperative HRT recommended if not contraindicated. The novel strategy consisted of RRS after the completion of childbearing and RRO at the age of 40 to 45 years (BRCA1-PV) or 45 to 50 years (BRCA2-PV). Some patients used hormonal therapy for menstrual cycle regulation after RRS. Patients could also opt for RRO within the currently recommended age range for RRSO. All procedures included peritoneal fluid sampling for cytologic assessment and thorough exploration of the abdominopelvic cavity. The fallopian tubes were embedded according to the sectioning and extensively examining the fimbriated end (SEE-FIM) protocol,12 and all surgical specimens were assessed by a gynecologic pathologist. Immunohistochemical staining (p53, Ki-67) was performed in case of abnormal morphologic findings. When carcinoma was found perioperatively, patients were excluded from further follow-up because surgery was therapeutic instead of risk reducing. When a serous tubal intraepithelial carcinoma was found, RRO was advised on short notice. According to Dutch guidelines, no surgical staging or chemotherapy was indicated for serous tubal intraepithelial carcinoma.
The primary outcome measure was menopause-specific QoL, quantified by the validated Greene Climacteric Scale (GCS), in which 21 symptoms are rated on a 4-point Likert scale (domains: depression/anxiety, somatic, vasomotor, and sexual problems).15 A higher sum represents more climacteric symptoms (range, 0-63). Invitations to complete this web-based questionnaire were sent at baseline, 3 months, and 1 year after surgery, and biennially thereafter. Data on most of the secondary outcome measures, such as health-related QoL, sexual functioning and distress, cancer worry, and decisional conflict and regret, were also collected by 2 web-based questionnaires used to assess sexual functioning.16 The first of these, the Female Sexual Functioning Index, is a validated questionnaire in which a higher total score (range, 2-36) represents better sexual functioning. The second, the Female Sexual Distress Scale, is a validated questionnaire in which a higher total score (range, 2-52) represents more sexual distress. eFigure 1 in Supplement 2 and the trial protocol (Supplement 1) include questionnaire details. Data on surgical complications and histopathologic findings were collected by obtaining reports on surgery, complications, and pathologic test results. All adverse events during the study period were registered in accordance with the Common Terminology Criteria for Adverse Events.17 Serious adverse events were defined as any medical occurrence or outcome that resulted in death, was life-threatening, or required hospitalization or prolongation of hospitalization, with the exception of elective hospital admissions. Occurrence of serious adverse events, with specification of serious adverse events, was monitored by local investigators and by screening the questionnaires. Follow-up on cardiovascular disease and cost-effectiveness has not been completed.
Statistical Analysis
The description of the sample size calculation can be found in the trial protocol.12 We tested for differences in baseline characteristics between treatment arms using t tests, Mann-Whitney tests, and χ2 tests. The primary end point was the mean increase in GCS total score after RRS compared with RRSO without HRT. Women in the RRSO group were analyzed based on their current HRT use, so women could potentially be included in the analysis without HRT at 3 months and also in the analysis with HRT at 1 year if HRT had been started within that period. The analysis of the primary outcome was restricted to women from both treatment arms without current HRT use. We carried out a mixed-model analysis on the change from baseline values in relation to treatment arm and visit, adjusting for the baseline value of the specific questionnaire, baseline age, type of BRCA-PV, baseline GCS score, baseline values of the 36-Item Short Form Health Survey (SF-36) subscales,18 and with a random-effects model for center or hospital in which participants are included and an unstructured correlation matrix for the within-participants correlation between repeated measurements (3 months and 1 year after surgery). The Satterthwaite approximation was used to calculate degrees of freedom. We started with a model with 2-way interactions for visit by treatment arm, age by treatment arm, and baseline GCS score by treatment arm, and main outcomes as mentioned above. One by one, all 2-way interaction terms were deleted, because all P values were >.10. Similar analyses were conducted comparing the RRS group with the RRSO group with current HRT and for the GCS subscales. The association between treatment arm and mean change from baseline in each of the secondary outcomes was analyzed with a mixed model, with an interaction term for time point by treatment, and adjusted for BRCA-PV group, age, and baseline values of the GCS and SF-36, and of the respective outcome, with random effects for center and an unstructured correlation matrix for the within-participant correlation between repeated measurements. The interaction term for time point by treatment was included independent of significance, because we were especially interested in change over time for these variables. The 2-sided significance level was set at P < .05. A correction for multiple testing was unnecessary because there is only 1 primary end point. Data analysis was conducted using SPSS for Windows, version 25 (IBM Corp).
Results
Study Population
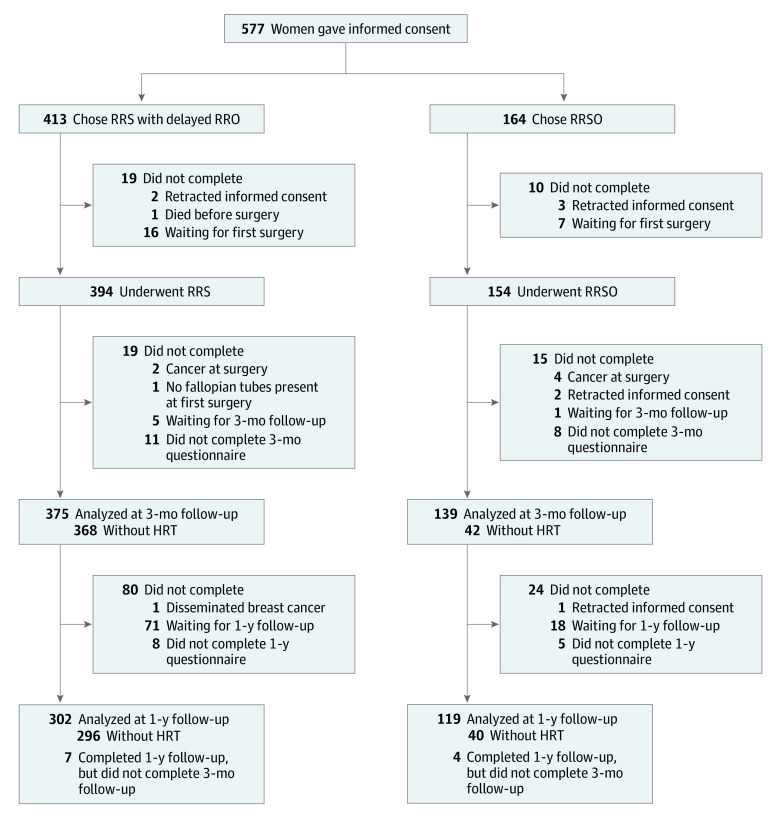
A total of 577 women who met the eligibility criteria gave written informed consent (Figure 1). The mean (SD) age was 37.2 (3.5) years; 297 women (51.5%) carried a BRCA1-PV, 280 (48.5%) carried a BRCA2-PV, and 413 (71.6%) chose RRS with delayed RRO.
Figure 1. Consolidated Standards of Reporting Trials Flow Diagram of Participants.
Women who underwent risk-reducing salpingectomy (RRS) used estrogen-based hormone replacement therapy (HRT) for menstrual cycle regulation after surgery. RRO indicates risk-reducing oophorectomy; RRSO, risk-reducing salpingo-oophorectomy.
Before the first surgery, 5 patients retracted consent and 1 patient died. Of all women who chose RRS (n = 413), a total of 394 (95.4%) underwent their surgery; of the women who chose RRSO (n = 164), a total of 154 (93.9%) underwent surgery; 23 women were still awaiting surgery (only 2.5% of participants who were supposed to have completed 1 year of follow-up). Except for age and type of BRCA-PV, no significant differences were observed between the groups (Table 1). The separate characteristics between women with and without HRT are displayed in eTable 1 in Supplement 2; only the number of women with prior breast cancer was higher among women without HRT after RRSO. Type and dosage of HRT can be found in eTable 2 in Supplement 2.
Table 1. Baseline Characteristics of Women Who Completed the First Surgery.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Total (n = 548) | RRS (n = 394) | RRSO (n = 154) | ||
| Age at inclusion, mean (SD), y | 37.2 (3.5) | 36.6 (3.6) | 38.7 (2.9) | <.001 |
| BRCA1 | 35.9 (2.9) | 35.3 (3.2) | 37.0 (1.9) | <.001 |
| BRCA2 | 38.7 (3.6) | 37.8 (3.5) | 41.5 (2.1) | <.001 |
| Age at first surgery, mean (SD), y | 37.5 (3.5) | 36.8 (3.5) | 38.8 (2.9) | <.001 |
| BRCA1 | 36.1 (2.9) | 35.5 (3.2) | 37.1 (1.8) | <.001 |
| BRCA2 | 38.8 (3.6) | 38.0 (3.5) | 41.5 (2.1) | <.001 |
| Type of BRCA pathogenic variant | ||||
| BRCA1 | 285 (52.0) | 190 (48.2) | 95 (61.7) | .005 |
| BRCA2 | 263 (48.0) | 204 (51.8) | 59 (38.3) | |
| Relationship status | ||||
| Married/partner | 487 (88.9) | 349 (88.6) | 138 (89.6) | .51 |
| Single | 54 (9.9) | 41 (10.4) | 13 (8.4) | |
| Unknown | 7 (1.3) | 4 (1.0) | 3 (1.9) | |
| Offspring | ||||
| No | 62 (11.3) | 46 (11.7) | 16 (10.4) | .72 |
| Yes | 476 (86.9) | 343 (87.1) | 133 (86.4) | |
| Unknown | 10 (1.8) | 5 (1.3) | 5 (3.2) | |
| Educational level | ||||
| Low | 62 (11.3) | 45 (11.4) | 17 (11.0) | .98 |
| Medium | 194 (35.4) | 139 (35.3) | 55 (35.7) | |
| High | 285 (52.0) | 206 (52.3) | 79 (51.3) | |
| Unknown | 7 (1.3) | 4 (1.0) | 3 (1.9) | |
| Breast cancer in personal history | ||||
| No | 462 (84.3) | 331 (84.0) | 131 (85.1) | .58 |
| Yes | 79 (14.4) | 59 (15.0) | 20 (13.0) | |
| Unknown | 7 (1.3) | 4 (1.0) | 3 (1.9) | |
| Risk-reducing mastectomy | ||||
| No | 328 (59.9) | 235 (59.6) | 93 (60.4) | .78 |
| Yes | 213 (38.9) | 155 (39.3) | 58 (37.7) | |
| Unknown | 7 (1.3) | 4 (1.0) | 3 (1.9) | |
| First-degree family member with ovarian cancer | ||||
| No | 463 (84.5) | 339 (86.0) | 124 (80.5) | .18 |
| Yes | 76 (13.9) | 50 (12.7) | 26 (16.9) | |
| Unknown | 9 (1.6) | 5 (1.3) | 4 (2.6) | |
Abbreviations: RRS, risk-reducing salpingectomy; RRSO, risk-reducing salpingo-oophorectomy.
Risk-reducing salpingo-oophorectomy was occasionally combined with hysterectomy (n = 5), breast surgery (n = 14), endometrial polyp resection (n = 1), large loop excisions of the cervical transformation zone (n = 2), or cesarean section (n = 1). Risk-reducing salpingectomy was combined with hysterectomy (n = 1) or breast surgery (n = 4). One woman was excluded from the study during RRS because she appeared to have already had bilateral salpingectomy. Another woman had laparoscopic removal of just 1 fallopian tube owing to firm adhesions; laparotomic oophorectomy with contralateral salpingectomy within the current guideline age was recommended. In 5 patients who opted for RRS, unilateral oophorectomy was performed because of macroscopic abnormalities (n = 4) or on the patient’s request (n = 1).
Outcomes
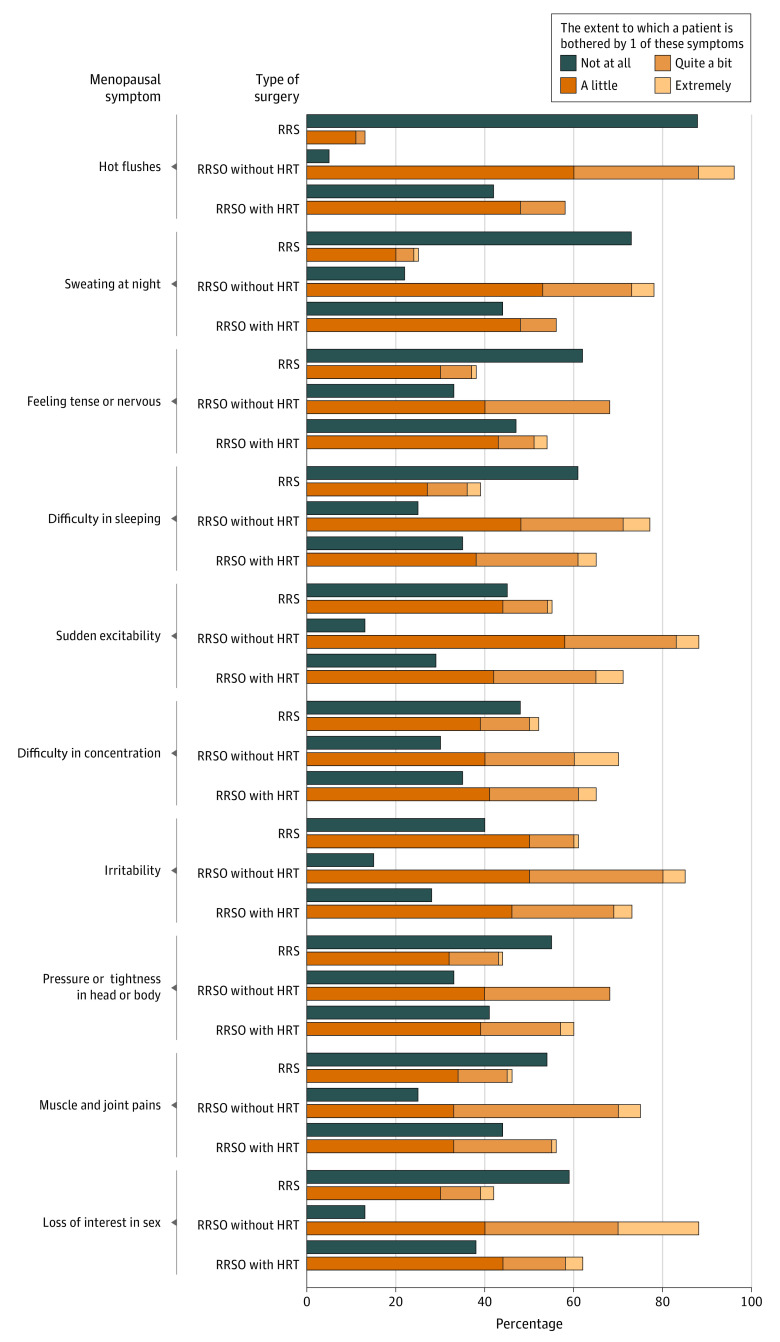
In evaluation of the primary outcome, without HRT, the observed mean (SD) increase from the baseline score on the GCS was 0.7 (6.3) points 1 year after RRS and 7.7 (8.3) points after RRSO. The adjusted mean difference between the treatment groups was 6.7 (95% CI, 5.0-8.4; P < .001) (Table 2; eFigure 2 in Supplement 2). The difference was present at both time points and on all subscales (Table 2; eTable 3 in Supplement 2). Hot flushes, sudden excitability, irritability, and loss of interest in sex were the most frequently reported symptoms (Figure 2).
Table 2. Total Score of the Greene Climacteric Scalea.
| Variable | RRS | RRSO | RRSO − RRS, adjusted MD (95% CI)b | P value | ||
|---|---|---|---|---|---|---|
| No. | Observed mean (SD) | No. | Observed mean (SD) | |||
| Women without HRT | ||||||
| Baseline GCS score, median (IQR) | 374 | 6.0 (3.0-11.0) | 44 | 9.5 (5.8-14.0) | 6.7 (5.0-8.4) | <.001 |
| CFB at 3 mo | 368 | 0.6 (5.5) | 42 | 6.9 (6.8) | ||
| CFB at 1 y | 296 | 0.7 (6.3) | 40 | 7.7 (8.3) | ||
| Women with HRT after RRSO c | ||||||
| Baseline GCS score, median (IQR) | 382 | 6.0 (3.0-12.0) | 99 | 7.0 (4.0-12.5) | 3.6 (2.3-4.8) | <.001 |
| CFB at 3 mo | 375 | 0.6 (5.5) | 97 | 3.5 (6.8) | ||
| CFB at 1 y | 302 | 0.8 (6.4) | 79 | 4.6 (7.7) | ||
Abbreviations: CFB, change from baseline; GCS, Greene Climacteric Scale; HRT, hormone replacement therapy; IQR, interquartile range; MD, mean difference; RRS, risk-reducing salpingectomy; RRSO, risk-reducing salpingo-oophorectomy.
Possible score range, 0 to 63; higher sum represents more climacteric symptoms.
Interaction between choice and time point was not statistically significant (P = .76); thus, the adjusted estimated difference was the same for both time points.
All women after RRS (with and without HRT) and only the women using HRT after RRSO are included.
Figure 2. Findings on the Female Sexual Functioning Index (FSFI) and Female Sexual Distress Scale (FSDS).
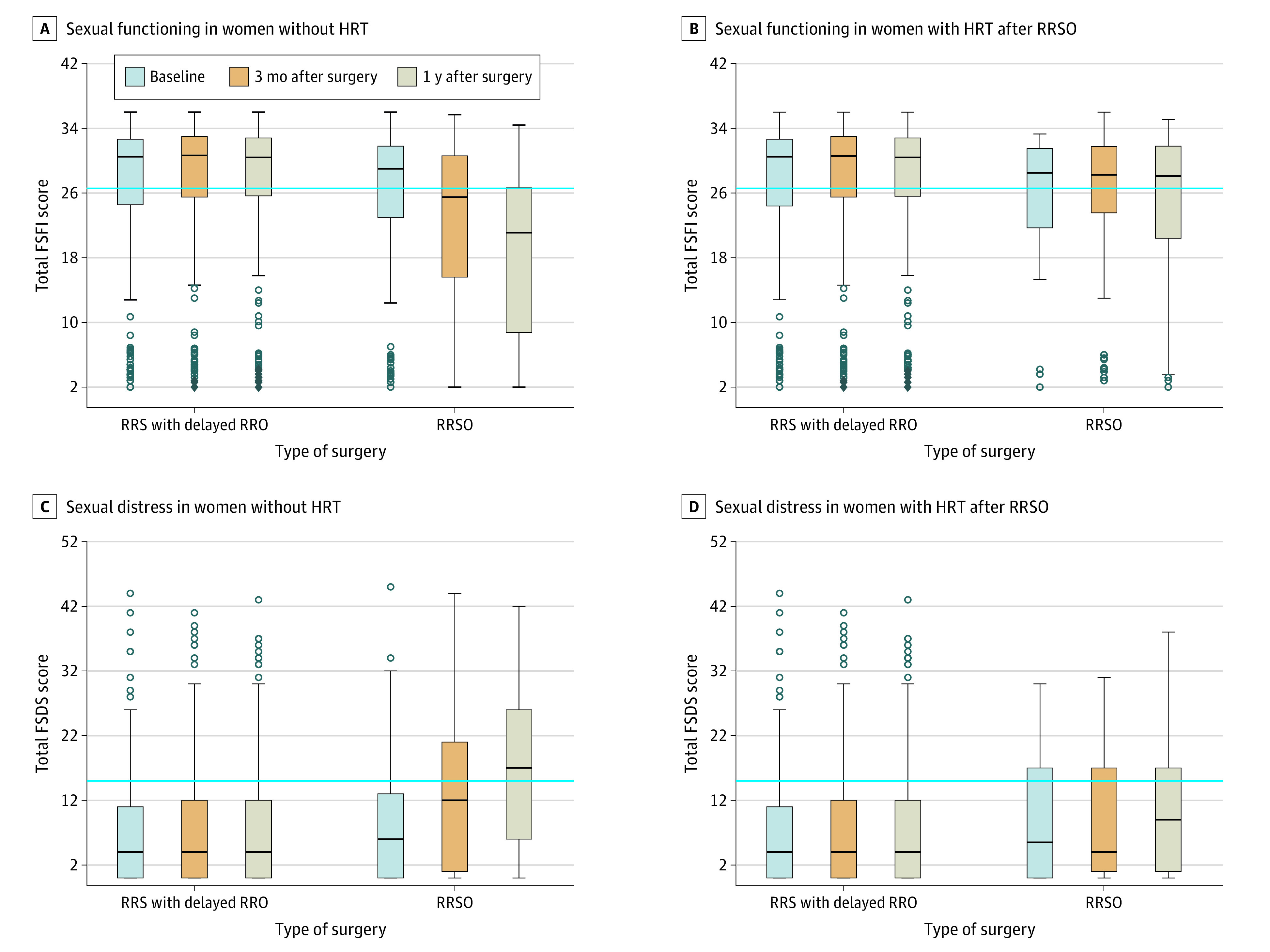
The blue lines indicate the validated cutoff scores of 26.55 points or less for the FSFI to identify women with impaired sexual functioning without hormone replacement therapy (HRT) (A) and with HRT after risk-reducing salpingo-oophorectomy (RRSO) (B), and 15 points or more for the FSDS to identify women with sexual distress without HRT (C) and with HRT after RRSO (D). Boxes indicate the 25th percentile, median, and 75th percentile. Dots represent outliers. Whiskers show the minimum and maximum ranges.Some bars do not have any whiskers as the used questionnaires had a minimum and maximum result. RRO indicates risk-reducing oophorectomy; RRS, risk-reducing salpingectomy.
In the secondary outcome regarding menopause-related QoL of women after RRSO with HRT compared with RRS, an adjusted mean difference in the GCS score of 3.6 points (95% CI, 2.3-4.8; P < .001) was found (Table 2; eFigure 2 in Supplement 2). The findings were similar at 3 and 12 months and on all GCS subscales (Table 2; eTable 4 in Supplement 2).
The only difference in health-related QoL was found in a significantly greater decrease in the physical component summary score of the SF-36 3 months after RRSO with HRT compared with RRS (eTable 5 in Supplement 2).
Patients experienced a mean (SD) decrease of 5.1 (8.2) points at 1 year on the Female Sexual Functioning Index after RRSO without HRT, whereas those who underwent RRS experienced an increase of 0.3 (7.1) points (eTable 5 in Supplement 2) (Figure 3). The difference became more pronounced over time. In women who used HRT after RRSO, a significant difference was found after 1 year (−2.0; 95% CI, −3.8 to −0.2; P = .03) (eTable 5 in Supplement 2). Based on the validated cutoff point of 26.55 points, impaired sexual functioning was present in 53 of 148 women (35.8%) in the RRSO group at baseline, increasing over 3 months (61 of 138 [44.2%]) and 1 year (65 of 117 [55.6%]). In the RRS group, corresponding findings were impaired sexual functioning in 121 of 388 women (31.2%) at baseline, 103 of 373 (27.6%) at 3 months, and 85 of 301 (28.2%) at 1 year.
Figure 3. The 10 Most Frequently Scored Items on the Greene Climacteric Scale 1 Year After Surgery.
HRT indicates hormone replacement therapy; RRS, risk-reducing salpingectomy; and RRSO, risk-reducing salpingo-oophorectomy.
Regarding sexual distress, patients experienced less increase from baseline on the Female Sexual Distress Scale after RRS compared with RRSO without HRT. This difference also became more pronounced over time (eTable 5 in Supplement 2) (Figure 2). No significant difference in change from baseline was found between patients after RRS and patients who used HRT after RRSO (eTable 5 in Supplement 2). Based on the validated cutoff point (≥15 points), 34 of 149 women (22.8%) in the RRSO group experienced relevant sexual distress at baseline, increasing to 45 of 139 women (32.4%) after 3 months and 50 of 119 (42.0%) after 1 year. In the RRS group, corresponding results were 69 of 388 (17.8%) experiencing sexual distress at baseline, 72 of 374 (19.3%) at 3 months, and 55 of 301 (18.3%) at 1 year.
A similar decline in cancer worry was found after RRS and RRSO, regardless of HRT use. There was no significant difference in decisional conflict or decisional regret (eTable 5 in Supplement 2).
The median surgical time was 44 minutes (range, 16-459) for RRS and 57 minutes (range, 24-310) for RRSO. In total, 319 women (82.4%) after RRS and 111 (74.0%) after RRSO were discharged on the day of surgery. The median blood loss was 0 mL in both groups (range, 0-350 mL for RRS; 0-250 mL for RRSO). An overview of all adverse events can be found in eTable 6 in Supplement 2. Perioperative complication rates (definitely or probably related to the study) were 3.3% in both groups. No cases of ovarian or peritoneal cancer occurred during follow-up. Eight patients developed breast cancer during follow-up and 2 had breast cancer recurrence (P = .57).
At the time of data analysis, 2 women had undergone RRO because of serous tubal intraepithelial carcinoma at initial salpingectomy. In total, 14 women underwent RRO at a later stage. Three BRCA2-PV carriers changed their preference and chose to undergo RRO within 2 years. Three other BRCA2-PV carriers underwent RRO 28 months (age, 40 years), 53 months (age, 49 years), and 54 months (age, 48 years) after RRS. Eight BRCA1-PV carriers underwent RRO 29 to 61 months after RRS: 1 at age 39 years and 7 aged 40 to 44 years.
One invasive carcinoma and 2 serous tubal intraepithelial carcinomas were found at RRS; of the serous tubal intraepithelial carcinomas, 1 invasive carcinoma was found at second-stage RRO. At RRSO, 1 isolated serous tubal intraepithelial carcinoma and 3 other carcinomas were found (Table 3). In the 14 women with previously normal histopathologic findings at RRS, no abnormalities were found in the ovaries and abdominal washings at RRO.
Table 3. Histopathologic Results.
| Variable | Age, y | BRCA-PV | Intraoperative findings | Pathologic findings | Localization | FIGO stage after surgical staging |
|---|---|---|---|---|---|---|
| RRS | 36 | 1 | Not suspicious (RRS) | STIC | Fallopian tube | NA |
| Not suspicious (RRO) | Benign | NA | NA | |||
| 42 | 2 | Not suspicious (RRS) | STIC | Fallopian tube | NA | |
| Not suspicious (RRO) | HGSC | Ovary | IA | |||
| 40 | 1 | Papillary lesion (RRS)a | HGSC | Fallopian tube and ovary | IIB | |
| RRSO | 35 | 1 | Not suspicious | STIC | Fallopian tube | NA |
| 39 | 1 | Not suspicious | HGSC | Fallopian tubes | IIA | |
| 38 | 1 | Suspicious lesions on diaphragm | Adenocarcinoma | Peritoneum | IIIB | |
| 38 | 1 | Suspicious for endometriosis | HGSC and STIC | Fallopian tubes and ovaries | IIIB | |
| 37 | 1 | Not suspicious | Severe atypia | Ovary | No carcinoma |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; HGSC, high-grade serous carcinoma; NA, not applicable; PV, pathogenic variant; RRO, delayed oophorectomy; RRS, risk-reducing salpingectomy; RRSO, risk-reducing salpingo-oophorectomy; STIC, serous tubal intraepithelial carcinoma.
Unilateral oophorectomy performed because of macroscopic lesion.
Discussion
In this nationwide, multicenter, nonrandomized controlled preference trial, BRCA1/2-PV carriers appeared to have better menopause-related QoL after RRS than after RRSO. Even when HRT was used, a significantly lower menopause-related QoL was observed after RRSO. Furthermore, women reported better sexual functioning after RRS compared with RRSO with and without HRT. The decline in cancer worry was similar in both groups. No significant differences were found in health-related QoL, decisional conflict, decisional regret, or surgical outcomes.
To our knowledge, this is the first trial to prospectively compare menopause-related QoL after RRS with delayed RRO and RRSO. The difference found in the GCS scores was larger than the reported difference of 5 points between premenopause and postmenopause status in the general population.15 This difference might be explained by the younger study population, resulting in lower baseline scores,15 and by the acute surgical onset of menopause.19 After RRS, the level of menopausal symptoms in BRCA1/2-PV carriers remained unchanged, consistent with a previous study.20 Women in our study reported more symptoms despite HRT use after RRSO, which supports previous findings that menopausal symptoms and sexual problems may be alleviated but not eliminated by HRT.21 Thus, the clinical benefit of delaying menopause is not limited to women with contraindications for HRT. The prescription of HRT after RRSO varies between countries. No current evidence is available of HRT being unsafe with regard to the breast cancer risk in presymptomatic women with a BRCA1/2-PV. However, randomized trials are lacking. As the supplemented amount of estrogen/progesterone in HRT is much lower than natural levels before surgery, it is expected that a presumed effect of HRT on and breast cancer risk is low. Optimization of HRT in terms of dosage and schedules might improve its performance; further studies of research and development could be useful, but HRT was not the focus of our study.
Potential drawbacks of RRS with delayed RRO are increased cancer worry, decisional regret, surgical complications, and cancer risk. A similar decline in cancer worry was found between groups, supporting previous findings.20 Low levels of decisional regret were present in both groups, and surgical complication rates were similar. We are not able to draw conclusions on complications of RRO. We did, however, find a higher incidence of occult (intraepithelial) carcinoma in the RRSO group; this difference might be explained by the higher age and proportion of BRCA1-PV carriers in that group. No interval ovarian or peritoneal cancers were observed, but follow-up is short and participants are relatively young. Nevertheless, our experience with serous tubal intraepithelial carcinoma at RRS and invasive carcinoma in the ovary at subsequent RRO underlines the importance of detecting serous tubal intraepithelial carcinoma lesions through meticulous examination by an experienced pathologist using the SEE-FIM protocol.22 Risk-reducing salpingo-oophorectomy was initially thought to significantly decrease breast cancer risk, but comparable breast cancer incidences in both groups support findings showing no or limited association with breast cancer risk after RRSO.23,24,25,26
For the near future, the priority is to examine whether RRS with delayed RRO may be used as a risk-reducing strategy with regard to oncological safety. The novel strategy can be offered as standard of care only when risks are known so women can make an informed choice between premature menopause and certain ovarian cancer risk. International collaboration is needed to collect these data. Therefore, the TUBA-WISP II study has recently been initiated in collaboration with the research group of the WISP trial27 and will combine long-term follow-up data of the study reported herein, the WISP trial, and prospectively collected data from several international centers.
Strengths and Limitations
The main strengths of our trial are its prospective multicenter design, large number of patients with nationwide coverage, and a low number of participants lost to follow-up (only 2.5% of participants who were supposed to have completed 1 year of follow-up). The trial was widely supported owing to the preparatory exploration of both patients’ and professionals’ needs and barriers.9,10 Standardized and validated questionnaires were used. Another strength of this study was our safety rule that was constantly updated and allowed us to respond in case of increasing ovarian cancer incidence.
The main limitation of this trial is the nonrandomized design. Although well considered and inevitable in this setting, this design may introduce confounding by individual patient preference.9,10 Owing to allocation based on patient preference, 72% of the patients chose RRS.9,10,20 Alternatively, this choice may be because women who preferred RRSO were less frequently referred to a participating hospital: RRSO can be performed in every hospital in the Netherlands, whereas performance of RRS is currently discouraged outside the context of a clinical trial. However, there is little reason to assume that these women differ from participants in our RRSO group.
Conclusions
In this nonrandomized controlled trial, patients who underwent RRS reported better menopause-related QoL and better sexual functioning compared with patients who received RRSO, with the difference more pronounced in women who did not receive HRT. The issue of oncologic safety of RRS with delayed RRO will be addressed in a recently started international follow-up study (TUBA-WISP).28
Trial Protocol
eTable 1. Baseline Characteristics per Group (With and Without Postoperative HRT)
eTable 2. Overview of Hormone Replacement Therapy at Three and Twelve Months After Surgery
eTable 3. Subscales of the Greene Climacteric Scale in Women Without Hormone Replacement Therapy
eTable 4. Subscales of the Greene Climacteric Scale in Women With Hormone Replacement Therapy After RRSO
eTable 5. Secondary Outcome Measures
eTable 6. Surgical Complications and Serious Adverse Events (SAE)
eFigure 1. A Schematic Overview of Data Collection and Timing of Questionnaires
eFigure 2. Box Plots of the Greene Climacteric Scale
References
- 1.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. ; BRCA1 and BRCA2 Cohort Consortium . Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402-2416. doi: 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- 2.Oei AL, Massuger LF, Bulten J, Ligtenberg MJ, Hoogerbrugge N, de Hullu JA. Surveillance of women at high risk for hereditary ovarian cancer is inefficient. Br J Cancer. 2006;94(6):814-819. doi: 10.1038/sj.bjc.6603015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Integraal Kankercentrum Nederland. Richtlijn Erfelijk en Familiair Ovariumcarcinoom [Guideline Hereditary and Familial Ovarian Carcinoma]. Published June 1, 2015. Accessed October 6, 2017. https://www.nvog.nl/wp-content/uploads/2018/02/Erfelijk-en-familiair-ovariumcarcinoom-1.0-28-05-2015.pdf
- 4.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967-975. doi: 10.1001/jama.2010.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeulen RFM, Beurden MV, Korse CM, Kenter GG. Impact of risk-reducing salpingo-oophorectomy in premenopausal women. Climacteric. 2017;20(3):212-221. doi: 10.1080/13697137.2017.1285879 [DOI] [PubMed] [Google Scholar]
- 6.Rocca WA, Gazzuola-Rocca L, Smith CY, et al. Accelerated accumulation of multimorbidity after bilateral oophorectomy: a population-based cohort study. Mayo Clin Proc. 2016;91(11):1577-1589. doi: 10.1016/j.mayocp.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piek JMJ, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195(4):451-456. doi: 10.1002/path.1000 [DOI] [PubMed] [Google Scholar]
- 8.Labidi-Galy SI, Papp E, Hallberg D, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8(1):1093. doi: 10.1038/s41467-017-00962-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arts-de Jong M, Harmsen MG, Hoogerbrugge N, Massuger LF, Hermens RP, de Hullu JA. Risk-reducing salpingectomy with delayed oophorectomy in BRCA1/2 mutation carriers: patients’ and professionals’ perspectives. Gynecol Oncol. 2015;136(2):305-310. doi: 10.1016/j.ygyno.2014.12.031 [DOI] [PubMed] [Google Scholar]
- 10.Holman LL, Friedman S, Daniels MS, Sun CC, Lu KH. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol Oncol. 2014;133(2):283-286. doi: 10.1016/j.ygyno.2014.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harmsen MG, Arts-de Jong M, Hoogerbrugge N, et al. Early salpingectomy (tubectomy) with delayed oophorectomy to improve quality of life as alternative for risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers (TUBA study): a prospective non-randomised multicentre study. BMC Cancer. 2015;15:593. doi: 10.1186/s12885-015-1597-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30(2):230-236. doi: 10.1097/01.pas.0000180854.28831.77 [DOI] [PubMed] [Google Scholar]
- 13.Harmsen MG, Arts-de Jong M, Horstik K, et al. Very high uptake of risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers: a single-center experience. Gynecol Oncol. 2016;143(1):113-119. doi: 10.1016/j.ygyno.2016.07.104 [DOI] [PubMed] [Google Scholar]
- 14.Harmsen MG, Steenbeek MP, Hoogerbrugge N, et al. A patient decision aid for risk-reducing surgery in premenopausal BRCA1/2 mutation carriers: Development process and pilot testing. Health Expect. 2018;21(3):659-667. doi: 10.1111/hex.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barentsen R, van de Weijer PH, van Gend S, Foekema H. Climacteric symptoms in a representative Dutch population sample as measured with the Greene Climacteric Scale. Maturitas. 2001;38(2):123-128. doi: 10.1016/S0378-5122(00)00212-7 [DOI] [PubMed] [Google Scholar]
- 16.ter Kuile MM, Brauer M, Laan E. The Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS): psychometric properties within a Dutch population. J Sex Marital Ther. 2006;32(4):289-304. doi: 10.1080/00926230600666261 [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Published May 28, 2009. Accessed April 18, 2021. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- 18.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 19.Gibson-Helm M, Teede H, Vincent A. Symptoms, health behavior and understanding of menopause therapy in women with premature menopause. Climacteric. 2014;17(6):666-673. doi: 10.3109/13697137.2014.913284 [DOI] [PubMed] [Google Scholar]
- 20.Nebgen DR, Hurteau J, Holman LL, et al. Bilateral salpingectomy with delayed oophorectomy for ovarian cancer risk reduction: a pilot study in women with BRCA1/2 mutations. Gynecol Oncol. 2018;150(1):79-84. doi: 10.1016/j.ygyno.2018.04.564 [DOI] [PubMed] [Google Scholar]
- 21.Madalinska JB, van Beurden M, Bleiker EM, et al. The impact of hormone replacement therapy on menopausal symptoms in younger high-risk women after prophylactic salpingo-oophorectomy. J Clin Oncol. 2006;24(22):3576-3582. doi: 10.1200/JCO.2005.05.1896 [DOI] [PubMed] [Google Scholar]
- 22.Visvanathan K, Vang R, Shaw P, et al. Diagnosis of serous tubal intraepithelial carcinoma based on morphologic and immunohistochemical features: a reproducibility study. Am J Surg Pathol. 2011;35(12):1766-1775. doi: 10.1097/PAS.0b013e31822f58bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heemskerk-Gerritsen BA, Seynaeve C, van Asperen CJ, et al. ; Hereditary Breast and Ovarian Cancer Research Group Netherlands . Breast cancer risk after salpingo-oophorectomy in healthy BRCA1/2 mutation carriers: revisiting the evidence for risk reduction. J Natl Cancer Inst. 2015;107(5):djv033. doi: 10.1093/jnci/djv033 [DOI] [PubMed] [Google Scholar]
- 24.Mavaddat N, Antoniou AC, Mooij TM, et al. ; GENEPSO; EMBRACE; HEBON; kConFab Investigators; IBCCS; kConFab; BCFR . Risk-reducing salpingo-oophorectomy, natural menopause, and breast cancer risk: an international prospective cohort of BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2020;22(1):8. doi: 10.1186/s13058-020-1247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai PL, Miller A, Gail MH, et al. Risk-reducing salpingo-oophorectomy and breast cancer risk reduction in the Gynecologic Oncology Group Protocol-0199 (GOG-0199). J Natl Cancer Inst Cancer Spectr. 2019;4(1):pkz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakkert IE, Mourits MJ, Jansen L, et al. Breast cancer incidence after risk-reducing salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers. Cancer Prev Res (Phila). 2012;5(11):1291-1297. doi: 10.1158/1940-6207.CAPR-12-0190 [DOI] [PubMed] [Google Scholar]
- 27.Surgery in preventing ovarian cancer in patients with genetic mutations. ClinicalTrials.gov identifier: NCT02760849. Updated March 11, 2021. Accessed April 27, 2021. https://clinicaltrials.gov/ct2/show/NCT02760849
- 28.TUBectomy with delayed oophorectomy in high risk women to assess the safety of prevention (TUBA-WISP-II). ClinicalTrials.gov identifier: NCT04294927. Updated June 5, 2020. Accessed April 27, 2021. https://clinicaltrials.gov/ct2/show/NCT04294927
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics per Group (With and Without Postoperative HRT)
eTable 2. Overview of Hormone Replacement Therapy at Three and Twelve Months After Surgery
eTable 3. Subscales of the Greene Climacteric Scale in Women Without Hormone Replacement Therapy
eTable 4. Subscales of the Greene Climacteric Scale in Women With Hormone Replacement Therapy After RRSO
eTable 5. Secondary Outcome Measures
eTable 6. Surgical Complications and Serious Adverse Events (SAE)
eFigure 1. A Schematic Overview of Data Collection and Timing of Questionnaires
eFigure 2. Box Plots of the Greene Climacteric Scale